Abstract
Objectives
Age-related hearing impairment, or presbycusis, is the most common communication disorder and neurodegenerative disease in the elderly. Its prevalence is expected to increase, due to the trend of growth of the elderly population. The current diagnostic test for detection of presbycusis is implemented after there has been a change in hearing sensitivity. Identification of a pre-diagnostic biomarker would raise the possibility of preserving hearing sensitivity before damage occurs. Mitochondrial dysfunction, including the production of reactive oxygen species and induction of expression of apoptotic genes, participates in the progression of presbycusis. Mitochondrial DNA sequence variation has a critical role in presbycusis. However, the nature of the relationship between mitochondrial DNA copy number, an important biomarker in many other diseases, and presbycusis is undetermined.
Methods
Fifty-four subjects with presbycusis and 29 healthy controls were selected after ear, nose, throat examination and pure-tone audiometry. DNA was extracted from peripheral blood samples. The copy number of mitochondrial DNA relative to the nuclear genome was measured by quantitative real-time polymerase chain reaction.
Results
Subjects with presbycusis had a lower median mitochondrial DNA copy number than healthy subjects and the difference was statistically significant (P=0.007). Mitochondrial DNA copy number was also significantly associated with degree of hearing impairment (P=0.025) and audiogram configuration (P=0.022).
Conclusion
The findings of this study suggest that lower mitochondrial DNA copy number is responsible for presbycusis through alteration of mitochondrial function. Moreover, the significant association of mitochondrial DNA copy number in peripheral blood samples with the degree of hearing impairment and audiogram configuration has potential for use as a standard test for presbycusis, providing the possibility of the development of an easy-to-use biomarker for the early detection of this condition.
Introduction
Presbycusis, or age-related hearing impairment, manifests as symmetric, bilateral, sensorineural hearing impairment, occurring slowly over a lifetime.Citation1 It is a major reason for hearing impairment in adults. Recently, Homans et al conducted a large cohort study that showed that 33% of men and 29% of women aged >65 years suffer from presbycusis.Citation2 Presbycusis affects communication skills, gradually leading to isolation, depression, and frustration. Thus, the condition greatly impacts the quality of human life and general health.Citation1,Citation3 Panza et al demonstrated that presbycusis is a risk factor for dementia and cognitive decline.Citation4 There is, as yet, no treatment for presbycusis, which develops over many years and people are usually identified after their hearing sensitivity change.Citation5 Various research groups are seeking a biomarker for this common impairment;Citation6–Citation9 development of a sensitive and specific biomarker for detection and prediction of presbycusis could reduce the impact of the condition on the health of society in the future.
Mitochondria are intracellular bodies, and their impairment contributes to presbycusis.Citation10 These are the only cellular organelles that contain their own genomes, known as mtDNA. According to the mitochondrial theory of aging proposed by Harman,Citation11 accumulation of reactive oxygen species (ROS) produced by mitochondria causes damage to intracellular macromolecules, particularly mtDNA. A lack of introns and protective histones make mtDNA more susceptible to ROS and other types of damage, which could lead to sequence mutations or copy number alterations.Citation12 Variation of both quality (mutations)Citation13,Citation14 and quantity (copy number)Citation15 of mtDNA has been associated with many human diseases, including neurodegenerative diseases,Citation16 metabolic diseases, and various types of cancer,Citation17 as well as in the process of aging.Citation18 Therefore, the application of mtDNA alteration as a biomarker in aging, age-related diseases, cancers, metabolic diseases, and even forensic science is currently being investigated.Citation19,Citation20
In recent years, studies have identified some chromosomal loci and genes associated with presbycusis.Citation3,Citation21,Citation22 A comparison of the mtDNA from temporal bones of presbycusis patients with that of healthy controls revealed a significant relationship between large-scale deletions in mtDNA and presbycusis.Citation23–Citation25 In addition to large deletions, there are reports of single nucleotide changes to mitochondrial genomes associated with presbycusis.Citation26 To determine the role of mtDNA copy number in the development of presbycusis and investigate the possibility of using mtDNA copy number as a biomarker for this impairment, we compared the mtDNA copy number in subjects with presbycusis and healthy controls by quantitative real-time polymerase chain reaction (PCR).
