Abstract
Background
Acute heart failure (AHF) is a serious condition that is associated with increased mortality in critically ill patients. Previous studies indicated that environmental exposure to cadmium increases mortality of general populations. However, the relationship of cadmium exposure and mortality is unclear for AHF patients.
Materials and methods
A total of 153 patients with AHF in intensive care units (ICUs) met the inclusion criteria and were followed up for 6 months. Demographic data, AHF etiology, hematological and biochemical data, and hospital mortality were recorded. The scores of two predictive systems (Sequential Organ Failure Assessment [SOFA], Acute Physiology and Chronic Health Evaluation II [APACHE II]) for mortality in critically ill patients were calculated, and urinary cadmium levels were recorded.
Results
At the end of the follow-up period, the mortality rate was 24.8%. The survivors (n=115) had higher urinary cadmium levels on day 1 (D1UCd) of ICU admission than non-survivors (n=38). A multiple linear regression analysis revealed a positive correlation between D1UCd and acute kidney injury, but a negative correlation between D1UCd and the level of serum albumin. A multivariate Cox analysis indicated that D1UCd was an independent predictor of mortality in AHF patients. For each increment of 1 μg of D1UCd, the hazard ratio for ICU mortality was 1.20 (95% confidence interval [CI]: 1.09–1.32, P<0.001). The area under the receiver operating characteristic curve for D1UCd was 0.84 (95% CI: 0.78–0.91), better than the values for the SOFA and APACHE II systems.
Conclusion
The D1UCd may serve as a single predictor of hospital mortality for AHF patients in the ICU. Because of the high mortality and smaller sample size, more investigations are required to confirm these observations and elucidate the underlying mechanisms.
Introduction
Acute heart failure (AHF) is a serious disease associated with high morbidity and mortality.Citation1 When the symptoms of AHF are severe in the patients of intensive care units (ICUs), aggressive medical treatment and organ support are required. Only a few studies have examined the epidemiology of ICU patients with AHF,Citation2,Citation3 although some studies have examined specific aspects of such patients.Citation4,Citation5
Cadmium is a well-known toxic metal.Citation6 Environmental and occupational exposure to cadmium is associated with several clinical diseases, including renal dysfunction, bone disease, and some cancers.Citation6 The half-life period for cadmium is approximately 10 to 30 years and the absorbed cadmium is eliminated from the body primarily in urine.Citation6 Because the urinary excretion of cadmium correlates well with cadmium accumulation in human bodies, the urinary cadmium levels may serve as an indicator of body cadmium burden.Citation7 However, the correlation between urinary cadmium levels and mortality is uncertain in AHF patients.
In the past 20 years, several scoring systems have been used to evaluate illness severity and predict mortality for critically ill patients, such as the Sequential Organ Failure Assessment (SOFA), the Acute Physiology and Chronic Health Evaluation II (APACHE II), the Simplified Acute Physiology Score, and the Multiple Organ Dysfunction Score.Citation8–Citation11 However, these scoring systems are complicated and time-consuming in clinical practice, and there may be significant inter-observer variability among the physicians who calculate these scores.Citation12 Moreover, there is still no single variable accurately predicting mortality in patients with AHF.
In this study, we evaluated the association between urinary cadmium levels and outcome of AHF patients and compared these results to the SOFA and APACHE II scoring systems.
Methods
This clinical study complied with the guidelines of the Declaration of Helsinki, and was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital, a tertiary referral center in Taiwan (IRB number: 101-3653C).
