Abstract
Introduction
Osteitis is one of the most serious complications in orthopedic surgery. Expert Tibia Nail (ETN) PROtect™ coated with a biodegradable layer of gentamicin-laden polymer was developed for prophylaxis of osteomyelitis. In systemic administration, gentamicin has only a small therapeutic index and serious side effects; it is potentially nephrotoxic as well as ototoxic. It is not yet known if relevant gentamicin concentrations are released into the systemic circulation after implantation of gentamicin-coated nails. In order to evaluate the patients’ risks profiles and increase patient safety, we measured gentamicin levels in pre- and postoperative serum samples of patients undergoing implantation of ETN PROtect.
Methods
Twenty-five patients who received ETN PROtect between March 2012 and August 2014 were included in this study. Collection of blood samples occurred before the operation, at weeks 1–4, 3 and 6 months, and up to 1 year after the implantation. Measurement of gentamicin levels in serum samples was performed at the central laboratory of Heidelberg University Hospital. Additionally, laboratory parameters, C-reactive protein, leukocyte number, urea and creatinine concentrations were analyzed in routine controls before and after operating and assessed for systemic side effects.
Results
Over the course of this prospective observational study, we were able to determine that gentamicin-coated nails do not release gentamicin into the systemic circulation above the lowest detectable level of 0.2 mg/dL. There were slight increases in the mean inflammation and renal retention markers, but no gentamicin-associated side effects could be linked to implantation. Furthermore, no allergic reactions could be detected during our study.
Conclusion
Our findings suggest that there is no relevant release of gentamicin into the systemic circulation causing a systemic effect, and serious side effects due to gentamicin-coated tibia nails should not be feared. Postoperative monitoring of renal function does not seem necessary because of the implantation of ETN PROtect.
Introduction
Osteitis is a serious complication in today’s bone reconstructive medicine.Citation1–Citation3 To date (July 2011), PROtect-coated tibial nails have been successfully implanted in over 100 patients. In general treatment of osteitis is rather difficult, with the danger for severe complications such as the threat of losing the extremity or even death in cases of sepsis.Citation3–Citation6 Especially, open fractures after high energy trauma are at risk of infections.Citation7–Citation10 Depending on the severity of the fracture, infection rates range from 6% to 33%.Citation1,Citation10,Citation11 Noteworthy is that up to 80% of all deep infections developed in Gustilo grade III open fractures.Citation12,Citation13
Systemic administration of antibiotics is a routine procedure for prophylaxis of infections in orthopedic surgery.Citation14 Also, systemic prophylaxis has been proven effective; different approaches for antibiotic delivery are becoming more important (antibiotic-impregnated collagen sponges, polymethylmethacrylate beads, and antibiotic-coated implants).Citation1,Citation15–Citation18 Furthermore, local application of antibiotics allows for higher doses of antibiotics without risking serious systemic side effects.Citation3 Antibiotic-coated implants could be an opportunity to end the vicious cycle of infection, multiple revision surgeries, and impaired healing by impeding bacterial colonization and reducing the risk of developing a biofilm on the implants’ surface.Citation3,Citation19–Citation21
For the treatment of extra-articular tibial defects, intramedullary nailing is currently the gold standard.Citation10,Citation22 SYNTHES (SYNTHES AG GmbH, DePuy Synthes Companies, Zuchwil, Switzerland) special antibiotic-coated nails were introduced in 2005 as a universal intramedullary implant for tibial fractures. Up to 2011, over 100 patients were treated with unreamerd tibia Nail (UTN) PROtect or Expert Tibia Nail (ETN) PROtect™. ETN PROtect (DePuy Synthes Companies, Zuchwil, Switzerland) consists of an alloy of titanium, aluminum, and niobium and is coated with an absorbable poly (d, l-lactide) (PDLLA) matrix in which gentamicin sulfate is incorporated. According to the manufacturer, the ETN PROtect is intended for use in open fractures (Gustilo–Anderson Grade I to III), revision surgeries after infection, after polytrauma and in immunosuppressed patients.Citation3
Gentamicin is an antibiotic of the aminoglycoside group.Citation23,Citation24 Its spectrum of action covers most Gram-negative bacteria as well as Staphylococcus aureus.Citation24 Therefore, its active spectrum includes the most common bacterial strains involved in infections after orthopedic surgeries or following open fractures,Citation25–Citation27 namely staphylococcus species, which was found in 50%–80% of infections in an orthopedic setting.Citation19,Citation27 However, gentamicin is potentially nephrotoxic and ototoxic, and renal function should be monitored during the time of systemic administration.Citation28,Citation29 Contraindications for the systemic administration of gentamicin are current pregnancy, inner ear damage, renal insufficiency, myasthenia gravis, and co-medication with other nephrotoxic or ototoxic drugs. Furthermore, a known allergy against gentamicin is a contraindication for use.