Materials and methods
All 50- to 90-year-old volunteers participating in the molecular investigation of presbycusis at the ENT and Head and Neck Research Center and Department, Iran University of Medical Sciences completed a questionnaire, based on that developed by the European presbycusis consortium, which included questions to determine a full medical history and details of exposure to environmental factors that affect hearing.Citation21,Citation27 Participants with confounding factors (any known reason for hearing loss, exposure to ototoxic drugs, and other pathologies correlated with hearing loss, such as cardiovascular disorder, liver cirrhosis, renal failure, and head and neck tumor), neurodegenerative diseases, and cancers were excluded. Otoscopic examination and pure-tone audiometry were performed on all volunteers. Air conduction was measured at octave frequencies of 250–8,000 Hz and bone conduction at 250–4,000 Hz using an Amplaid 319 audiometer (Milan, Italy). Calibration was performed according to the American National Standards Institute (ANSI, 2004a). Subjects with any kind of middle ear pathology affecting hearing sensitivity, conductive hearing loss (mean air-bone differences at 500, 1,000, and 2,000 Hz of ≥15 dB), and extensive noise exposure (self-reported) were excluded.
Presbycusis patients (n=54) with symmetric, bilateral hearing impairment with the pure-tone average of mean frequencies 1, 2, 4, and 8 kHz of ≥30 dB HL were selected. Healthy subjects (n=29) with normal ear examination and the pure-tone average of mean frequencies ≥25 dB HL were selected. The mean age of patients was 66.87±8.9 years and that of healthy subjects was 63±5.3 years. The gender ratio was 54.2% male and 45.8% female, and there were no statistically significant differences in age and gender between presbycusis and healthy subjects (P>0.05). This study was approved by the Ethics Committee of Iran University of Medical Sciences, and all subjects provided signed informed consent.
Genomic DNA was extracted from peripheral blood samples using the QIAamp DNA Mini Kit (Cat No 51304; Qiagen, Hilden, Germany) according to the manufacturer’s protocol.
Relative mtDNA copy number was determined through simultaneous assessment of the nuclear gene, TMIE (NC_000003.12), and the mitochondrial gene, MT-RNR1 (NC_012920.1), using quantitative PCR (qPCR). Primers were as follows: MT-RNR1-F, 5′-AGTAGAGTGCTTAGTTGAGC-3′; MT-RNR1-R, 5′-AGTACACTTCCATGTTACG-3′; TMIEF, 5′-CCATTCCTTGGGTCTCTGAA-3′; and TMIER, 5′-AGCAGAGGAACAGGGTGAC-3′. qPCR was performed using a Rotor-gene 6000 instrument (Corbett Life Science, Sydney, Australia) and QuantiFast SYBR Green PCR master mix (Cat No 204054, Qiagen). All reactions were performed in duplicate with total reaction volumes of 10 μL, containing 5 μL of 1X Quanti Fast SYBR Green PCR master mix, 0.12 μL of each primer (10 pmol), 0.7 μL of DNA, and 4 μL of RNase-free water. After initial heating for 1 min at 95°C as an activation step, 40 cycles of denaturation at 95°C for 10 s, followed by annealing and extension at 60°C for 45 s, were performed.
The threshold cycle (Ct) of each sample was determined for MT-RNR1 and TMIE. Relative mtDNA copy number was determined using the following equation: 2−ΔCt (Δ Ct = CtMT-RNR1 − CtTMIE).
Standard curves were prepared for both nuclear and mitochondrial PCR. R2 correlation for each standard curve was ≥0.99. Melting curve analysis of qPCR and running of PCR products on agarose gels (1.5%) were performed to confirm amplification of a specific product without primer dimmer or unwanted PCR product.
Statistical analyses were performed using SPSS 22 for Windows (IBM, Chicago, IL, USA). All numerical variables were checked for normal distribution using the one-sample Kolmogorov–Smirnov test. The association between the mtDNA copy number in presbycusis and healthy subjects was evaluated using the Mann–Whitney U-test. The associations between mtDNA copy number and degree of hearing impairment and audiogram configuration were evaluated using Kruskal–Wallis H-test. Statistical significance was defined as P<0.05.