Patient information and data collection
All participants were at least 18 years old and admitted to the cardiac ICU or medical ICU. The included patients presented fatigue, limitation of physical activity, orthopnea and dyspnea, which were compatible with the typical symptoms of heart failure of the New York Heart Association Functional class III or IV.Citation13 The diagnosis of AHF was based on a detailed taking of their history, the assessment of signs/symptoms of congestion and/or hypoperfusion of organs, and confirmed by appropriate additional studies, including electrocardiography, chest roentgenogram and echocardiography, which were recommended by the guidelines of the American College of Cardiology/American Heart Association and the European Society of Cardiology.Citation14,Citation15 The exclusion criteria were as follows: total urine less than 300 mL/day on admission day 1; duration of ICU stay less than 1 day; duration of hospital stay more than 120 days; presence of end-stage renal disease; readmission to the ICU; history of occupational, residential, or other exposure to cadmium or history of intoxication from other heavy metals. In this study, acute respiratory failure was defined as acute onset of respiratory failure that required mechanical ventilator support. Acute kidney injury (AKI) was defined as serum creatinine (SCr) above 2.0 mg/dL and/or daily urine amount less than 500 mL.
For each patient we collected demographic data, laboratory data, length of stay in the ICU, pre-existing chronic disease, etiology of AHF, clinical condition upon ICU admission, data for scoring predictive indices (at least 12 h before death in non-survivors), and outcome. The blood samples were taken within 24 h after ICU admission. The urine samples were collected during the first 24 h to determine the total amount of urinary cadmium on day 1 (D1UCd) of ICU admission.
This study was approved by the Institutional Review Committee of our hospital and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from every study patient.
Clinical scoring systems
The APACHE II and SOFA scoring models were used to assess the severity of illness for every enrolled participant. We calculated APACHE II scores as described previously;Citation8 physiological assessments were performed using the highest abnormal physiological values obtained on day 1 of ICU admission. We evaluated SOFA scores as described by Vincent et al;Citation11 the highest abnormal value was recorded for each of the six organ systems on day 1 of ICU admission.
Measurement of urinary cadmium
The urine samples of study patients were collected in cadmium-free bottles. The urinary cadmium levels were measured by the method proposed by Jin et al.Citation16 Briefly, 100 μL of urine and 500 μL of trace-metal-grade distilled 0.8 M HNO3 were added to a 1.5 mL Eppendorf vessel, which was shaken immediately. After overnight storage in a refrigerator, these vessels were permitted to warm to room temperature and whirl-mixed for 5–10 s. Then, the vessels were spun in an Eppendorf centrifuge for 5 min at 11,500 rpm. The clear supernatant samples were obtained after the above procedures and transferred directly to graphite furnace containers. We employed electrothermal atomic absorption spectrometry (SpectrAA-220 Zeeman; Varian, Palo Alto, CA, USA) to determine the cadmium levels in these samples. We confirmed the quality control consistently by internal and external quality control procedures. In addition, we used a certified commercially prepared product (Seronorm Trace Elements; Sero AS, Billingstad, Norway) to evaluate the intra-batch accuracy and ensure inter-batch standardization. The coefficient of variation for measurements of cadmium was ≤5.0%.
Statistical analysis
The Kolmogorov-Smirnov test was used to determine the normal distribution for continuous variables. Continuous variables are expressed as means ± standard deviations or medians with interquartile ranges (IQRs), and categorical variables as numbers and percentages. Continuous variables were compared using the Student’s t-test or Mann–Whitney U test, as appropriate. Categorical variables were compared using the chi-squared test or Fisher’s exact test. We used the linear regression model to identify factors associated with D1UCd. All potential variables (P<0.05) in a simple linear regression model were entered into a multiple linear regression model with backward stepwise procedures. We used the Cox proportional hazard model to measure all variables and determine their significance for prediction of mortality. The hazard ratios (HRs) and 95% confidence intervals (CIs) for death were obtained with this model. All potential variables (P<0.05) in a univariate model were entered into a multivariate Cox model with forward stepwise procedures. We applied the Hosmer-Lemeshow (HL) test to evaluate the goodness-of-fit of these predictive models.Citation17 We plotted receiver operating characteristic (ROC) curves and calculated the area under curve (AUC) to evaluate the performance of different predictors.Citation18 The SPSS 18.0 for Windows XP (SPSS Inc., Chicago, IL,USA) software package was used in all analyses, and a two-sided P-value <0.05 was considered significant.