Although gentamicin would be suitable for treating osteitis,Citation18 it is not a standard drug for systemic antibiotic prophylaxis in orthopedic surgery because of serious dose-dependent side effects and a small therapeutic index.Citation14,Citation23 A systemic administration of gentamicin is limited to cases of serious infections, when other antibiotics do not work. To hinder complications, therapeutical drug monitoring of gentamicin levels in serum is established.
It has already been shown in experimental research that locally administrated gentamicin does not influence bone healing in a negative way.Citation30 However, it is not known if gentamicin-coated implants cause a relevant systemic exposure to gentamicin. If so, possible contraindications and side effects have to be considered before surgery, and renal function should be monitored after implantation. Previously, we observed a successful application in patients at risk for infection.Citation3
In this study, we investigated how safe ETN PROtect is in a clinical setting, especially its systemic impact, nephrotoxic side effects, and allergenicity. Therefore, gentamicin levels were determined in serum samples taken before and after implantation of ETN PROtect.
Patients and methods
Patients
This study was conducted in accordance with the Declaration of Helsinki.Citation31 All individuals followed the study protocol. The study was approved by the ethics committee of the Ruprecht-Karls-University of Heidelberg (S-636/2011). Patients (n=24) underwent implantation of ETN PROtect/gentamicin-coated tibia nails between March 2012 and August 2014 at our clinic, and were included in the study. Patients were over the age of 18 and gave formal written consent for surgical treatment. Due to fracture of the nail, one patient had to undergo two operations within an interval of 8 months, and both times a coated nail was used. From this patient at both operations tissue and blood samples were collected; therefore in total 25 cases (n=25) from 24 different patients were taken into account.
Serum samples
Peripheral blood samples were drawn over a period of 1 year following a standardized time pattern, as seen in .
Blood was taken by venipuncture (S-Monovette®; SARSTEDT, Nümbrecht, Germany) followed by centrifugation for 10 minutes at 15°C at 3,000 rpm in a Rotixa 50 RS (Fa. Hettich, Tuttlingen, Germany). Supernatant was pipetted into 1.5 mL Eppendorf® reaction vessels and a minimum of 0.6 mL was aliquoted to each vessel. Serum samples were stored at −80°C and only transported once on dry ice for measurement.Citation32,Citation33
Analysis of serum samples was performed in the central laboratory of the Heidelberg University Hospital. Gentamicin concentrations were determined in thoroughly thawed serum samples using the Siemens ADVIA Centaur Gentamicin assay with a Siemens Advia Centaur XPT (Siemens Healthcare Gmbh Eschborn, Germany). Gentamicin quantitation was achieved by a competitive immunoassay using direct chemiluminescent technology – acridinium ester-labeled gentamicin derivative in the reagent for a limited amount of monoclonal mouse anti-gentamicin antibody, which was coupled to paramagnetic particles in the solid phase (REF 05223979; Siemens Healthcare Diagnostics, Erlangen, Germany). Using this method, the lowest possible detectable level of gentamicin was 0.2 mg/L. Literature indicates that doses below 2.0 mg/L seems to be harmless.Citation34,Citation35
Markers of renal retention and inflammation
Urea and creatinine were examined as markers of renal retention. For inflammation, C-reactive protein (CRP) and leukocyte count were used. These parameters were gathered from routine controls before and after the operation and were accessible through patient charts. An initial burst of gentamicin was expected as noted in previous literature; therefore, we analyzed relevant laboratory parameters up to 6 days after the operation.Citation36,Citation37
Statistics
Mean values of laboratory parameters for all patients at different time points were descriptively analyzed. Standard deviations (SDs) for the assessed parameters and range were described.