Results
The degree of hearing impairment of subjects with presbycusis was classified into five categories according to the American Speech–Language–Hearing Association: mild, moderate, moderately severe, severe, and profound.Citation28 Patients in this study suffered from mild, moderate, and moderately severe hearing impairment ().
Figure 1 (A) Classification of presbycusis patients by degree of hearing impairment (n=54). (B) Classification of patients by shape of audiogram.
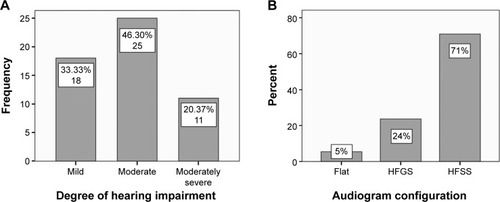
Based on audiogram configuration described previously, patients were divided into five groups: flat, high-frequency gently sloping (HFGS), high-frequency steeply sloping (HFSS), low-frequency ascending, and mid-frequency U-shape.Citation29 Presbycusis subjects in this study exhibited flat, HFGS, and HFSS shapes ().
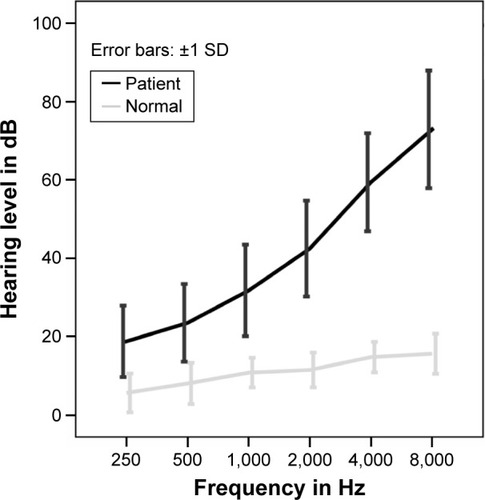
The mean audiograms of presbycusis patients and healthy subjects are presented in .
Figure 2 Mean audiograms of patients with presbycusis and healthy subjects. Error bars represent SDs.
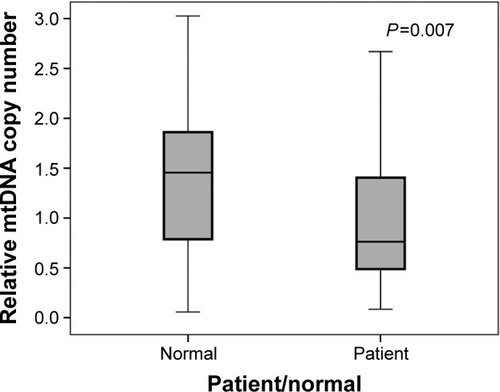
The mitochondrial copy number of study participants was evaluated by measuring the proportion of mtDNA relative to nuclear DNA using the level of MT-RNR1 as an evaluation index of the mitochondrial genome and that of TMIE for nuclear DNA. Median mtDNA copy number for subjects with presbycusis was 0.76, whereas that of healthy subjects was 1.45, indicating that the mitochondrial copy number of subjects with presbycusis was almost halved compared with the healthy controls (). The difference in mtDNA copy number between the two groups was statistically significant (P=0.007).
Figure 3 Distribution of peripheral blood mtDNA copy number in Iranian patients with presbycusis and healthy controls. The relative mtDNA copy number was evaluated by measuring the proportion of mtDNA relative to nuclear DNA. The difference between mtDNA copy number in the presbycusis and healthy subject groups was statistically significant (Mann–Whitney U-test, P=0.007). The box plots show the median (solid line across the box), inter-quartile range (shaded boxes), and range (whiskers).
A Kruskal–Wallis H-test showed that there was a statistically significant difference in mtDNA copy number between the different hearing degrees, χ2(3) =9.33, P=0.025, with a mean rank mtDNA copy number of 50.79 for normal, 29.72 for mild, 38.20 for moderate, and 44.64 for moderately severe.
