Abstract
Objective
In focal cartilage lesions, multipotent mesenchymal stem cells in bone marrow are aimed to be moved into the defect area using subchondral drilling or microfracture method. However, repaired tissue insufficiently fills the defect area or cannot meet natural hyaline tissue functions, due to fibrous structure. We investigated the effect of a combined solution of sodium hyaluronate + chondroitin sulfate (HA+CS) administered intra-articularly after subchondral drilling on newly formed cartilage in rabbits with focal osteochondral defects.
Materials and methods
A total of 32 New Zealand White mature rabbits, whose weights ranged from 2.5 to 3 kg, were randomly divided into four groups. Full-thickness osteochondral defect was formed in the left-knee medial femur condyles of all rabbits. Subchondral drilling was then performed. The following treatment protocol was administered intra-articularly on knee joints on days 7, 14, and 21 after surgery: group 1, 0.3 mL combined solution of HA+CS (20 mg CS combined with 16 mg HA/mL); group 2, 0.3 mL HA (16 mg/mL); group 3, 0.3 mL CS (20 mg/mL); and group 4 (control group), 0.3 mL saline solution. In the sixth week, all animals were killed and then evaluated histopathologically and biochemically.
Results
There was significant articular cartilage formation in the HA+CS group compared to the HA, CS, and control groups. Hyaline cartilage formation was observed only in the HA+CS group. Cartilage-surface continuity and smoothness were significantly higher in the HA+CS and HA groups compared to the other groups. Normal cartilage mineralization was found to be significantly higher in the HA+CS group compared to the other groups. Increased levels of VEGFA and IL-1β in synovial fluid were observed in the HA+CS group.
Conclusion
After subchondral drilling, intra-articular HA-CS combination therapy is a good choice to promote better quality new cartilage-tissue formation in the treatment of focal osteochondral defects.
Introduction
Cartilage injuries usually occur due to high-impact mechanical load exposure on the articular surface during routine activities, and often affect young people with athletic lifestyles.Citation1 Although articular cartilage, with its highly differentiated structure, is a metabolically active tissue, chondrocytes in the matrix have a relatively slow turnover, and self-repair capacity is limited, due to insufficient blood-circulation support. A minor injury occurring in articular cartilage may lead to progressive injury and degeneration.Citation2,Citation3 Injuries particularly result in increased tissue wear, loss of articular surface smoothness, and focal cartilage defects. This triggers a series of subchondral changes leading to joint pain and dysfunction, and may lead to osteoarthritis at different severity levels in the long term if not treated.Citation4
Effective cartilage therapy should both improve the activity level of the patient by reducing pain, and delay and reduce the development of osteoarthritis.Citation5 Although many cartilage-repair options have been developed for the treatment of osteochondral defects, such as open-surgery procedures, cartilage transplantation, tissue engineering, autologous osteochondral transplantation, autologous chondrocyte implantation, microfracture and drilling, perichondral and periosteal grafting, fetal membrane application, and intra-articular hyaluronic acid injections,Citation2,Citation6–Citation9 normal cartilage tissue has not been exactly achieved.Citation10–Citation13
We think that the combined use of sodium hyaluronate (HA)Citation8 and chondroitin sulfate (CS)Citation14 in a single injectable form (HA+CS), positive results of which have been reported when used separately in the treatment of osteoarthritis and chondral lesions, can result in a synergistic effect on cartilage healing; this combined treatment protocol may lead to new cartilage formation of good quality in the treatment of osteochondral defects. Therefore, we investigated the effect of HA+CS solution on newly formed cartilage after subchondral drilling in femur condyles of rabbits with focal osteochondral defects.
Materials and methods
This study was conducted with the approval of the institutional ethical board of animal assays of Fırat University (2014/111). The study was conducted in accordance with the Guide for the Use and Care of Laboratory Animals. A total of 32 mature male New Zealand White rabbits weighing 2.5–3 kg were divided randomly into four equal groups, with eight animals in each group.