Results
Patients’ characteristics
Patients demographic details are shown in . The dates of the blood collections for each patient are given in . Body mass index in the patients’ cohort ranged from 18.75 to 52.16 kg/m2. The mean body mass index was 30.81 kg/m2 with an SD of 7.23 kg/m2.
Table 1 Patient demographics
Table 2 Dates (dd/mm/yy) of blood collection for gentamicin detection for each patient
Measurement of gentamicin levels
We were not able to detect gentamicin levels higher than the lowest detectable level of 0.2 mg/L in any of the 139 serum samples.
Evaluation of markers for renal retention and inflammation markers
The preoperative mean values of the collected laboratory parameters were 31.80 mg/dL with an SD of 5.82 mg/dL for urea, 0.95±0.14 mg/dL for creatinine, 7.53±2.22 nL−1 for leukocyte count, and 8.29±9.21 mg/L for CRP. Except for CRP, which has an upper reference range limit of 5 mg/L, all initial values were within the reference range.
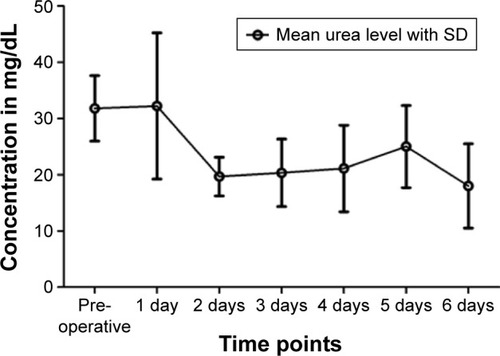
On average, the concentration of urea in patients’ serum decreased from 32.22±13.00 mg/dL on the first day after operation to 19.67±3.43 mg/dL on the second day. It leveled between 25.00±7.29 mg/dL and 18.00±7.48 mg/dL on the following days ().
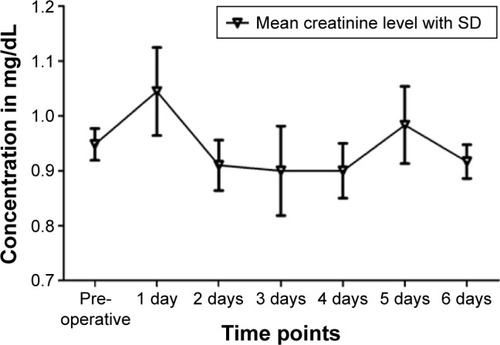
The average creatinine levels increased from the time before the operation (0.95±0.15 mg/dL) to the first day after the operation (1.04±0.24 mg/dL), but dropped below the initial value on the following days, except on the fifth postoperative day (0.98±0.17 mg/dL) ().
In one patient, renal retention markers showed a noticeable increase: creatinine level rose from 1.1 mg/dL pre-operatively to 1.4 mg/dL (reference range <1.3 mg/dL) postoperatively, the urea level likewise from 37 to 55 mg/dL (reference range <45 mg/dL). This was the only sample in which urea and creatinine levels above the upper reference range limit were detected.