A Kruskal–Wallis H-test showed that there was a statistically significant difference in mtDNA copy number between the different audiogram configurations, χ2(2) =7.62, P=0.022, with a mean rank mtDNA copy number of 51.23 for flat shape, 32.96 for HFGS shape, and 37.68 for HFSS shape.
Discussion
Presbycusis is categorized as a complex or multifactorial disorder.Citation30,Citation31 Factors affecting its development include genetic background, environmental factors, ototoxic drugs, medical conditions, and hormonal factors.Citation32–Citation34 Presbycusis occurs as a result of degeneration of both the inner ear structure and the central auditory system of the brain.Citation35–Citation37 Mitochondria play a key role in cellular damage and death in the aging process of hearing.Citation38,Citation39 Mitochondrial dysfunction is related to the development of certain neurodegenerative, age-related diseases, and cancer.Citation14,Citation40 Mutations in the MT-RNR1 and MT-TF genes can cause hearing loss.Citation26,Citation41 Investigation of the temporal bone of presbycusis patients showed an increase in deletions and point mutations in mtDNA. Bai et al detected a 4,977 bp deletion, known as the common deletion, in the mtDNA of human cochlear tissue of presbycusis patients.Citation23,Citation24 Markaryan demonstrated that the expression of the MT-CO3 gene is decreased in spiral ganglion cells from individuals with presbycusis, suggesting that deficits in the electron transport chain of spiral ganglion cells contribute to presbycusis.Citation25
The results of this study indicate a statistically significant decrease in mtDNA copy number in presbycusis subjects. Our study is the first to investigate the relationship between mtDNA copy number and presbycusis. Previously, Santos et al proposed a decrease in mtDNA copy number as an important marker for oocyte quality, having shown that such a decrease is due to insufficient mitochondrial biogenesis or cytoplasmic maturation, causing fertilization failure.Citation42 Studies of the relationship between neurodegenerative disease and mtDNA content have produced similar results to those of our study. Podlesniy et al suggested low content of mtDNA in cerebrospinal fluid as a novel biomarker for the early detection of preclinical Alzheimer’s disease.Citation43 Petersen et al demonstrated reduced mtDNA copy number in blood leukocytes from patients with Huntington’s disease.Citation16
Our results are consistent with previous studies demonstrating that presbycusis is commonly a high-frequency hearing impairment, as HFSS was the most common configuration in patients in this study (70.9%), followed by HFGS (23.64%) ().Citation1,Citation44
According to previous studies, a decrease in the number of mitochondrial genomes can cause impaired or reduced mitochondrial activity. A study using HeLa cells indicated that depletion of the mitochondrial genome by decreasing catalase expression enhances oxidative damage to nuclear DNA and influences nuclear genome stability.Citation45 Oxidative stress is associated with excessive ROS production in the mitochondrial matrix; ROS increases death of cellular structures of the cochlear, leading to an increase in mutations in the mitochondrial genome.Citation40 In transgenic mice, accumulation of mtDNA mutations by mitochondrial dysfunction influences energy metabolism. The consequent reduction in energy production induces apoptosis, leading to a loss of critical cochlear cells and resulting in age-related hearing impairment.Citation46
The results of this study, together with those of previous reports demonstrating the relationship between mtDNA sequence variation and presbycusis,Citation23,Citation26,Citation47 highlight the role of mtDNA in presbycusis progression. A significant reduction in the number of mitochondrial genomes in presbycusis subjects, due to mitochondrial dysfunction, may be one of the molecular mechanisms involved in the development of this impairment. In addition, quantification of mtDNA in peripheral blood samples may constitute an easily assessed biomarker of presbycusis, since the high-sensitivity qPCR-based assay can detect even minute variations in mtDNA copy number. This blood biomarker could easily be incorporated into regular physical examinations as it employs a simple and cheap screening method. Moreover, the results of this study demonstrate that mtDNA copy number is statistically significantly associated with the degree of hearing (P=0.025) and audiogram configuration (P=0.022). Clearly, these results require further confirmation in a larger study population to determine whether the method is appropriate for clinical application. In addition, more research is required to understand the relationship between the decrease in mtDNA copy number and the onset of presbycusis.
