Abstract
Background
Thoracic endovascular aortic repair (TEVAR) has become an emerging treatment modality for acute type B aortic dissection (TBAD) patients in recent years. The risk factors and impacts of acute kidney injury (AKI) after percutaneous TEVAR, however, have not been widely established.
Methods
We retrospectively studied the clinical records of 305 consecutive patients who admitted to our institution and had TEVAR for TBAD between December 2009 and June 2013. The patients were routinely monitored for their renal functions preoperatively until 7 days after TEVAR. The Kidney Disease Improving Global Guidelines (KDIGO) criteria were used for AKI.
Results
Of the total 305 consecutive patients, 84 (27.5%) developed AKI after TEVAR, comprising 66 (21.6%) patients in KDIGO stage 1, 6 (2.0%) patients in stage 2 and 12 (3.9%) patients in stage 3. From the logistic regression analysis, systolic blood pressure (SBP) on admission >140 mmHg (odds ratio [OR], 2.288; 95% CI, 1.319–3.969) and supra-aortic branches graft bypass hybrid surgery (OR, 3.228; 95% CI, 1.526–6.831) were independent risk factors for AKI after TEVAR. Local anesthesia tended to be a protective factor (OR, 0.563; 95% CI, 0.316–1.001). The preoperative renal function, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker or statin administration, volume of contrast agent, range of TBAD and false lumen involving renal artery were not associated with post-operation AKI. The in-hospital mortality and major adverse events were markedly increased with the occurrence of AKI (7.1% vs 0.9%, P=0.006; 14.3% vs 3.2%, P<0.001, respectively).
Conclusions
TEVAR for TBAD has a high incidence of AKI, which is associated with worse in-hospital outcomes. SBP on admission and supra-aortic branches graft bypass hybrid surgery were the most significant risk factors. Renopreventive measures should be considered in high-risk patients.
Introduction
Despite its infrequent progression to permanent dialysis dependence, acute kidney injury (AKI) is associated with extended intensive care, prolonged hospital stay, diminished quality of life and shorter long-term survival.Citation1–Citation4 AKI after thoracic endovascular aortic repair (TEVAR) of thoracic aortic diseases has been documented in several studies, with incidences ranging from 1.5% to 34%.Citation5–Citation7
TEVAR is emerging as an important treatment option for type B aortic dissection (TBAD) to induce aortic remodeling by sealing the proximal entry tear, to prevent late complications and to avoid the risk associated with open surgery at the same time.Citation8–Citation12 Several less invasive novel techniques have been introduced in aortic dissection endovascular repair in recent years, such as percutaneous technique, hybrid approach and rapid artificial cardiac pacing for inducing hypotension during stent deployment. However, the impact of these techniques on the renal dysfunction after TEVAR is still an area of ongoing research.Citation13–Citation15
As far as we know, little research has been focused on the risk factors and impacts of AKI after TEVAR on TBAD. Most studies have focused on the types of conditions requiring TEVAR, including aneurysms, dissections and transections to penetrating ulcers.Citation3,Citation7 However, TBAD presents with other pathologic states that may influence renal function in a number of ways: 1) the blood supply for the side branch of aorta from the true lumen (TL)/false lumen (FL) may influence renal perfusion. Side branch artery occlusions caused by obstruction from the dissection flap, which can either prolapse across a vessel origin without entering it (dynamic obstruction) or directly extend into a vessel (static obstruction);Citation16 2) the TL/FL hemodynamic change and inflammatory response after aortic stent graft deployment may impact on the renal function postoperatively;Citation17 3) because of a complicated TL and FL association, the contrast agent may be administrated more to make sure whether the stent graft is implanted in the TL and at the right place and 4) the blood pressure (BP) change during procedure such as controlling depressurization during stent deployment may reduce the renal perfusion. All of these features may lead to postoperative renal dysfunction, such as contrast-induced nephropathy, renal ischemia, renal artery embolization or occlusion, renal infarction or ischemia–reperfusion injury.Citation18 The purpose of this paper is to determine the risk factors and impact of AKI on TEVAR for TBAD.