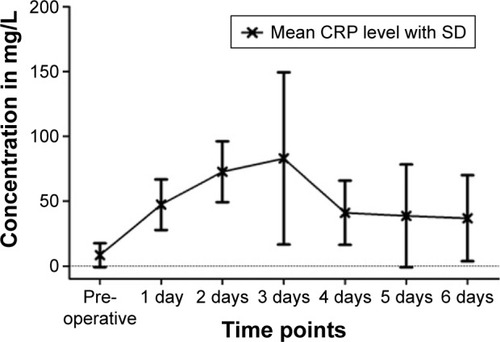
CRP as a marker of inflammation showed on average an increase from 8.29±9.21 mg/L (reference range <5 mg/L) preoperatively to 47.29±19.51 mg/L on the first day, 72.66±23.48 mg/L on the second, and 82.96±66.41 mg/L on the third day postoperatively. A significant decrease was already apparent on the fourth day at 41.01±24.75 mg/L. Subsequently, average CRP values approached the initial value. Several patients showed a CRP above 100 mg/L, mostly on the second or third day after the operation, but all showed declining infection parameters and a decrease within 1 or 2 days thereafter. The highest registered CRP value was 183.3 mg/L ().
Figure 4 Mean values of CRP at different time points.
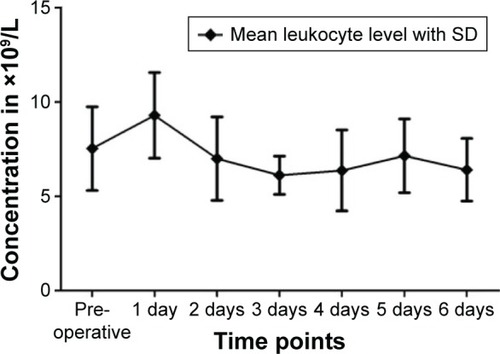
The mean leukocyte count preoperatively was 7.53±2.22 nL−1; on the first postoperative day, it was 9.30±2.27 nL−1. It declined in the following days, but stayed within the reference range.
Leukocyte count differed from the reference range of 4–10 nL−1 in some patients. One patient showed intermittent leukopenia preoperatively as well as postoperatively. Six other patients showed slight leukocytosis as high as 12.74 nL−1 (). These values normalized within 1–2 days.
All patients could be released within 15 days after surgery, with declining infection parameters. No allergic reactions or serious side effects were observed.
Discussion
In this prospective observational study with 25 patients treated with ETN PROtect, we confirmed that gentamicin-coated nails do not release gentamicin above the lowest detectable level of 0.2 mg/dL into the systemic circulation.
The major strengths of this study are its prospective approach and the inclusion of serum samples from the first day after implantation. Osteitis and osteomyelitis are feared complications of orthopedic surgery. Metallic implants are especially prone to infections.Citation8,Citation11 Routine prophylaxis involves the perioperative administration of antibiotics, but this has the downside of systemic side effects and the danger of insufficient doses of antibiotics at the operation site.Citation4,Citation38 An alternative is the local application of antibiotics through drug-coated implants like ETN PROtect. The gentamicin sulfate (up to 20–50 mg) incorporated in the amorphous PDLLA matrix is released in an initial burst followed by a continuous phase, as in vivo and in vitro experiments have shown.Citation37 We were able to demonstrate successful treatment with ETN PROtect in patients with increased risk for infections in the clinical setting.Citation3 Local application of gentamicin has been proven successful and effective in the prophylaxis of infection.Citation1,Citation18,Citation37,Citation39 Local concentrations high enough for prophylaxis do not affect bone healing in a negative way.Citation30 But, in order to increase patient safety and adequately educate patients preoperatively on the risks and effects of the treatment with ETN PROtect, we need to know if a systemic effect is possible. Consequences could be an indication for therapeutic drug monitoring and monitoring of renal function after implantation.
A serious rise of CRP above 200 mg/L within a few hours would have been suspicious for severe inflammation or bacterial infection.Citation40 Since all increased CRP values remained below 200 mg/L and declined within a short period of time, there were no signs of postoperative infections.Citation41,Citation42 Leukocytosis is not only a sign of chronic inflammation, but also a sign of metabolic crisis, acute blood loss, acute hemolysis as well as pain or the body’s reaction to stress.Citation43 We have observed the change in inflammation markers due to trauma during surgery.Citation42,Citation44 We could observe a slight average increase of urea and creatinine that remained within the standard range. Generally, urea may rise due to fever, trauma, hypovolemia, or a catabolic state of metabolism.Citation42,Citation45 Creatinine is primarily a sign of acute renal dysfunction, possibly severe blood loss, damages due to drugs, hemolysis, and chronic renal damage.Citation42 Both parameters can rise to values above the upper reference range limit shortly after a surgery, due to trauma and blood loss. Altogether, the analysis of laboratory parameters showed changes matching a physiological response to surgical trauma.