After full-thickness osteochondral defect and subchondral drilling, all experimental animals underwent the following treatment protocol by a blinded practitioner: group 1 (n=8), 0.3 mL combined solution of HA+CS (20 mg CS combined with 16 mg HA/mL; Ialuril; IBSA Farmaceutici Italia SRL, Lodi, Italy) was administered intra-articularly to the knee joint; group 2 (n=8), 0.3 mL HA (16 mg/mL; Sinovial Forte; IBSA Farmaceutici) was administered intra-articularly to the knee joint; group 3 (n=8), 0.3 mL CS (20 mg/mL; Uropol; Medac GmbH, Wedel, Germany) was administered intra-articularly to the knee joint; and group 4 (n=8, control group), 0.3 mL saline solution was administered intra-articularly to the knee joint. Starting 1 week after the wound was closed, the knee joint of each experimental animal underwent the treatment protocol at the same dose once a week for 3 weeks.
After receiving postoperative care, the experimental animals were fed with appropriate pellet feed and water, and they were allowed to move freely in their cages during all steps. At the end of the sixth week, all animals were killed, and then samples were evaluated macroscopically, histopathologically, and biochemically.
Surgical procedure
General anesthesia was induced with an intramuscular injection of 50 mg/kg ketamine HCl and was maintained with an intramuscular injection of 5 mg/kg xylazine HCl and 0.3 mg/kg ketamine HCl. The left hind limbs of the rabbits were shaved, scrubbed with 10% povidone iodine, and draped in a sterile fashion. The left-knee medial parapatellar incision was performed and the patella dislocated laterally. A full-thickness osteochondral defect of 3–4 mm in diameter and 4 mm in depth extending to subchondral bone in left medial femur condyles was then created. Subsequently, three sub-chondral drills were performed on the defect area with the help of Kirschner wire in triangular configuration, passing the subchondral bone by at least 3 mm (as far as bleeding and fat were observed). The wound was then closed properly. In accordance with study protocol, HA+CS, CS, HA, and saline solution were administered again into the knee joints of experimental animals at 7, 14, and 21 days after surgery. All experimental animals were killed at the end of the sixth week using the carbon dioxide-inhalation method.
Histopathological evaluation
Articular cartilage, synovial tissue, and synovial fluid of knees of 31 rabbits were collected. The knee articular cartilage of all rabbits was assessed according to the macroscopic scoring system identified by Rudert et alCitation15 ().
Table 1 Macroscopic scoring system for cartilage healing
The samples collected were fixed with 10% formalin solution, decalcified with 10% nitric oxide, and placed in paraffin blocks. All samples were divided into sections with a thickness of 4 μm and stained with hematoxylin and eosin for morphological analysis. For immunohistochemical analysis, all samples were also stained with proliferating cell nuclear antigen (Dako, Glostrup, Denmark) using an automatic staining system with poly-l-lysine-coated microscope slides (SN 712299, ref 750-700; Ventana Medical Systems, Tucson, AZ, USA). The collagen type in resulting cartilage-tissue matrix was determined by immunoperoxidase staining. All preparations were evaluated by a blinded pathologist using light microscopy (BX51; Olympus, Tokyo, Japan). Histological criteria identified by Dorotka et alCitation16 were used to show the type of tissue developing in the osteochondral defect area. The samples were also evaluated according to the International Cartilage Repair Society visual histological assessment scale modified by Mainil-Varlet et alCitation17 ().
Table 2 Modified ICRS visual histological assessment scale
Biochemical evaluation
Before all animals were killed, 0.2 mL saline solution was injected into the knee joint to obtain sufficient articular synovial fluid, and then synovial fluid was aspirated. Approximately 0.3–0.6 mL synovial fluid obtained from each knee joint was centrifuged at 5,000 rpm for 30 minutes. TNFα, neopterin, collagenase type 2, TGF1β, TIMP1, VEGFA, and IL-1β levels in synovial fluid were determined using enzyme-linked immunosorbent-assay kits (Sunred Biological Technology, Shanghai, China).
Statistical analysis
All data were evaluated using the SPSS 17.0 package program (SPSS Inc, Chicago, IL, USA). A 95% confidence interval and P-value less than 0.05 were considered significant for all analyses. Numerical data were analyzed using the Shapiro–Wilk test to assess whether the data were parametric. Numerical data between groups were evaluated by Kruskal–Wallis test. If the difference between groups was statistically significant, Bonferroni-corrected Mann–Whitney U tests were used to assess which groups were different. All numerical data are represented as median, minimum, and maximum values. Differences in categorical variables were compared with Pearson’s χ2 test and are represented as percentage and number.