Methods
Patient population
Data from all patients admitted to the Guangdong Cardiovascular Institute, Guangdong General Hospital, who underwent percutaneous TEVAR for TBAD between December 2009 and June 2013 were retrospectively analyzed. Patients who received TEVAR, including complicated or uncomplicated TBAD, with definite detecting tear entry were included into the study. Data were collected by cardiologists and were entered by EpiData software 3.1 (The EpiData Association, Odense, Denmark) using consistency check on 2 copies. The study was approved by the ethics committees of Guangdong General Hospital, and written informed consent was obtained from all the patients participated in the study.
Exclusion criteria included 1) previous endovascular repair for aortic disease, 2) medical history of dialysis-dependent renal failure, 3) patients who have received extra renal artery revascularization, 4) associated on-pump or off-pump bypass graft surgery, 5) patients who are deemed technically unsuccessful, 6) connective tissue disorders and 7) age <18 years or pregnant. Patients were also excluded in case of death in the operating room before or during procedure (n=3), lack of test of renal function postoperatively (n=12) or lack of computed tomography (CT) image in the institution’s medical record system (n=6). About 305 patients met the criteria and were recruited into the study.
Perioperative management
Patients presenting with a nonemergent status and a preoperative creatinine >2.0 mg/dL underwent preoperative hydration. We gave the patients isotonic saline (1 mL/kg/hour) from 12 hours preprocedure to 24 hours postprocedure. We routinely administered prophylactic antibiotics (cefuroxime) 30 minutes to 1 hour before procedure and then again 12 hours after the procedure.
Description of procedure
Percutaneous TEVAR was conducted following standard procedures described previously,Citation13 which began with 18-gauge needle puncture of the common femoral arteries (CFAs) under fluoroscopy. According to CT image, the entry points were based on the anatomy relationship between CFA and femoral head. Two or multiple 6F Perclose ProGlide devices were deployed in the CFA before upsizing to a 20–25F sheath. The sutures were secured to close the arteriotomy at the end of the procedure. The construction of the pathway in the TL for stent graft delivery was guided and confirmed by sectional angiography or intravascular ultrasound. The thoracic stent grafts used included Medtronic Talent (Medtronic, Minneapolis, MN, USA), Zenith TX2 (COOK, Bjaeverskov, Denmark) and Hercules (Microport, Shanghai, China), which were approved by the State Food and Drug Administration during the period of the study. Briefly controlled hypotension was utilized during device deployment, and then again if balloon aortoplasty was performed by using the rapid artificial cardiac pacing technique.Citation15,Citation19 Since November 2009, stent size selection for all cases has been oversized by 10%–15%. To prevent posterior circulation ischemia, we performed supra-aortic branch graft bypass surgery before TEVAR in cases of right vertebral artery dominance with insufficient aortic arch landing zone.Citation20 The patients underwent regional anesthesia unless needed additional supra-aortic branches graft bypass surgery.
Definitions
Aortic dissection was defined as disruption of the medial layer provoked by intramural bleeding, resulting in separation of the aortic wall layers and subsequent formation of a TL and an FL with or without communication. The AKI was diagnosed according to the KDIGO criteria from Kidney Disease Improving Global Guidelines.Citation21 We used the maximum change in serum creatinine level in the first 7 days after surgery to classify patients according to the KDIGO criteria. The estimated glomerular filtration rate (eGFR) was calculated with the chronic kidney disease epidemiology collaboration formular.Citation22 The major adverse events were defined as death, stroke, paraplegia and the need for renal replacement therapy (RRT).
Statistical analysis
Mean ± SD or medians (interquartile range) were used to describe continuous variables; intergroup differences were evaluated by Student’s t-test or nonparametric Mann–Whitney U-test, depending on the distribution of variables. Categorical variables were presented as frequencies, and percentages were compared by Fisher’s exact test or χ2 test. Stepwise multivariate logistic regression variables were fitted from variables found to have marginal associations with AKI on univariate testing (P<0.10). Odds ratios (ORs), 95% CIs and probability values are reported. All statistical analyses were performed using SPSS software, version 19.0 (IBM Inc., Chicago, IL, USA).