One patient showed a conspicuous change in creatinine and urea. Infection parameters were also increased, but clinical course of the patient was inconspicuous. Ten days after the operation, renal retention markers were controlled and had returned to the initial value. The patient was released 14 days after surgery, with declining infection parameters. The patient’s records showed a medical history of hypercholesterolemia, overweight, heartburn, back pain, and burnout, but no renal diseases. Preoperative and postoperative medication included analgesics (Voltaren®, Novalgin®), proton-pump inhibitor (Pantozol® 40), low-molecular-weight heparin (Clexane® 60 subcutan), and single-shot antibiotic Zinacef® (cefuroxime) as prophylaxis during surgery. Due to the fact that no gentamicin was detectable in the patient’s blood and renal injury is dose-dependent, we, as well as the attending physicians, do not link the deviation in laboratory parameters to the release of gentamicin. Furthermore, we could not see any clinical signs of renal failure, such as a decrease in urine production, edema, or pleural effusions. We see the intraoperative application of cefuroxime as the cause for the changes in the renal retention markers. Manufacturer’s specifications and the literature indicate that cefuroxime as well as gentamicin can induce acute renal failure.Citation46,Citation47 Overall, no severe side effects occurred, which could be linked to systemic gentamicin exposure.
In the course of this prospective, observational study, we were able to confirm that gentamicin-coated nails do not release gentamicin into the systemic circulation above the lowest detectable level of 0.2 mg/dL. Our findings suggest that no relevant amount of gentamicin for causing a systemic effect is released into the systemic circulation, although in vivo studies showed high local concentrations of gentamicin on the implant site.Citation11,Citation18,Citation30 This fits our observation that no severe side effects could be linked to implantation. Postoperative monitoring of renal function does not seem necessary after implantation of ETN PROtect. Furthermore, no allergic reactions were observed during our study.
The most important strength of this study is its prospective approach. Furthermore, we not only measured gentamicin levels, but also analyzed parameters for infection and renal failure. One advantage of our study is that we were able to take serum samples before operation and on the first day after the operation. In vivo and in vitro studies showed that the release of gentamicin from PDLLA-coating occurs in an initial burst so one can state that evidence of gentamicin in the systemic circulation is most likely seen within hours or on the first day after implantation.Citation37 Fuchs et al already tried to detect gentamicin in serum samples but only analyzed samples from follow-ups on days 4 and 7, week 5, and months 3 and 6 after implantation.Citation11 This could still be improved by analyzing blood samples directly after implantation. Further point for criticism can be that there are more sensitive tools for detecting the actual plasma levels of gentamicin such as high-performance liquid chromatography. However, a study by Walenkamp et al on gentamicin-coated beads could not prove systemic side effects for gentamicin levels up to 0.4 μg/mL; therefore, we consider 0.2 μg/mL to be sensitive enough.Citation48
Conclusion
Our findings suggest that no relevant amount of gentamicin is released into the circulation that could cause a systemic effect. We conclude that serious side effects due to systemic gentamicin release by gentamicin-coated tibia nails are not to be feared. Postoperative monitoring of renal function does not seem necessary because of the implantation of ETN PROtect. In our opinion, ETN PROtect is an important strategy for infection prophylaxis and safe in clinical use.
Acknowledgments
The authors have revealed all financial and personal relationships to other persons and organizations that could inappropriately influence (bias) this work.
Disclosure
GS is a consultant for SYNTHES USA and owns a patent on gentamicin coating of implants. The authors report no other conflicts of interest in this work.
