Abstract
Background
The pathology of osteoarthritis (OA) is partly attributed to genetic factors; however, the role of ADAM12 polymorphism is still controversial. It is necessary to perform a meta-analysis to investigate this possible correlation.
Methods
Case–control studies on the association between OA susceptibility and ADAM12 polymorphism were comprehensively collected by searching PubMed, Embase, and Web of Science. Odds ratios (ORs) and 95% confidence intervals (CIs) were pooled to evaluate OA risk that was possibly conferred by ADAM12 variant. The analyses were performed not only among general population but also in male and female groups.
Results
A total of 8 studies with 10 populations were finally included in this meta-analysis. In the general population, 4 comparisons were carried out (C allele vs G allele, CC vs GG, GC + CC vs GG, and CC vs GC + GG) and found that ADAM12 rs3740199 polymorphism was not associated with increased OA vulnerability. On the other hand, the analyses stratified by gender made 5 comparisons (C allele vs G allele, CC vs GG, GC vs GG, GC + CC vs GG, and CC vs GC + GG). It was shown that rs3740199 polymorphism (GC + CC vs GG) was a risk factor for OA among male patients (OR =1.45, 95% CI =1.04–2.01). Sensitivity analysis indicated that it was an unstable outcome. No correlation was identified in women. Neither heterogeneity nor publication bias was detected in the analyses mentioned above.
Conclusion
ADAM12 rs3740199 polymorphism is likely to be associated with OA susceptibility among male patients, other than the general population. More studies are needed to confirm this observation. The mechanism by which ADAM12 variant plays a role in OA pathogenesis is also warranted and important for interpreting this possible correlation.
Introduction
Osteoarthritis (OA) is a degenerative joint disease that results from breakdown of joint cartilages and bones. This disease is a major cause of disability worldwide, with poor functional outcomes and limited lifespan even after effective treatment. As a result, the focus is shifting to risk factor identification and disease prevention.Citation1 A number of risk factors for OA exist, including physical activity, obesity, gender, aging, and genetic predisposition.Citation2 In recent years, genetic risk factors associated with OA susceptibility have been extensively studied. At present, 21 independent OA susceptibility loci have been established from candidate gene studies, linkage studies, and genome-wide association studies (GWASs).Citation3 Apart from that, more specific genes which conferred vulnerability of OA are still being identified. Evidence has been published for the association of OA predisposition with single-nucleotide polymorphisms (SNPs) of a member from disintegrin and metalloprotease (ADAM) family – ADAM12.Citation4–Citation6
The ADAM family comprises >30 zinc-dependent proteases that contribute to proteolysis, adhesion, fusion, and cell signaling.Citation7 ADAM12 is one of the splice forms of ADAMs and the gene for human ADAM12 is located on chromosome 10q26.3.Citation8 Two SNPs of the ADAM12 gene, rs3740199 and rs1871054, have been studied most frequently with OA susceptibility. It has been demonstrated that rs3740199 is associated with OA susceptibility among the general population,Citation9 or certain groups of the studied participants.Citation6,Citation10 Apart from disease vulnerability, ADAM12 mutation has also been linked to OA severity.Citation5 However, studies that did not observe any positive association of ADAM12 SNP with OA also exist among various races.Citation11,Citation12 As a result, until now, no consensus has been reached regarding the proposal that ADAM12 polymorphisms are related to disease predisposition, severity, or phenotype. In addition, the power of genetic association study is of importance. Individual study, especially with limited sample size, provides insufficient power. Therefore, it is necessary to collect the up-to-date evidence, perform a meta-analysis, and present with the latest and robust overview of this subject. To better understand the association of OA susceptibility with ADAM12 SNPs, a meta-analysis was conducted by a comprehensive literature search and synthesis of data from eligible case–control studies.
Materials and methods
Identification of eligible studies and data collection
The latest literature search was conducted in January 2017 on PubMed, Web of Science, and Embase. The following keywords were used: “osteoarthritis,” “OA,” “ADAM12,” and “polymorphism.” Case–control studies about the association of ADAM12 polymorphisms with OA susceptibility were collected. Relative references were further reviewed to identify potential extra studies. No restrictions of race and publication year were imposed. Published articles with full text, other than reviews or records from conference proceedings, were included. To exclude overlapping data observed from the same cohort, only the newest study or the study with the largest sample size was selected. Records were dropped to remove flawed studies when the genotypic distribution deviated from Hardy–Weinberg equilibrium (HWE).