Results
Patient characteristics
A total of 153 critically ill patients with AHF (94 males and 59 females) were enrolled in this study (). The mean patient age was 70.1±14.2 years. The median duration of ICU stay was 7.0 days (IQR: 4.0–20.0), and the median duration of hospital stay was 21.0 days (IQR: 13.0–36.5). The median score of APACHE II was 15.0 (IQR: 10.0–20.0) and the median score of SOFA was 5.0 (IQR: 3.0–8.0). The median D1UCd was 0.998 μg/day (IQR: 0.475–2.287) and the mean D1UCd was 2.15±3.86 μg/day. The main three etiologies were ischemic heart disease (64.1%, n=98), rheumatic heart disease (9.2%, n=14), and valvular heart disease (8.5%, n=13). The initial status at ICU admission was as follows: 79 (51.6%) patients had acute respiratory failure requiring mechanical ventilation due to acute pulmonary edema, 54 (35.3%) had cardiogenic shock and received inotropic agents, 47 (30.7%) had acute myocardial infarction (AMI) or unstable angina, and 57 (37.3%) had AKI. The overall mortality rate was 24.8% (n=38). As shown in and , the baseline characteristics and laboratory parameters of the survivors (n=115) and non-survivors (n=38) were compared. These results indicated that non-survivors had longer ICU stays, higher prevalence of AMI and AKI, higher APACHE II and SOFA scores, higher levels of D1UCd, and higher levels of blood urea nitrogen (BUN) and SCr. More of the survivors were smokers and survivors had higher levels of serum albumin (Alb) and hemoglobin (Hgb).
Table 1 Baseline characteristics of acute heart failure patients on admission to the ICU (n=153)
Table 2 Baseline vital signs and biochemical data of acute heart failure patients on admission to the ICU (n=153)
Determinants of urinary cadmium excretion
As shown in , simple linear regression analysis demonstrated D1UCd was positively related to AKI, but negatively related to the serum levels of Alb and Hgb. Stepwise multiple linear regression analysis indicated that D1UCd was positively related to AKI (Beta ± standard error: 0.807±0.444, P=0.046), but negatively related to the serum level of Alb (Beta ± standard error: −1.294±0.421, P=0.025).
Table 3 Determinants of urinary cadmium excretion in acute heart failure patients on day 1 of ICU admission (n=153)
Cox regression analysis for mortality
Univariate Cox regression analysis indicated that AKI, high respiratory rate, high BUN, high APACHE II score, and high D1UCd were potential predictors of mortality. As shown in , we used a multivariate Cox regression analysis with forward stepwise methods to determine the independent effects of these potential predictors. The results indicated that high respiratory rate and high D1UCd were independent predictors of ICU mortality. The HR of mortality for each increment of 1 time/min in respiratory rate was 1.11 (95% CI: 1.03–1.20, P=0.004) and the HR of mortality for each increment of 1 μg/day in urinary cadmium was 1.20 (95% CI: 1.09–1.32, P<0.001).
Table 4 Forward multivariate Cox analysis of risk factors for hospital mortality in acute heart failure patients on day 1 of ICU admission (n=153)
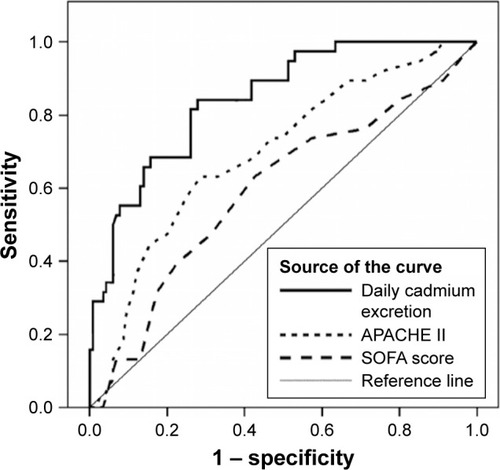
Goodness-of-fit and ROC curves of scoring systems and D1UCd
The HL chi-square test was used to evaluate the goodness-of-fit and the results were obtained as follows: (1) D1UCd (HL chi-square =8.32, 8 degrees of freedom [df], P=0.403); (2) APACHE II (HL chi-square =5.93, 8 df, P=0.655); (3) SOFA (HL chi-square =3.84, 7 df, P=0.798). As shown in , the ROC curves were plotted for the two scoring models and D1UCd, and AUCs were also calculated. The results demonstrated that D1UCd (AUC =0.84±0.03, 95% CI: 0.78–0.91, P<0.001) had better discriminatory performance than the APACHE II score (AUC =0.70±0.05, 95% CI: 0.60–0.77, P<0.001) or the SOFA score (AUC =0.60±0.06, 95% CI: 0.49–0.70, P=0.080).