References
- SchmidmaierGLuckeMWildemannBHaasNPRaschkeMProphylaxis and treatment of implant-related infections by antibiotic-coated implants: a reviewInjury200637Suppl 2S105S11216651063
- SchmidmaierGMoghaddamALong bone nonunionZ Orthop Unfall2015153665967626670151
- MoghaddamAZietzschmannSBrucknerTSchmidmaierGTreatment of atrophic tibia non-unions according to ‘diamond concept: Results of one- and two-step treatmentInjury201546Suppl 4S39S5026542865
- LimaALOliveiraPRCarvalhoVCCimermanSSavioEDiretrizes Panamericanas para el Tratamiento de las Osteomielitis e Infecciones de Tejidos Blandos GroupRecommendations for the treatment of osteomyelitisBraz J Infect Dis201418552653424698709
- MiskaMFindeisenSTannerMTreatment of nonunions in fractures of the humeral shaft according to the Diamond ConceptBone Joint J201698-B1818726733519
- MoghaddamAErmischCSchmidmaierGNon-union current treatment conceptShafa Ortho JIn Press2015
- HalawiMJMorwoodMPAcute management of open fractures: An evidence-based reviewOrthopedics20153811e1025e103326558667
- MakridisKGTosounidisTGiannoudisPVManagement of infection after intramedullary nailing of long bone fractures: treatment protocols and outcomesOpen Orthop J2013721922623919097
- MoghaddamAZimmermannGHammerKBrucknerTGrütznerPAvon RecumJCigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fracturesInjury201142121435144221665205
- Moghaddam-AlvandiAZimmermannGHammerKBrucknerTGrütznerPAvon RecumJCigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fracturesInjury201344111670167122935593
- FuchsTStangeRSchmidmaierGRaschkeMJThe use of gentamicin-coated nails in the tibia: preliminary results of a prospective studyArch Orthop Trauma Surg2011131101419142521617934
- GaeblerCBergerUSchandelmaierPRates and odds ratios for complications in closed and open tibial fractures treated with unreamed, small diameter tibial nails: a multicenter analysis of 467 casesJ Orthop Trauma200115641542311514768
- NoumiTYokoyamaKOhtsukaHNakamuraKItomanMIntramedullary nailing for open fractures of the femoral shaft: evaluation of contributing factors on deep infection and nonunion using multivariate analysisInjury20053691085109316054148
- DhammiIKUl HaqRKumarSProphylactic antibiotics in orthopedic surgery: Controversial issues in its useIndian J Orthop201549437337626229155
- MendelVSimanowskiHJScholzHCHeymannHTherapy with gentamicin-PMMA beads, gentamicin-collagen sponge, and cefazolin for experimental osteomyelitis due to Staphylococcus aureus in ratsArch Orthop Trauma Surg2005125636336815864679
- LocaDSokolovaMLocsJSmirnovaAIrbeZCalcium phosphate bone cements for local vancomycin deliveryMater Sci Eng C Mater Biol Appl20154910611325686933
- LetschRRosenthalEJokaTLocal antibiotic administration in osteomyelitis treatment – a comparative study with two different carrier substancesAktuelle Traumatol19932373243297906085
- LuckeMWildemannBSadoniSSystemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat modelBone200536577077815794930
- KnaeplerHLocal application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection in orthopaedic surgeryInt J Surg201210Suppl 1S15S2022659311
- FolschCFedermannMKuehnKDCoating with a novel gentamicin palmitate formulation prevents implant-associated osteomyelitis induced by methicillin-susceptible Staphylococcus aureus in a rat modelInt Orthop201539598198825380688
- InzanaJASchwarzEMKatesSLAwadHABiomaterials approaches to treating implant-associated osteomyelitisBiomaterials201681587126724454
- WesthauserFZimmermannGMoghaddamSReaming in treatment of non-unions in long bones: cytokine expression course as a tool for evaluation of non-union therapyArch Orthop Trauma Surg201513581107111626085339