Two reviewers extracted data independently, and disagreements were solved by discussion. The following data were collected: first author, publication year, race, characteristics of cases and controls, diagnosis criteria, SNP position, methods of genotyping, genotypic distributions, and odds ratios (ORs). Additional information regarding the characteristics of the participants was required if stratification analyses were needed.
Statistical analysis
Chi-square test was applied to assess whether the genotypic distribution was in concordance with HWE and whether the original article did not mention the outcome of the HWE test. Q-statistic was performed to evaluate the heterogeneity across studies.Citation13 The magnitude of heterogeneity was measured by I2-value, indicating the proportion of the total variance across studies due to heterogeneity, other than chance. I2≥50%, 50%>I2≥25%, and 25%>I2 were defined as high, medium, and low heterogeneity, respectively.Citation14 If no inconsistency was detected, ORs were aggregated by Mantel–Haenszel’s method in fixed-effect model. Otherwise, Dersimonian–Laird method in random-effect model was applied.Citation15 Publication bias was detected by Egger’s linear regression test.Citation13 Sensi-tivity analysis was performed by omitting one study in each turn and then checking statistical significance. All statistical analyses were conducted by Stata 9.0 (Stata Crop LP, College Station, TX, USA). All P-values were two-sided, with <0.05 being statistically significant.
Results
Characteristics of the eligible studies
The process of study inclusion is illustrated in . Overall, 8 studies with 10 populations, including 8,553 cases and 6,349 controls, were eligible for this meta-analysis.Citation4–Citation6,Citation9–Citation12,Citation16 The majority of the cases were individuals who were diagnosed with knee, hip, and hand OA from various races, according to the criteria of American College of Rheumatology (ACR).Citation4–Citation6,Citation9,Citation11,Citation12 Four candidate gene positions (rs3740199, rs1871054, rs1278279, and rs1044122) were investigated; among which rs3740199 was the most frequently studied locus; therefore, the original data regarding the association of rs3740199 polymorphism with OA susceptibility were primarily extracted. The genotypic distributions from all the included studies did not deviate from HWE according to the descriptions from the corresponding articles ().
Table 1 Main characteristics of the included studies
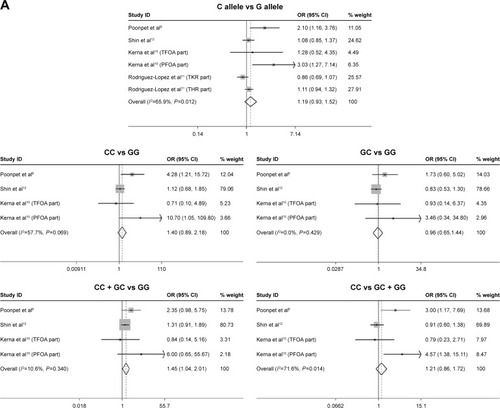
The information obtained from the studies revealed that the most common comparison was between carriers of rs3740199 C allele and carriers of rs3740199 G allele.Citation4–Citation6,Citation10–Citation12,Citation16 Besides, the available genetic models that were introduced in the trials were CC versus GG, GC + CC versus GG, and CC versus GC + GG.Citation4–Citation6,Citation9–Citation12,Citation16 Thus, the ORs with 95% confidence intervals (95% CIs) regarding these comparisons were extracted and pooled into the meta-analysis. Stratification analyses dependent on gender were performed among 6 populationsCitation6,Citation10–Citation12; thus, these data were also obtained ().
Table 2 Main results of the studies about the association of OA susceptibility with rs3740199
ADAM12 rs3740199 polymorphism was not associated with OA susceptibility in the general population
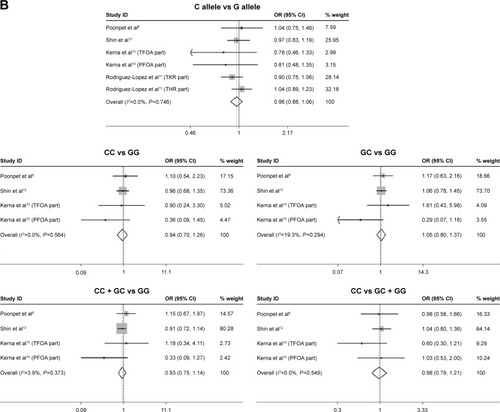
The comparison of risk for OA susceptibility between rs3740199 C allele carriers and G allele carriers was performed among 9 of 10 populations. As shown in , no significant association was observed. Sensitivity analyses showed that all these insignificant results would not be influenced when any population was omitted. Neither heterogeneity across studies nor publication bias was detected. Similarly, the investigations on CC versus GG, GC + CC versus GG, and CC versus GG + GC genetic models did not yield any remarkable relationship, either. These results were also proven stable by sensitivity analyses. Heterogeneity and publication bias were not found, either ().