Figure 1 Receiver operating characteristic curves based on intensive care unit day 1 urinary cadmium excretion (AUC =0.84±0.03, 95% CI: 0.78–0.91), APACHE II score (AUC =0.70±0.05, 95% CI: 0.60–0.77), and SOFA score (AUC =0.60±0.06, 95% CI: 0.49–0.70).
Discussion
This 6-month observational study demonstrated that the main cause of AHF in these critically ill patients was ischemic heart disease (64.1%), similar to two previous studies of AHF patients (70% and 61%).Citation2,Citation3 The mortality rate was 24.8%, also similar to previous studies of ICU patients with AHF (30% and 27.4%).Citation2,Citation3 Notably, we found that D1UCd was remarkably related to ICU mortality after adjusting for potential variables, including APACHE II and SOFA scores. The HR of mortality for each increment of 1 μg/day in urinary cadmium levels was 1.20. A review of the literature indicates this study is the first to reveal that urinary cadmium level was remarkably correlated with mortality of AHF patients.
There are several scoring modelsCitation8–Citation11 which are used to evaluate disease severity and predict the outcome of critically ill patients, including APACHE II and SOFA. Although these two models are widely applied in clinical practice, they are not user-friendly systems. In addition, such models are subject to significant inter-observer differences.Citation19 Therefore, a single, reliable, and unbiased predictor of mortality is needed for critical patients because these patients often require prompt and aggressive treatments. Based on the study results, we found that the D1UCd is a simple predictor of mortality for critically ill AHF patients.
The general population (without occupational exposure to cadmium) has a urinary excretion of cadmium less than 2 μg/day,Citation20 but there are no similar data of urinary cadmium levels in AHF patients, after reviewing the previous reports. In this study, the mean D1UCd of critically ill AHF patients was 2.15±3.86 μg/day, which was higher than that (1.07±1.45 μg/day)Citation21 of the general population in Taiwan. Although the physiological mechanism underlying the connection between elevated cadmium levels in biologic fluids and heart failure is unknown, several studies provide some insight. In rats, the heart muscle cells could be deformed by cadmium poisoning because of the increment of free radicals and lipid peroxidation, based on ultrastructural examination.Citation22 Another rat study indicated that cadmium acted solely as an inhibitor of heart pyruvate-malate-supported mitochondrial respiration, suggesting that disturbances in myocardial metabolism and function may occur following cadmium exposure.Citation23 Furthermore, an epidemiologic study of 12,049 US participants demonstrated that urinary cadmium levels were significantly correlated with self-reported prevalence of heart failure.Citation24 A prospective cohort study of 3,348 adults in the US also revealed a significant association between urine cadmium and increased incidence of heart failure.Citation25 Meanwhile, a study of 4,378 participants in Sweden with a 17-year follow-up period demonstrated that the participants in the fourth quartile of blood cadmium levels had significantly higher incidence of heart failure than those in the first quartile.Citation26 All of these findings suggest that elevated cadmium levels may exacerbate the critical condition and increase hospital mortality in AHF patients. However, further investigations are needed to identify the mechanism and confirm the causal effect.