- KumarCGHimabinduMJettyAMicrobial biosynthesis and applications of gentamicin: a critical appraisalCrit Rev Biotechnol200828317321218937107
- HariprasadGKumarMRaniKKaurPSrinivasanAAminoglycoside induced nephrotoxicity: molecular modeling studies of calreticulin-gentamicin complexJ Mol Model20121862645265222086461
- LuckeMSchmidmaierGSadoniSA new model of implant-related osteomyelitis in ratsJ Biomed Mater Res B Appl Biomater200367159360214528456
- TaylorGJBannisterGCCalderSPerioperative wound infection in elective orthopaedic surgeryJ Hosp Infect19901632412471979574
- DapuntUSprangerOGantzSAre atrophic long-bone nonunions associated with low-grade infections?Ther Clin Risk Manag2015111843185226719698
- Lopez-NovoaJMQuirosYVicenteLMoralesAILopez-HernandezFJNew insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of viewKidney Int2011791334520861826
- LernerSASchmittBASeligsohnRMatzGJComparative study of ototoxicity and nephrotoxicity in patients randomly assigned to treatment with amikacin or gentamicinAm J Med1986806b981043524221
- FassbenderMMinkwitzSKronbachZLocal gentamicin application does not interfere with bone healing in a rat modelBone201355229830423631877
- World Medical AssociationWorld Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjectsJAMA2013310202191219424141714
- MoghaddamABreierLHaubruckPNon-unions treated with bone morphogenic protein 7: introducing the quantitative measurement of human serum cytokine levels as promising tool in evaluation of adjunct non-union therapyJ Inflamm (Lond)201613326807043
- BenderDHaubruckPBoxrikerSKorffSSchmidmaierGMoghaddamAValidity of subjective smoking status in orthopedic patientsTher Clin Risk Manag2015111297130326345646
- DahlgrenJGAndersonETHewittWLGentamicin blood levels: A guide to nephrotoxicityAntimicrob Agents Chemother19758158621164007
- van RaaijTMVisserLEVultoAGVerhaarJAAcute renal failure after local gentamicin treatment in an infected total knee arthroplastyJ Arthroplasty200217794895012375257
- NastSFassbenderMBormannNIn vivo quantification of gentamicin released from an implant coatingJ Biomater Appl Epub2016210
- VesterHWildemannBSchmidmaierGStöckleULuckeMGentamycin delivered from a PDLLA coating of metallic implants: In vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitisInjury201041101053105920541756
- DiefenbeckMMuckleyTHofmannGOProphylaxis and treatment of implant-related infections by local application of antibioticsInjury200637Suppl 2S95S10416651078
- LuckeMSchmidmaierGSadoniSGentamicin coating of metallic implants reduces implant-related osteomyelitis in ratsBone200332552153112753868
- ClyneBOlshakerJSThe C-reactive proteinJ Emerg Med19991761019102510595891
- PepysMBHirschfieldGMC-reactive protein: a critical updateJ Clin Invest2003111121805181212813013
- GressnerAMArndtTLexikon der Medizinischen Laboratoriumsdiagnostik: Band 1 Klinische Chemie. [Dictionary for Medical Laboratory Diagnostic: Volume I Clinical Chemistry]SpringerLink: Bücher2007Berlin, HeidelbergSpringer
- MunkerRHillerEGlassJPaquetteRModern Hematology: Biology and Clinical Management (Contemporary Hematology)2nd edTotowa, NJHumana Press2007
- CzaplickiAPBorgerJEPolitiJRChambersBTTaylorBCEvaluation of postoperative fever and leukocytosis in patients after total hip and knee arthroplastyJ Arthroplasty20112681387138921353453
- GowdaSDesaiPBKulkarniSSHullVVMathAAVernekarSNMarkers of renal function testsN Am J Med Sci20102417017322624135
- LeongCLThiruventhiranTCefuroxime-induced acute renal failureNephron200084218510657721
- TrollforsBSuurkulaMPriceJDNorrbyRRenal function during cefuroxime treatment in patients with pre-existing renal impairmentJ Antimicrob Chemother1980656656706773920
- WalenkampGHVreeTBvan RensTJGentamicin-PMMA beadsPharmacokinetic and nephrotoxicological studyClin Orthop Relat Res19862051711833516500