Results
There was no infection in any experimental animals. One experimental animal was excluded from the study due to death. A total of 31 experimental animals completed the study: eight in the HA+CS group (25.8%), eight in the HA group (25.8%), eight in the CS group (25.8%), and seven in the control group (22.6%).
In macroscopic evaluation, there was synovial hypertrophy and excessive synovial fluid accumulation in the knee joint in three cases. One of these cases was in the HA+CS group, and two were in the CS group. In the macroscopic examination of the defect area, there were no statistical differences between the groups in terms of filling, color, or surface roughness of newly formed cartilage tissue (, ).
Figure 1 Macroscopic view of osteochondral healing.
Abbreviations: CS, chondroitin sulfate; HA, sodium hyaluronate.
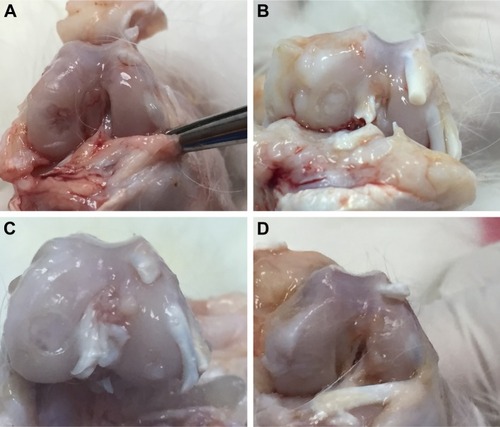
Table 3 Macroscopic comparison of newly formed cartilage between groups
There was a statistically significant difference between groups in terms of newly formed cartilage-tissue type according to Dorotka histological scoring (). There was no cartilage formation in fibrous tissue in any of the groups. There was statistically significant articular cartilage formation in the HA+CS group compared to the HA, CS, and control groups (P=0.041, 0.01, and 0.029, respectively). Hyaline cartilage formation was observed in a single case in the HA+CS group (–).
Figure 2 Histopathological appearance of cartilage formed in the control group.
Abbreviation: H&E, hematoxylin and eosin.
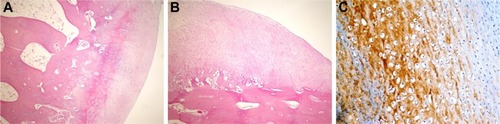
Figure 3 Histopathological appearance of cartilage formed in the CS group.
Abbreviations: CS, chondroitin sulfate; H&E, hematoxylin and eosin.
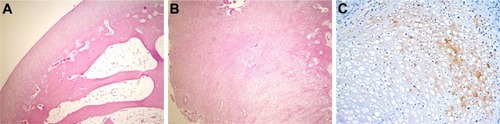
Figure 4 Histopathological appearance of cartilage formed in the HA group.
Abbreviations: HA, sodium hyaluronate; H&E, hematoxylin and eosin.
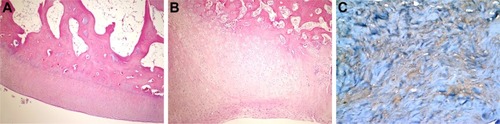
Figure 5 Histopathological appearance of cartilage formed in the HA+CS group.
Abbreviations: CS, chondroitin sulfate; HA, sodium hyaluronate; H&E, hematoxylin and eosin.
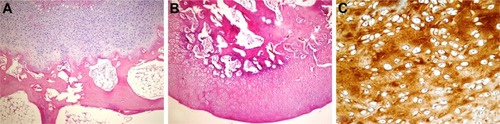
Table 4 Histological tissue type according to Dorotka et al’s classificationCitation16
The characteristics of the groups according to the modified International Cartilage Repair Society visual histological assessment scale are shown in . Cartilage-surface continuity and smoothness were observed to be statistically higher in the HA+CS and HA groups compared to the CS and control groups (P=0.041 for HA+CS vs CS; P=0.007 for HA+CS vs control; P=0.041 for HA vs CS; P=0.041 for HA vs control). There was significantly higher mixed-type (hyaline/fibrocartilage) cartilage-structure formation in cartilage matrix in the HA+CS, HA, and CS groups compared to the control group (P=0.049). In terms of cell distribution, there was a statistically significant level of cartilaginous clusters in mixed/column types in other groups compared to the control group (P=0.009). There was no difference among the groups in terms of number of living cells in newly formed cartilage. The living cell count was dominant in all groups. There was no statistically significant difference between the groups in terms of subchondral bone characteristics in the defect area. Normal cartilage mineralization (calcified cartilage) was found to be significantly higher in the HA+CS group compared to the HA, CS, and control groups (P=0.041, 0.01, and 0.01, respectively). There was a similarity among groups in terms of proliferating cell nuclear antigen and type of collagen (type I and II) ().