Figure 2 Forest plots of the analyses on the association of OA susceptibility with rs3740199 polymorphism. (A) C allele versus G allele; (B) CC versus GG; (C) CC + GC versus GG; (D) CC versus GC + GG.
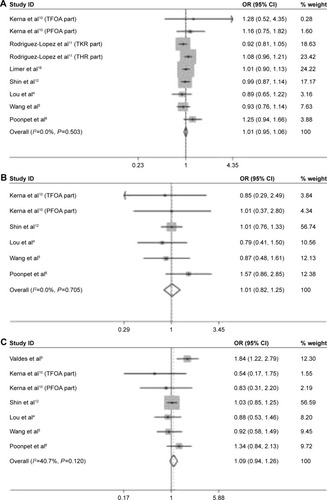
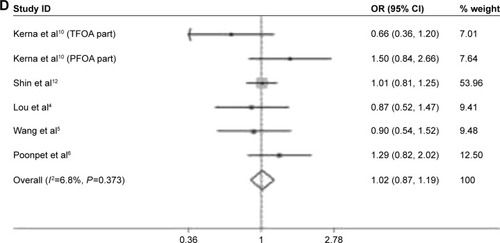
Table 3 Main results of the meta-analysis
ADAM12 rs3740199 polymorphism was likely to be associated with OA susceptibility among males
In some studies, the additional risk of OA conferred by rs3740199 polymorphism was exclusively observed in a specific group of peopleCitation10,Citation11,Citation17; thus, further investigation into this hypothesis was done by analyzing data that were stratified by gender. As illustrated in , the only significant association was identified among males, indicating that the risk of OA was 45% higher (95% CI: 1.04–2.01, P=0.028) in carriers of CG and CC, compared with carriers of GG. In this comparison, neither inconsistency nor publication bias was detected. However, sensitivity analysis demonstrated that the exclusion of study from Poonpet et alCitation6 would reverse this relationship into insignificant.
Figure 3 Forest plots of the analyses on the gender-dependent associations of OA susceptibility with rs3740199 polymorphism. (A) Different comparisons among males; (B) different comparisons among females.
Abbreviations: TFOA, tibiofemoral knee OA; PFOA, patellofemoral knee OA; OA, osteoarthritis; CI, confidence interval.
No remarkable and stable correlation was found for the remaining comparisons among men. Specifically, in the study of C allele versus G allele, the exclusion of one populationCitation11 would result in a statistically significant association. The same scenario also happened when CC versus GG and CC versus GG + GC were studied, obtaining positive correlations between OA risk and rs3740199 polymorphism.
In women patients, this meta-analysis demonstrated that rs3740199 polymorphism was unlikely to confer extra risk for OA to this group of people, regardless of evaluating various genetic models. Sensitivity analyses confirm the stability of these outcomes. Neither heterogeneity nor publication bias was found ().
Table 4 Results of the meta-analysis dependent on gender
Discussion
OA is a complex disease, induced by a combination of three main risk factors: genetics, environmental factors, and aging.Citation17 Numerous candidate genes have been screened to identify susceptible loci of OA, and ADAM12 is one of them. This is, to the authors’ knowledge, the first meta-analysis to investigate the association between ADAM12 (rs3740199) polymorphism and OA predisposition. In this study, case–control studies on ADAM12 polymorphism and OA susceptibility were comprehensively collected and 8 trials with 10 populations were obtained. The most frequently researched gene locus on ADAM12 (rs3740199) was selected and analyzed. It was noteworthy that the unfavorable effect of rs3740199 polymorphism (GC + CC vs GG) was exclusively observed among male. Unfortunately, this result was unstable; therefore, the evidence was not strong. On the other hand, although comparisons concerning other genetic models did not yield significant correlations, these results were also unstable, heavily affected by the data from a single population. Therefore, until now, the available data indicated that ADAM12 rs3740199 polymorphism was likely to be associated with OA vulnerability among male patients, other than the general population, but more studies are needed to draw a stable and safe conclusion.