Age-related hearing impairment is the most common sensory disease in the elderly population.Citation10 With the growth of the aging population, the prevalence of age-related disorders such as presbycusis is increasing.Citation1 The World Health Organization estimates that in 2025, more than 500 million individuals will suffer from presbycusis.Citation48 Presbycusis begins slowly, without clinical manifestation. Further human studies are required to elucidate methods for early diagnosis and prevention.
Acknowledgments
We appreciate the participation of all the volunteers in this research and would also like to thank those in Audiometry and Clinical Department, Rasoul Akram Hospital, Iran University of Medical Sciences for their kind cooperation during this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- HuangQTangJAge-related hearing loss or presbycusisEur Arch Otorhinolaryngol201026781179119120464410
- HomansNCMetselaarRMDingemanseJGPrevalence of age-related hearing loss, including sex differences, in older adults in a large cohort studyLaryngoscope Epub201675
- CiorbaAHatzopoulosSBianchiniCAimoniCSkarzynskiHSkarzynskiPHGenetics of presbycusis and presbystasisInt J Immunopathol Pharmacol2015281293525816403
- PanzaFSolfrizziVLogroscinoGAge-related hearing impairment-a risk factor and frailty marker for dementia and ADNat Rev Neurol201511316617525686757
- CarsonAJ“What brings you here today?” The role of self-assessment in help-seeking for age-related hearing lossJ Aging Stud2005192185200
- MichikawaTNakamuraTImamuraHMarkers of overall nutritional status and incident hearing impairment in community-dwelling older Japanese: the Kurabuchi StudyJ Am Geriatr Soc20166471480148527310369
- WilliamsonTTZhuXWaltonJPFrisinaRDAuditory brainstem gap responses start to decline in mice in middle age: a novel physiological biomarker for age-related hearing lossCell Tissue Res2015361135936925307161
- PangJXiongHYangHCirculating miR-34a levels correlate with age-related hearing loss in mice and humansExp Gerontol201676586726802970
- KuchinskySEAhlstromJBCuteSLHumesLEDubnoJREckertMASpeech-perception training for older adults with hearing loss impacts word recognition and effortPsychophysiology201451101046105724909603
- FujimotoCYamasobaTOxidative stresses and mitochondrial dysfunction in age-related hearing lossOxid Med Cell Longev2014201458284925110550
- HarmanDThe biologic clock: the mitochondria?J Am Geriatr Soc19722041451475016631
- LeeHCWeiYHMitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stressInt J Biochem Cell Biol200537482283415694841
- DowlatiMADerakhshandeh-PeykarPHoushmandMNovel nucleotide changes in mutational analysis of mitochondrial 12S rRNA gene in patients with nonsyndromic and aminoglycoside-induced hearing lossMol Biol Rep20134032689269523242658
- GreavesLCReeveAKTaylorRWTurnbullDMMitochondrial DNA and diseaseJ Pathol2012226227428621989606
- Clay MontierLLDengJJBaiYNumber matters: control of mammalian mitochondrial DNA copy numberJ Genet Genomics200936312513119302968
- PetersenMHBudtz-JørgensenESørensenSAReduction in mitochondrial DNA copy number in peripheral leukocytes after onset of Huntington’s diseaseMitochondrion201417142124836434
- ZhouWZhuMGuiMPeripheral blood mitochondrial DNA copy number is associated with prostate cancer risk and tumor burdenPLoS One2014910e10947025279731
- Zabihi DibaLMohaddes ArdebiliSMGharesouranJHoushmandMAge-related decrease in mtDNA content as a consequence of mtDNA 4977 bp deletionMitochondrial DNA A DNA Mapp Seq Anal20162743008301226152346
- WallaceDCA mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicineAnnu Rev Genet20053935940716285865
- MeissnerCBrusePMohamedSAThe 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more?Exp Gerontol200843764565218439778
- Van LaerLHuygheJRHannulaSA genome-wide association study for age-related hearing impairment in the SaamiEur J Hum Genet201018668569320068591