Figure 6 Histopathologic appearance of specimens according to PCNA staining.
Abbreviation: PCNA, proliferating cell nuclear antigen.
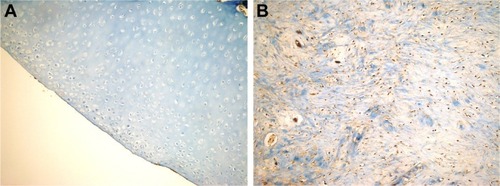
Table 5 Changes between groups according to modified ICRS visual histological assessment scale
In biochemical analysis, mean TNFα, neopterin, collagenase type 2, TGF1β, and TIMP1 levels in synovial fluid were similar among groups. However, there was a significant difference among the groups in terms of mean VEGFA and IL-1β levels in synovial fluid. The mean VEGFA level in synovial fluid was significantly higher in the HA+CS group compared to the HA, CS, and control groups (P<0.001, P=0.03, and P=0.001, respectively). The mean IL-1β level in the HA+CS group was significantly higher than the HA, CS, and control groups (P=0.01, 0.01, and 0.04, respectively) ().
Table 6 Comparison of changes in synovial fluid between groups
Discussion
This is the first study investigating the effect of intra-articular HA+CS combination therapy on newly formed cartilage after subchondral drilling in the osteochondral defect model. There was no cartilage formation in fibrous tissue structure in any group in this study. In our study, there was significantly higher hyaline-like articular cartilage formation in the HA+CS group compared to other groups, and hyaline cartilage formation was observed only in the HA+CS group. We suggest that this combined treatment enhances cartilage proliferation by creating a synergistic effect compared to the use of HA or CS alone. There was significantly higher normal cartilage mineralization in the HA+CS group compared to other groups, the continuity and surface smoothness of cartilage were better, and there was more mixed/columnar cluster-type cell distribution. Therefore, this combination may be a good option for cartilage lesions.
Subchondral drilling and microfracture are common procedures in focal cartilage lesions. The aim of these methods is to move the multipotent mesenchymal stem cells in the bone marrow to the defect area. However, the repaired tissue obtained by this procedure has a fibrocartilaginous structure. Since this repaired tissue in fibrocartilaginous structure is rich in type 1 collagen and contains fewer proteoglycans, it is less stable compared to normal hyaline cartilage, which is rich in type 2 collagen.Citation5,Citation7,Citation18,Citation19 For this reason, additional treatment interventions have been studied to improve the quality of the repaired tissue and obtain long-term functional and stable repaired tissue that is similar to hyaline cartilage.Citation12 In most experimental animal studies, glycosaminoglycans after subchondral drilling or microfracture have been used frequently to obtain better-quality cartilage formation.Citation8,Citation12,Citation20,Citation21
Glycosaminoglycans, such as HA and CS are amino sugar-containing polysaccharides found in the extracellular matrix of all vertebrates.Citation22 HA plays an important role in the feeding of cartilage, and is responsible for the viscoelastic properties of synovial fluid (shock absorption and lubrication).Citation23 The anticatabolic effect of HA has been demonstrated in in vitro and in vivo models.Citation24,Citation25 Intra-articular injections have been shown to reduce articular cartilage damage by inhibiting the production of IL-1β, metalloproteinases,Citation26 and NOCitation13, which cause cartilage-matrix degradation. Besides increasing synthesis of cartilage components, such as sulfated glycosaminoglycans, CS, proteoglycans, and type 2 collagen,Citation27 hyaluronic acid treatment also contributes to the formation of hyaline-like cartilage by increasing chondrocyte proliferation.Citation8,Citation28 CS, which increases chondrocyte production in joint cartilage, has been reported to have beneficial effects, such as stimulation of extracellular matrix (proteoglycan, type 2 collagen production), inhibition of inflammatory mediators and degradative enzymes (myeloperoxidase, N-acetyl glucosaminidase, collagenase, hyaluronidase, elastase, IL-1β, and PGE2), reduction of chondrocyte apoptosis, and inhibition of cartilage degeneration.Citation28–Citation32 It was reported in a meta-analysis conducted by HochbergCitation33 that the use of CS was effective in both reducing symptoms and preventing reduction of knee-joint spacing in patients with knee osteoarthritis.