Results
Population
A total of 305 consecutive patients were recruited: 84 (27.5%) developed AKI after TEVAR, consisting of 66 (21.6%) patients with KDIGO stage 1, 6 (2.0%) patients in stage 2 and 12 (3.9%) patients in stage 3. The mean age was 54.5±10.3 years, and 269 (88.2%) of patients were men. About 252 (82.6%) patients had a history of hypertension. Forty-four (14.4%) patients were on angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, with no significant difference between the 2 groups. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) tended to be higher in the AKI group than in the non-AKI group (SBP: 148.63±24.57 mmHg vs 138.45±22.31 mmHg, P=0.001; DBP: 83.21±13.43 mmHg vs 79.34±13.13 mmHg, P=0.013). There was no significant difference between the 2 groups in terms of pre-operation renal function (creatinine: 130.60±141.04 μmol/L vs 116.08±80.18 μmol/L, P=0.620; blood urea nitrogen (BUN): 7.00±4.40 mmol/L vs 6.61±3.91 mmol/L, P=0.593 and eGFR: 72.27±28.48 mL/min/1.73 m2 vs 72.02±24.6 mL/min/1.73 m2, P=0.743, respectively). The results of the white blood cell count, hemoglobin (HGB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), uric acid (URIC), and D-dimer (DDI) were similar in both groups, as shown in .
Table 1 Baseline clinical characteristics of patient population
Aortic dissection features
About 144 (47.1%) patients were presented with pleural effusion, which was comparable between the AKI group and the non-AKI group (47.5% vs 46.4%, P=0.699). There was no significant difference between the AKI group and the non-AKI group in terms of FL involvement of celiac trunk, superior mesenteric artery, inferior mesenteric artery and renal artery. The characteristics of the vascular situation of aortic dissection are presented in .
Table 2 Aortic dissection features and AKI
Operative data and outcome
Thirty-eight (12.4%) patients received supra-aortic branch bypass with a higher incidence in the AKI group compared with the non-AKI group (23.8% vs 8.1%, P=0.004). There was no significant difference in the volume of contrast medium and the number of cover stent observed between the AKI group and the non-AKI group. The in-hospital mortality, RRT and major adverse events were markedly increased with the occurrence of AKI (death: 7.1% vs 0.9%, P=0.006; RRT: 6.0% vs 0.5%, P=0.007, major adverse events: 14.3% vs 3.2%, P<0.001, respectively; ). The in-hospital mortality (P<0.001) and major adverse events (P<0.001) were associated with the severity of AKI ().
Figure 1 In-hospital mortality (A) and major adverse events (B) with AKI stages.
Abbreviations: AKI, acute kidney injury; RRT, renal replacement therapy.
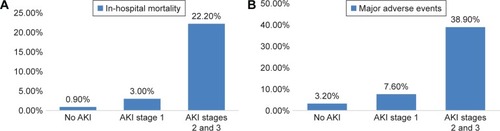
Table 3 Operative data and outcome
Risk factors for AKI
Owing to the limited positive events, we performed the logistic regression analysis with the following variables: age >60 years, male gender, SBP when admitted to hospital (>140 mmHg), local anesthesia, supra-aortic branches graft bypass hybrid surgery, diabetes mellitus, coronary artery disease and at least one side of renal artery involvement by FL. SBP >140 mmHg (OR, 2.288; 95% CI, 1.319–3.969) on admission and supra-aortic branches graft bypass hybrid surgery (OR, 3.228; 95% CI, 1.526–6.831) were independent risk factors for AKI after TEVAR. Local anesthesia tended to be a protective factor (OR, 0.563; 95% CI, 0.316–1.001). The FL involving renal artery was not an independent risk factor ().