- NewmanDLFisherLMOhmenJGRM7 variants associated with age-related hearing loss based on auditory perceptionHear Res20122941–212513223102807
- MarkaryanANelsonEGHinojosaRQuantification of the mitochondrial DNA common deletion in presbycusisLaryngoscope200911961184118919358252
- BaiUSeidmanMDHinojosaRQuirkWSMitochondrial DNA deletions associated with aging and possibly presbycusis: a human archival temporal bone studyAm J Otol19971844494539233484
- MarkaryanANelsonEGHinojosaRMajor arc mitochondrial DNA deletions in cytochrome c oxidase-deficient human cochlear spiral ganglion cellsActa Otolaryngol2010130778078720085441
- ZhuYZhaoJFengBMutations in the mitochondrial 12S rRNA gene in elderly Chinese peopleActa Otolaryngol20151351263425376778
- Van EykenEVan LaerLFransenEKCNQ4: a gene for age-related hearing impairment?Hum Mutat200627101007101616917933
- ClarkJGUses and abuses of hearing loss classificationASHA19812374935007052898
- MostafaHSaadMEl-AttarAMitochondrial DNA (mtDNA) haplotypes and dysfunctions in presbycusisActa Otorhinolaryngol Ital2014341546124711684
- OhlemillerKKAge-related hearing loss: the status of Schuknecht’s typologyCurr Opin Otolaryngol Head Neck Surg200412543944315377958
- FalahMHoushmandMFarhadiMPresbycusis: From current knowledge to future treatment prospectsJ Isfahan Med School201634382526535 Persian
- LinFRThorpeRGordon-SalantSFerrucciLHearing loss prevalence and risk factors among older adults in the United StatesJ Gerontol A Biol Sci Med Sci201166558259021357188
- CharitidiKFrisinaRDVasilyevaONZhuXCanlonBExpression patterns of estrogen receptors in the central auditory system change in prepubertal and aged miceNeuroscience201017041270128120736049
- TadrosSFD’SouzaMZettelMLZhuXLynch-ErhardtMFrisinaRDSerotonin 2B receptor: upregulated with age and hearing loss in mouse auditory systemNeurobiol Aging20072871112112316822592
- OhlemillerKKFrisinaRDAge-related hearing loss and its cellular and molecular basesSchachtJPopperANFayRRAuditory Trauma, Protection, and RepairBoston, MASpringer US2008145194
- FrisinaDRFrisinaRDSpeech recognition in noise and presbycusis: relations to possible neural mechanismsHear Res19971061–2951049112109
- ZettelMLZhuXO’NeillWEFrisinaRDAge-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent systemJ Assoc Res Otolaryngol20078228029317453307
- WongACRyanAFMechanisms of sensorineural cell damage, death and survival in the cochleaFront Aging Neurosci201575825954196
- FalahMNajafiMHoushmandMFarhadiMExpression levels of the BAK1 and BCL2 genes highlight the role of apoptosis in age-related hearing impairmentClin Interv Aging2016111003100827555755
- KamogashiraTFujimotoCYamasobaTReactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing lossBiomed Res Int2015201561720725874222
- BalaliMKamalidehghanBFarhadiMAssociation of nuclear and mitochondrial genes with audiological examinations in Iranian patients with nonaminoglycoside antibiotics-induced hearing lossTher Clin Risk Manag20161211712826889084
- SantosTAEl ShourbagySSt JohnJCMitochondrial content reflects oocyte variability and fertilization outcomeFertil Steril200685358459116500323
- PodlesniyPFigueiro-SilvaJLladoALow cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer diseaseAnn Neurol201374565566823794434
- UchidaYSugiuraSSoneMUedaHNakashimaTProgress and prospects in human genetic research into age-related hearing impairmentBiomed Res Int2014201439060125140308
- DelsiteRLRasmussenLJRasmussenAKKalenAGoswamiPCSinghKKMitochondrial impairment is accompanied by impaired oxidative DNA repair in the nucleusMutagenesis200318649750314614184
- SomeyaSYamasobaTKujothGCThe role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gammaNeurobiol Aging20082971080109217363114
- ManwaringNJonesMMWangJJMitochondrial DNA haplogroups and age-related hearing lossArch Otolaryngol Head Neck Surg2007133992993317875861
- SprinzlGMRiechelmannHCurrent trends in treating hearing loss in elderly people: a review of the technology and treatment options – a mini-reviewGerontology201056335135820090297