Strauss et alCitation8 stated that intra-articular HA injection for 3 weeks after microfracture was positively effective in repaired tissue developing in chondral defects in the early period. They observed anti-inflammatory and chondroprotective effects of HA support, reducing the development of degenerative changes in the knee joint. Öztemür et alCitation34 found significant differences between HA and a control group in terms of both cartilage quality and type 2 collagen expression in their animal study with a cartilage-defect model (without subchondral drilling). In their study, the expression of VEGFA and IL-1β in synovial fluid was high in the HA group; on the other hand, these levels were normal in the HA group in our study. However, there was a significant increase in VEGFA and IL-1β levels in the HA+CS group in our study. We think that this was the result of inflammatory response due to drug intensity.
Suwannaloet et alCitation12 reported that HA injection promoted migration of chondrocytes and increased type 2 collagen content in fibrocartilage regeneration induced by subchondral drilling in rabbits with full-thickness osteochondral defects. Kim et alCitation20 indicated that 3-week HA injection with intra-articular single mesenchymal stem cell injection had a very effective role in the healing of osteochondral defects. In our study, there was significant articular cartilage formation of hyaline and/or hyaline cartilage-like structures in the HA+CS group. The continuity and smoothness of the articular surface were better in both the HA+CS and HA groups compared to other groups. In addition, although it was not statistically significant, newly formed cartilage tissue filled the defect area better in the HA+CS group compared to other groups, and it was similar in color and smoothness to its surrounding cartilage. We think that the use of intraarticular glycosaminoglycan after subchondral drilling or microfracture increases chondrocyte proliferation and affects cartilage healing positively in osteochondral defect treatment.
Naito et alCitation35 demonstrated in their experimental study that glucosamine increases type 2 collagen synthesis in joint cartilage and exhibits a chondroprotective effect in OA, inhibiting type 2 collagen degradation. Although type 2 collagen density in newly formed cartilaginous tissue was higher in the HA+CS group, it was not significant in our study. Gonçalves et alCitation14 evaluated CS and HA effects in animal experiments in which degenerative joints were formed. Although there was no significant difference between groups, there were nucleolar organizer regions promoting proliferation of chondrocytes and an increase in cell amounts in the HA group. However, they indicated that the high nucleolar organizer region:cell ratio observed in the group treated with CS was an indication of stimulated metabolic activity of chondrocytes and that CS may be helpful in reducing degenerative joint disease.
It has been reported that CS and glucosamine combination enhances type 2 collagen and proteoglycan synthesis in human articular chondrocytes, reduces the production of some proinflammatory mediators and proteases, can reduce cell-death processes, and can stabilize the anabolic– catabolic balance of extracellular cartilage matrix.Citation30 Tosun et alCitation36 observed cartilaginous metaplasia formation after local combined HA+CS administration after Achilles tendon repair, and expressed the view that combined HA+CS administration might be useful in cartilage injuries.
The combined use of glucosamine sulfate and CS has become a popular supportive protocol in the treatment of degenerative joint disease.Citation37 Henrotin et alCitation23 emphasized that both pain and functional impairment decreased throughout their pilot study, in which patients with knee osteoarthritis were administered intra-articular HA+CS combination injections. Rivera et alCitation38 indicated that intra-articular HA+CS is effective and safe in both reduction of analgesic consumption and improvement in joint mobility in patients with knee osteoarthritis.
There have been insufficient studies on the use of the HA+CS combination as intra-articular injection in the treatment of osteoarthritis and chondral defects.Citation18,Citation23,Citation36–Citation38 Chen et alCitation18 compared groups of HA, CS, and HA+CS with a control group in rabbits with experimentally formed osteoarthritis. They reported that HA+CS injections had a chondroprotective effect, and synovial membrane healing was best in this combination group. Although there was no statistical difference among the treatment groups, they indicated that IL-1β, TNFα, TIMP1, and NO levels synthesized in synovial fluid were suppressed and cartilage healing was better in treatment groups compared to the control group. In our study, mean TNFα, neopterin, collagenase type 2, TGF1β, and TIMP1 levels in synovial fluid were similar among groups. On the other hand, VEGFA and IL-1β levels in synovial fluid were higher in the HA+CS group compared to other groups. The IL-1β level in synovial fluid in the HA+CS group was lower in Chen et al,Citation18 but higher in our study compared to other groups. The TNFα level in the HA+CS group was observed to be lower in Chen et al,Citation18 but there was no difference in our study. We suggest no relationship between changes in inflammatory mediators and new-cartilage formation, but it is necessary to conduct an extensive and long-term study investigating the effect of biochemical molecules, known as inflammatory markers, in the healing of acute-onset lesions.