In general, AUC lower than 0.7 was considered as an indicator of poor performance, and higher than 0.8 as good performance. The analytic results indicated that D1UCd outperformed the APACHE II and SOFA scoring models and had the highest AUC (0.84±0.03, 95% CI: 0.78–0.91). We also used the HL goodness-of-fit test to evaluate the calibration of models. Generally, HL statistics lower than 15 and P-values of 0.2 to 0.8 indicate a good fit.Citation27,Citation28 The study results showed D1UCd had a good calibration (HL chi-square =8.32, P=0.403). These findings revealed that D1UCd had a high discriminatory power and good calibration as a predictor of mortality for AHF patients, and was better than the established predictive scoring systems. Therefore, D1UCd may serve as a single predictor of mortality for AHF patients. It remains to be demonstrated whether lowering the body cadmium levels of AHF patients by chelation therapy will reduce their disease severity and improve their survival rate.
Patients admitted for AHF often develop exacerbated clinical renal function,Citation29 which is mediated through several mechanisms, including renal hypoperfusion caused by low cardiac output, elevated central venous pressure, activation of renin-angiotensin-aldosterone system, endothelial dysfunction, and others.Citation29 Moreover, some drugs for treating AHF and AMI are also associated with impairment of renal function, especially loop diuretics.Citation29 In addition, hypoalbuminemia is common in patients with AHF,Citation30 which may result from enhanced transcapillary escape rate of Alb from the intravascular to extravascular space, loss of nutrients due to increased hepatic venous congestion, decreased synthesis of Alb by liver and protein-losing enteropathy.Citation30 Furthermore, AKI itself would accelerate protein breakdown, which is attributed to acute disease process, uremic toxins, inflammatory mediators, metabolic acidosis, hormonal imbalance and other factors, resulting in hypoalbuminemia.Citation31 In the current study, we demonstrated D1UCd was positively correlated with AKI, but negatively correlated with serum Alb level in AHF patients. Cadmium exposure has well-known toxic effects on the kidneysCitation16 and severe AHF may induce AKI in critically ill patients, so higher urinary cadmium levels might be observed in this population. Moreover, the increased cadmium levels in biologic fluids may influence the synthesis and metabolism of Alb, based on several in vitro studies. For example, Wan et alCitation32 found lower Alb production in rat hepatocytes treated with cadmium and these perturbations were not alleviated after the cells were returned to cadmium-free medium. Gena et alCitation33 reported that cadmium can lead to albuminuria in a pig proximal tubular cell model by impairing reabsorption and secretion. However, further studies are needed to explore the causal relationships between cadmium, AKI, and serum Alb in patients with AHF.
Cadmium has a long biological half-life (10–30 years) in humans and mainly accumulates in the liver and kidney.Citation6,Citation34 After uptake from the lung or the gastrointestinal tract, cadmium in blood is bound to Alb and high molecular weight proteins and transported to the liver,Citation35 where it is bound to metallothionein. After a few days of cadmium exposure, cadmium-metallothionein compounds appear in the blood.Citation35 Because of the small molecular size, these compounds are efficiently filtered by the glomeruli and reabsorbed by the proximal tubule cells.Citation6,Citation35,Citation36 Then, the absorbed cadmium accumulates in the kidneys for a long time with a low rate of urinary excretion.Citation6 Although cadmium exposure is a risk factor of cardiovascular diseases in humans,Citation25,Citation26 and may disturb myocardial metabolism (suggested by animal studies),Citation22,Citation23 it is uncertain whether the basic metabolism of cadmium would be changed in patients with cardiac diseases, including heart failure. Hence, further studies are needed to elucidate its specific role in AHF patients.