Table 4 Risk factors of AKI
Discussion
The current study indicates that AKI is a common problem after percutaneous TEVAR, which is associated with in-hospital mortality and complications. In our study, we found that the incidence of AKI after TEVAR was 27.5%, including 21.6% in KDIGO stage 1, 2.0% in stage 2 and 3.9% in stage 3. The in-hospital outcomes of death and events were associated with the severity of AKI. Patients in stages 2 and 3 had significant higher rates of in-hospital mortality and major adverse events. Similar incidence of AKI was found in the study by Zhu et al,Citation23 which identified AKI in 48 (30.8%) of 156 TEVAR for TBAD patients according to RIFLE (risk, injury, failure, lose and end-stage renal disease) classification, with 7 (14.5%) patients requiring continuous RRT. Other previous studies, however, have suggested the rates of AKI after endovascular repair varying from 1.5% to 34%.Citation5–Citation7 The observed differences in incidence rates could be due to a couple of reasons. First, the lack of consistent definition of renal failure used in these studies could account for the differences in the published rates of AKI after endovascular repair. In this study, we chose the criteria of KDIGO AKI which referenced the AKIN criteria from Acute Kidney Injury Network to define the renal dysfunction as it is a more sensitive indicator of renal function and can be used as a risk factor to predict long-term survival.Citation24,Citation25 Second, the differences in various study populations may be another reason. The previous studies focusing on AKI after endovascular repair included patients of abdominal aortic aneurysm, thoracic aortic aneurysms, Stanford TBADs, penetrating thoracic ulcers and traumatic aortic transection.Citation5–Citation7 The rates of postprocedure AKI after endovascular repair for thoracic or abdominal aortic aneurysm alone were relatively low ranging from 2% to 17%,Citation26–Citation29 when compared with TEVAR for TBAD which ranged from 27.5% to 30.8%.Citation23 The differences in pathophysiology between aortic dissection and aneurysm may also be factor to consider, including hemodynamic changes by the TL/FL, the dissection flap, the load of contrast agent and the controlled BP depressurization during stent deployment.
For the risk factors of AKI, SBP on admission >140 mmHg and supra-aortic branch graft bypass hybrid surgery were identified as independent risk factors by multivariate regression analysis. Local anesthesia tended to be a protective factor (OR, 0.563; 95% CI, 0.316–1.001). Previous studies have found risk factors of AKI after endovascular aortic repair to include chronic kidney disease, acute dissection, complicated dissection, mal-perfusion complications,Citation18 thoracoabdominal extention, postoperative transfusion,Citation7 intraoperative hypotension, stroke, sepsis, lengthy procedures and number of stents.Citation30 Again, the different findings may be because of the different aortic pathological changes, operative strategies and perioperative administration.
Higher BP was associated with the occurrence of AKI. In a previous study,Citation31 multivariate analysis showed that SBP on admission and bilateral renal artery involvement were strong predictors of preoperative AKI for TBAD. Higher SBP on admission may be associated with renal artery involvement because the renin–angiotensin–aldosterone system, which is activated because of renal artery involvement, induces a dramatic increase in BP. Furthermore, higher SBP may lead to sustainable expansion of the FL, causing generalized ischemia to the kidney leading to AKI. It was found that BP variability is an independent risk factor for the prognosis of aortic dissection. We could not identify BP variability in this current study from the retrospective design. However, our data indicate that higher BP on admission is not only a risk factor for AKI preoperatively but also post-TEVAR.
Supra-aortic branches graft bypass hybrid surgery often require general anesthesia, lengthy procedures, more blood loss, postoperative transfusion, higher rates of intraoperative hypotension, more severe inflammatory response and assisted mechanical ventilation. These factors may take the responsibility for a higher incidence of AKI after TEVAR.Citation23,Citation30,Citation32,Citation33
The most important features of this current study are taking the changes of aortic dissection anatomy into account. Effusion and partial thrombosis were reported to be associated with unfavorable prognosis.Citation34,Citation35 However, it seems they may not be related to AKI in this current study. The FL directly extending to the renal artery may be associated with AKI after TEVAR. However, the TL/FL relationship between renal artery may not be an independent risk factor. Owing to the limited sample size of this study, we could not provide enough evidence to confirm or refute this issue. Further studies are needed to explore the real implication of renal artery involvement by the FL. It may be easier to predict that the end-organ malperfusion caused by TBAD will turn better after TEVAR with improvement in the blood supply by the TL after TEVAR. However, the perfusion of side branch artery after TEVAR may be a little more complicated as the end-organ ischemia may deteriorate when blood is supplied by TL and FL at the same time or just FL. Therefore, the renal malperfusion will not always be relieved after TEVAR. Similar findings documented in previous reports corroborate the fact that patients, who had AKI before TEVAR was performed, had no improvements in their renal function but rather had higher incidences of renal failure after TEVAR.Citation31 In conclusion, it is vitally important that we pay more attention to the hemodynamic changes of side branch artery involvement by an FL.