It has been reported that there are some differences in pathogenesis among different animals. Breinan et alCitation10 reported that a less meaningful effect of autologous chondrocyte implantation was found in a canine-model study than observed in their previous work in the rabbit. They emphasized that this condition may be explained by species differences, relative age of the animals, location of the defect (patella vs trochlea), and aspects of the surgical techniques. Although this study was an animal study, there may be some minor differences compared to practices in humans, but we think that these positive results cannot be overlooked.
Conclusion
In light of this information, we propose that intra-articular HA+CS combination therapy, which is administered in addition to subchondral drilling causing mesenchymal stem cell stimulation, affects hyaline-like articular cartilage formation positively in the treatment of focal osteochondral defects. This combination can be used as a safe and an effective treatment for all cartilage lesions.
Author contributions
HBT, MG, SAG, AU and SS performed the operation, wrote the manuscript. HBT, SÇ, SS, and SAG contributed to the discussion, reviewed/edited the manuscript, and researched data. HBT, MG, SÇ, and AU helped to draft the manuscript. HBT, SS, SAG, MG, and AU researched data and contributed to discussion. The histopathological evaluation of samples was made by ÖÜ. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Professor Dr Bilal Üstündağ from Fırat University Clinical Biochemistry Department, who provided assistance in biochemical analysis and evaluation in this study. This study received financial support from the Scientific and Technological Research Council of Turkey (TUBITAK).
Disclosure
The authors report no conflicts of interest in this work.
References
- FlaniganDCHarrisJDTrinhTQSistonRABrophyRHPrevalence of chondral defects in athletes’ knees: a systematic reviewMed Sci Sports Exerc201042101795180120216470
- GilloglySDVoightMBlackburnTTreatment of articular cartilage defects of the knee with autologous chondrocyte implantationJ Orthop Sports Phys Ther19982842412519785259
- SagliyanAKarabulutEUnsaldiEYamanIEvaluation of the activity of intraarticular hyaluronic acid in the repair of experimentally induced osteochondral defects of the stifle joint in dogsVet Med (Praha)20095413340
- MessnerKGillquistJCartilage repair: a critical reviewActa Orthop Scand19966755235298948264
- SharmaBFermanianSGibsonMHuman cartilage repair with a photoreactive adhesive-hydrogel compositeSci Transl Med20135167167ra6
- AltmanRDIntra-articular sodium hyaluronate in osteoarthritis of the kneeSemin Arthritis Rheum2000302111811071577
- KhanWSJohnsonDSHardinghamTEThe potential of stem cells in the treatment of knee cartilage defectsKnee201017636937420051319
- StraussESchachterAFrenkelSRosenJThe efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesionsAm J Sports Med200937472072619204370
- TanidehNDehghaniNSMojtahedJFThe healing effect of bioglue in articular cartilage defect of femoral condyle in experimental rabbit modelIran Red Crescent Med J201113962963322737537
- BreinanHAMinasTHsuHPNehrerSShortkroffSSpectorMAutologous chondrocyte implantation in a canine model: change in composition of reparative tissue with timeJ Orthop Res200119348249211398864
- MithoeferKMcAdamsTWilliamsRJKreuzPCMandelbaumBRClinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysisAm J Sports Med200937102053206319251676
- SuwannaloetWLaupattarakasemWSukonPOng-ChaiSLaupattarakasemPCombined effect of subchondral drilling and hyaluronic acid with/without diacerein in full-thickness articular cartilage lesion in rabbitsScientificWorldJournal2012201231074522666105
- TakahashiKHashimotoSKuboTHirasawaYLotzMAmielDHyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis modelJ Orthop Res200119350050311398866
- GonçalvesGMeloEGGomesMGNunesVARezendeCMEffects of chondroitin sulfate and sodium hyaluronate on chondrocytes and extracellular matrix of articular cartilage in dogs with degenerative joint diseaseArq Bras Med Vet Zootec200860193102