Cigarettes are one of the most prominent sources of cadmium exposure, and are associated with an increased mortality due to cardiovascular diseases.Citation6,Citation37 Cadmium oxide produced during the burning of tobacco has a high bioavailability in humans.Citation38 Approximately 30%–40% of the inhaled cadmium oxide is absorbed into the blood circulatory system.Citation38 Compared with non-smokers, smokers have 4–5 times higher cadmium levels in their blood and 2–3 times higher contents of cadmium in their kidneys.Citation38 Although previous studies demonstrated that smoking was a well-known risk factor of mortality and significantly correlated with blood cadmium level, we observed that survivors were more likely to be smokers than non-survivors (50.4% vs 28.9%, P=0.024) in these AHF patients. In addition, we found that D1UCd remained independently associated with increased mortality after adjustment for smoking in this study, which indicated that other causes except smoking may be the sources of cadmium exposure in these study patients. Since patients with history of occupational exposure to cadmium or history of intoxication from other heavy metals were excluded, the source of cadmium is most likely from the exposure in daily life such as contaminated food, drinking water, or passive smoking. Further studies are required to explore whether reducing cadmium exposure reduces the mortality of AHF patients.
There are several limitations of this study. First, although D1UCd was significantly associated with hospital mortality in critical AHF patients, the causal relationship is uncertain. Future investigations are required to clarify the underlying mechanisms. Second, not having serial assessments of B-type natriuretic peptide (BNP) is a limitation because a BNP immunoassay was not available to us at the time of this study. BNP reflects cardiac structure and function and can assist in the diagnosis of AHF. However, whether BNP can predict short-term outcome of AHF remains unclear.Citation39 Third, due to the fact that this study was conducted at a single institution with a heterogeneous group of subjects, the study results may not apply to other hospitals. Hence, a multi-center investigation with more patients is warranted to verify the current findings in the future.
Conclusion
This is the first study to demonstrate that the D1UCd was significantly and independently associated with hospital mortality of critical AHF patients. Notably, the D1UCd has good calibration and discriminatory power, and outperformed the established predictive scoring systems. Moreover, D1UCd is a single variable with minimal inter-observer differences. Therefore, D1UCd should be considered a good predictor of mortality for critical AHF patients. Further investigations are needed to clarify these observations.
Disclosure
The authors report no conflicts of interest in this work.
References
- Siirilä-WarisKLassusJMelinJPeuhkurinenKNieminenMSHarjolaVPFINN-AKVA Study GroupCharacteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failureEur Heart J200627243011301717127708
- RudigerABusingerFStreitMSchmidERMaggioriniMFollathFPresentation and outcome of critically ill medical and cardiac-surgery patients with acute heart failureSwiss Med Wkly20091397–811011619234879
- ZannadFMebazaaAJuilliereYClinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: The EFICA studyEur J Heart Fail20068769770516516552
- NieminenMSBrutsaertDDicksteinKEuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of populationEur Heart J200627222725273617000631
- YancyCWLopatinMStevensonLWDe MarcoTFonarowGCADHERE Scientific Advisory Committee and InvestigatorsClinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) DatabaseJ Am Coll Cardiol2006471768416386668
- JärupLBerglundMElinderCGNordbergGVahterMHealth effects of cadmium exposure – a review of the literature and a risk estimateScand J Work Environ Health199824Suppl 1151
- BuchetJPLauwerysRRoelsHRenal effects of cadmium body burden of the general populationLancet199033687176997021975890
- KnausWADraperEAWagnerDPZimmermanJEAPACHE II: a severity of disease classification systemCrit Care Med198513108188293928249
- Le GallJRLemeshowSSaulnierFA new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter studyJAMA199327024295729638254858