Several limitations exist in this study. It was a retrospective, uncontrolled study and therefore subject to inherent limitations in the study design. The study is also limited by the lack of urine output measurements. Moreover, as we did not monitor the blood flow of renal artery, we could not define the exact time of renal hypoperfusion. The short- and long-term mortality was also unclear in AKI patients with TBAD; thus, in-hospital mortality may have been underestimated.
Conclusion
In summary, our data indicate that AKI is still a common problem after percutaneous TEVAR for TBAD, which is associated with worse in-hospital outcomes. Specifically, SBP on admission >140 mmHg and supra-aortic branches graft bypass hybrid surgery were the most relevant predictive factors of AKI after TEVAR. However, the extents of dissection and branch artery involvements, the renal function preoperatively, AECI/ARB or statin administration, volume of contrast agent and range of TBAD were not related to AKI. Renal preventive measures should be considered in high-risk patients. The significance of imaging and anatomic changes in AKI after TEVAR are worth further studies.
Acknowledgments
The authors thank Nianjin Xie and Jie Li for their great help in contribution to conception, design and acquisition of data. They also thank Lambert Tetteh Appiah (Komfo Anokye Teaching Hospital, Kumasi, Ghana) for the revision of text.
The study was supported by the Science and Technology Programme of Guangdong Province (2014A020215023), Science and Technology Programme of Guangzhou (201508020114) and Science and Technology Programme of Guangzhou (201300000180).
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkBMavanurADreznerADGallagherJMenzoianJOClinical impact of chronic renal insufficiency on endovascular aneurysm repairVasc Endovascular Surg2006406437445
- BoyleJRAcute kidney injury predicts mortality after endovascular aortic repairEur J Vasc Endovasc Surg201550443126073045
- PisimisisGTBecharaCFBarshesNRLinPHLaiWSKougiasPRisk factors and impact of proximal fixation on acute and chronic renal dysfunction after endovascular aortic aneurysm repair using glomerular filtration rate criteriaAnn Vasc Surg2013271162223088805
- LokCEAustinPCWangHTuJVImpact of renal insufficiency on short- and long-term outcomes after cardiac surgeryAm Heart J2004148343043815389229
- EggebrechtHBreuckmannFMartiniSFrequency and outcomes of acute renal failure following thoracic aortic stent-graft placementAm J Cardiol200698445846316893697
- FairmanRMCriadoFFarberMVALOR InvestigatorsPivotal results of the medtronic vascular talent thoracic stent graft system: the VALOR trialJ Vasc Surg200848354655418572352
- PiffarettiGMariscalcoGBonardelliSPredictors and outcomes of acute kidney injury after thoracic aortic endograft repairJ Vasc Surg20125661527153423058721
- SongTKDonayreCEWalotIEndograft exclusion of acute and chronic descending thoracic aortic dissectionsJ Vasc Surg200643224725816476595
- NienaberCARousseauHEggebrechtHRandomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trialCirculation2009120252519252819996018
- NienaberCAKischeSRousseauHINSTEAD-XL trialEndovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trialCirc Cardio vasc Interv201364407416
- FattoriRMontgomeryDLovatoLSurvival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD)JACC Cardio vasc Interv201368876882
- ErbelRAboyansVBoileauCESC Committee for Practice Guidelines2014ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC)Eur Heart J201435412873292625173340
- NiZHLuoJFHuangWHTotally percutaneous thoracic endovascular aortic repair with the preclosing technique: a case-control studyChin Med J (Engl)2011124685185521518591