- RudertMWilmsUHobergMWirthCJCell-based treatment of osteochondral defects in the rabbit knee with natural and synthetic matrices: cellular seeding determines the outcomeArch Orthop Trauma Surg2005125959860816075272
- DorotkaRWindbergerUMacfeldaKBindreiterUTomaCNehrerSRepair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrixBiomaterials200526173617362915621252
- Mainil-VarletPAignerTBrittbergMHistological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS)J Bone Joint Surg Am200385ASuppl 24557
- ChenLLingPXJinYZhangTMHyaluronic acid in combination with chondroitin sulfate and hyaluronic acid improved the degeneration of synovium and cartilage equally in rabbits with osteoarthritisDrug Discov Ther20115419019422466300
- SteadmanJRMillerBSKarasSGSchlegelTFBriggsKKHawkinsRJThe microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League playersJ Knee Surg2003162838612741420
- KimSSKangMSLeeKYLeeMJWangLKimHJTherapeutic effects of mesenchymal stem cells and hyaluronic acid injection on osteochondral defects in rabbits’ kneesKnee Surg Relat Res201224316417222977794
- SawKYHussinPLokeSCArticular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat modelArthroscopy200925121391140019962065
- FraserJRLaurentTCLaurentUBHyaluronan: its nature, distribution, functions and turnoverJ Intern Med1997242127339260563
- HenrotinYHauzeurJPBruelPAppelboomTIntra-articular use of a medical device composed of hyaluronic acid and chondroitin sulfate (Structovial CS): effects on clinical, ultrasonographic and biological parametersBMC Res Notes2012540722862789
- HiraokaNTakahashiKAAraiYIntra-articular injection of hyaluronan restores the aberrant expression of matrix metalloproteinase-13 in osteoarthritic subchondral boneJ Orthop Res201129335436020886647
- SantangeloKSJohnsonALRuppertASBertoneALEffects of hyaluronan treatment on lipopolysaccharide-challenged fibroblast-like synovial cellsArthritis Res Ther200791R117214881
- TakahashiKGoomerRSHarwoodFKuboTHirasawaYAmielDThe effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1β (IL-1β), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritisOsteoarthritis Cartilage19997218219010222217
- AkmalMSinghAAnandAThe effects of hyaluronic acid on articular chondrocytesJ Bone Joint Surg Br20058781143114916049255
- KuboMAndoKMimuraTMatsusueYMoriKChondroitin sulfate for the treatment of hip and knee osteoarthritis: current status and future trendsLife Sci20098513–1447748319695267
- GuidolinDDRonchettiIPLiniEGuerraDFrizzieroLMorphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500–730 kDa sodium hyaluronate (Hyalgan) and methylprednisolone acetate on primary osteoarthritis of the kneeOsteoarthritis Cartilage20019437138111399102
- HenrotinYMartyMMobasheriAWhat is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis?Maturitas201478318418724861964
- SmithMMGhoshPThe synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environmentRheumatol Int1987731131223671989
- TakahashiKHashimotoSKuboTHirasawaYLotzMAmielDEffect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritisJ Rheumatol20002771713172010914857
- HochbergMCStructure-modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta-analysis of randomized placebo-controlled trials of 2-year durationOsteoarthritis Cartilage201018Suppl 1S28S3120399895
- ÖztemürZÖzerHGölgeUHAltuntaşEEBulutOComparing extracorporeal shock wave and hyaluronic acid in a rabbit cartilage defect model: the effects of ESW on cartilage defectTurk J Med Sci201343484492
- NaitoKWatariTFuruhataAEvaluation of the effect of glucosamine on an experimental rat osteoarthritis modelLife Sci20108613–1453854320188111
- TosunHBGümüştaşSAKomMUludağASerbestSEröksüzYThe effect of sodium hyaluronate plus sodium chondroitin sulfate solution on peritendinous adhesion and tendon healing: an experimental studyBalkan Med J201633325826627308069
- KellyGSThe role of glucosamine sulfate and chondroitin sulfates in the treatment of degenerative joint diseaseAltern Med Rev19983127399600024
- RiveraFBertignoneLGrandiGEffectiveness of intra-articular injections of sodium hyaluronate-chondroitin sulfate in knee osteoarthritis: a multicenter prospective studyJ Orthop Traumatol2016171273326577936