- MarshallJCCookDJChristouNVBernardGRSprungCLSibbaldWJMultiple organ dysfunction score: a reliable descriptor of a complex clinical outcomeCrit Care Med19952310163816527587228
- VincentJLMorenoRTakalaJThe SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care MedicineIntensive Care Med19962277077108844239
- PoldermanKHGirbesARThijsLGStrack van SchijndelRJAccuracy and reliability of APACHE II scoring in two intensive care units. Problems and pitfalls in the use of APACHE II and suggestions for improvementAnaesthesia2001561475011167435
- BennettJARiegelBBittnerVNicholsJValidity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac diseaseHeart Lung200231426227012122390
- HuntSAAmerican College of CardiologyAmerican Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure)ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure)J Am Coll Cardiol2005466e1e8216168273
- NieminenMSBohmMCowieMRExecutive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of CardiologyEur Heart J200526438441615681577
- JinTNordbergMFrechWCadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad)Biometals200215439741012405535
- LemeshowSHosmerDWJrA review of goodness of fit statistics for use in the development of logistic regression modelsAm J Epidemiol19821151921067055134
- HanleyJAMcNeilBJThe meaning and use of the area under a receiver operating characteristic (ROC) curveRadiology1982143129367063747
- PoldermanKHJornaEMGirbesARInter-observer variability in APACHE II scoring: effect of strict guidelines and trainingIntensive Care Med20012781365136911511950
- BernardALauwerysREffects of cadmium exposure in humansFoulkesECHandbook of experimental pharmacology198680135177
- LinJLLuFHYehKHIncreased body cadmium burden in Chinese women without smoking and occupational exposureJ Toxicol Clin Toxicol19953366396448523485
- OzturkIMBuyukakilliBBalliECimenBGunesSErdoganSDetermination of acute and chronic effects of cadmium on the cardiovascular system of ratsToxicol Mech Methods200919430831719778222
- KislingGMKoppSJPaulsonDJHawleyPLTowJPInhibition of rat heart mitochondrial respiration by cadmium chlorideToxicol Appl Pharmacol19878932953043603562
- PetersJLPerlsteinTSPerryMJMcNeelyEWeuveJCadmium exposure in association with history of stroke and heart failureEnviron Res2010110219920620060521
- Tellez-PlazaMGuallarEHowardBVCadmium exposure and incident cardiovascular diseaseEpidemiology201324342142923514838
- BorneYBarregardLPerssonMHedbladBFagerbergBEngstromGCadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort studyBMJ Open201556e007366
- AngusDCClermontGKramerDJLinde-ZwirbleWTPinskyMRShort-term and long-term outcome prediction with the Acute Physiology and Chronic Health Evaluation II system after orthotopic liver transplantationCrit Care Med200028115015610667515
- RosenbergALRecent innovations in intensive care unit risk-prediction modelsCurr Opin Crit Care20028432133012386493
- CarubelliVMetraMLombardiCRenal dysfunction in acute heart failure: epidemiology, mechanisms and assessmentHeart Fail Rev201217227128221748453
- UthamalingamSKandalaJDaleyMSerum albumin and mortality in acutely decompensated heart failureAm Heart J201016061149115521146671
- WiedermannCJWiedermannWJoannidisMHypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studiesIntensive Care Med201036101657166520517593
- WanXLachapelleMMarionMFournierMDenizeauFRecovery potential of hepatocytes from inhibition of albumin secretion by cadmiumJ Toxicol Environ Health19933843813928478980
- GenaPCalamitaGGugginoWBCadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cellsEnviron Health Perspect2010118111551155620576581
- JärupLCadmium overload and toxicityNephrol Dial Transplant200217Suppl 23539
- NordbergMGeneral aspects of cadmium: transport, uptake and metabolism by the kidneyEnviron Health Perspect19845413206734552
- Tellez-PlazaMJonesMRDominguez-LucasAGuallarENavas-AcienACadmium exposure and clinical cardiovascular disease: a systematic reviewCurr Atheroscler Rep2013151035623955722
- HsuCWYenTHChenKHEffect of blood cadmium level on mortality in patients undergoing maintenance hemodialysisMedicine (Baltimore)20159442e175526496294
- SatarugSMooreMRAdverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smokeEnviron Health Perspect2004112101099110315238284
- CarpenterCRKeimSMWorsterARosenPBEEM (Best Evidence in Emergency Medicine)Brain natriuretic peptide in the evaluation of emergency department dyspnea: is there a role?J Emerg Med201242219720522123173