- ZhangTJiangWLuHLiuJThoracic endovascular aortic repair combined with assistant techniques and devices for the treatment of acute complicated Stanford type B aortic dissections involving aortic archAnn Vasc Surg201632889726806251
- ChenJHuangWLuoSYangDXuZLuoJApplication of rapid artificial cardiac pacing in thoracic endovascular aortic repair in aged patientsClin Interv Aging20149737824403824
- HiratzkaLFBakrisGLBeckmanJA2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular MedicineJ Am Coll Cardiol20105514e27e12920359588
- ChangCKChuterTANiemannCUSystemic inflammation, coagulopathy, and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repairJ Vasc Surg20094951140114619394543
- TwineCPBoyleJRRenal dysfunction after EVAR: time for a standard definitionJ Endovasc Ther201320333133323731305
- HuangWHHePCLuoJFA randomized controlled trial of rapid artificial cardiac pacing in thoracic endovascular aortic repairZhonghua Yi Xue Za Zhi2011912416681672 Chinese21914313
- FuWGDongZHWangYQStrategies for managing the insufficiency of the proximal landing zone during endovascular thoracic aortic repairChin Med J (Engl)2005118131066107116098257
- KellumJALameireNDiagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1)Crit Care201317120423394211
- MatsushitaKMahmoodiBKWoodwardMComparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rateJAMA2012307181941195122570462
- ZhuJChenSJinGAcute renal injury after thoracic endovascular aortic repair of Stanford type B aortic dissection: incidence, risk factors, and prognosisJ Formos Med Assoc2014113961261924613460
- KhwajaAKDIGO clinical practice guidelines for acute kidney injuryNephron Clin Pract20121204c179c18422890468
- JamesMBouchardJHoJCanadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injuryAm J Kidney Dis201361567368523518195
- SaratzisAMelasNMahmoodASarafidisPIncidence of Acute Kidney Injury (AKI) after Endovascular Abdominal Aortic Aneurysm Repair (EVAR) and impact on outcomeEur J Vasc Endovasc Surg201549553454025736516
- RuanZBZhuLYinYGChenGCRisk factors of early and late mortality after thoracic endovascular aortic repair for complicated stanford B acute aortic dissectionJ Card Surg201429450150624863011
- DrewsJDPatelHJWilliamsDMDasikaNLDeebGMThe impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repairAnn Thorac Surg201497620272033 discussion 203324726602
- KimMBradyJELiGAnesthetic technique and acute kidney injury in endovascular abdominal aortic aneurysm repairJ Cardiothorac Vasc Anesth201428357257824321848
- PisimisisGTKhoynezhadABashirKKruseMJDonayreCEWhiteRAIncidence and risk factors of renal dysfunction after thoracic endovascular aortic repairJ Thorac Cardiovasc Surg2010140Suppl 6S161S16721092786
- RenHMWangXHuCYRelationship between acute kidney injury before thoracic endovascular aneurysm repair and in-hospital outcomes in patients with type B acute aortic dissectionJ Geriatr Cardiol201512323223826089846
- WaldRWaikarSSLiangosOPereiraBJChertowGMJaberBLAcute renal failure after endovascular vs open repair of abdominal aortic aneurysmJ Vasc Surg2006433460466 discussion 46616520155
- AmblerGKCoughlinPAHayesPDVartyKGohelMSBoyleJRIncidence and outcomes of severe renal impairment following ruptured abdominal aortic aneurysm repairEur J Vasc Endovasc Surg201550444344926188721
- TsaiTTEvangelistaANienaberCAInternational Registry of Acute Aortic DissectionPartial thrombosis of the false lumen in patients with acute type B aortic dissectionN Engl J Med2007357434935917652650
- MukherjeeDEvangelistaANienaberCAImplications of periaortic hematoma in patients with acute aortic dissection (from the International Registry of Acute Aortic Dissection)Am J Cardiol200596121734173816360367