In addition to the SNPs of ADAM12, its haplotypes have been related to OA predisposition, with much more remarkable effect. In the genetic study conducted among Chinese Han individuals, haplotype analysis revealed that 5 haplotypes (TATC, TATG, TACG, CATG, and CGTC) were associated with the increased risk of OA.Citation4 In line with that, haplotype CAAT has been found to elevate the risks for OA by as high as 6.10-fold among Caucasian women and 1.54-fold among Caucasian men.Citation18 The sexual difference is also found in the haplotype study. It has been reported that CCAA haplotype increased the risk for tibiofemoral joint OA among males, and GCGG haplotype increased the risk for joint space narrowing among females.Citation19 In contrast, some haplotypes may be protective against OA. rs3740199/rs1871054 GC decreased the risk for patellofemoral joint OA among male patients (OR =0.10) and the risk for osteophytes among overall people (OR =0.24).Citation10 Besides, haplotype CGAT and CGGT were also related with smaller risk for knee OA predisposition.Citation18
Apart from the genetic association studies about ADAM12 SNPs and OA, some clinical trials investigating the role of ADAM12 protein and mRNA also provide valuable supporting evidence. In overall, it has been observed that overexpression of ADAM12 is related with OA. The level of ADAM12-L mRNA expression was remarkably higher in OA cartilage than in normal samples. In situ hybridization demonstrated that clustered chondrocytes in OA cartilage were the main source of ADAM12-L mRNA. The expression of this protein was visualized on clustered chondrocyte membranes by immunohistochemistry.Citation20 The expressions of ADAM12 mRNA and protein in synovium were significantly linked to the severity of histological synovitis.Citation21 ADAM12-S protein in serum could be increased in some OA patients, correlating with the grade of this disease and osteophyte occurrence.Citation22 However, the amount of ADAM12 protein may not be linked with gene polymorphism, and since it has been reported that the functional protein level is not determined by genetic variants (rs3740199, rs1871054, rs1278279, and rs1044122),Citation23 the involvement of ADAM12 gene and protein in OA pathogenesis needs further research.
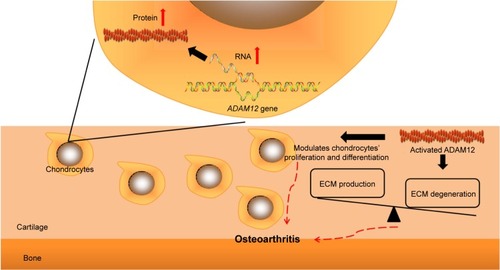
Despite the fact that so many observational studies have been conducted, little is known about the molecular biological role of ADAM12 on OA pathogenesis. The possible mechanism by which ADAM12 participates in OA is illustrated in . Human ADAM12 consists of two forms produced by alternative splicing gene, which are secreted, short form (ADAM12-S) and larger, membrane-bounding form (ADAM12-L).Citation8 Similar to the archetypical structure of ADAM proteins, the amino acid sequence of ADAM12 includes proprotease, metalloprotease, disintegrin, cysteine-rich domains, and in the case of L form, a transmembrane and cytoplasmic domains.Citation8 The metalloprotease domain converts from latent state into functional state after cleavage of the prodomain, resulting in an ADAM12 protein with catalytic activity.Citation24 Activated ADAM12 has the capacity to degrade extracellular matrix (ECM) components, including gelatin, fibronectin, and type IV collagen.Citation25 The molecular interaction between ADAM12 and β1 integrins may change cell–matrix contacts in the growth plate and modulate proliferation and differentiation of chondrocyte.Citation26 As it is known, articular cartilage is a narrow layer of specialized ECM that is elaborated and maintained by articular chondrocytes. In normal cartilage, chondrocytes maintain a balance between the production and the degeneration of ECM, resulting in stability of the tissue. This equilibrium is disturbed in degenerative joint diseases such as OA.Citation20 Therefore, it is plausible that ADAM12 may contribute to the pathogenesis of OA by the influence on ECM and chondrocytes. Existing evidence has demonstrated that OA chondrocytes produce more ADAM12-L mRNA and protein after administration with TGF-β in a dose-dependent fashion.Citation20 The overexpression of ADAM12-L could result in chondrocyte proliferation via insulin-like growth factor signaling.Citation20
Figure 4 Possible mechanism by which ADAM12 participates in OA pathogenesis.
There are some limitations in this study, so the results should be treated with caution. The adverse impact of rs3740199 polymorphism was demonstrated by insufficient studies. Only 3 trials with 4 groups of individuals, involving 1,043 cases and 2,008 controls, were pooled together to address this issue. Furthermore, this positive correlation was proven to be unstable since it was remarkably influenced by the exclusion of study from Poonpet et al.Citation6 In Poonpet’s study,Citation6 limited male cases and controls were recruited. It was reported that CC male carriers had 328% higher risk than GG male carriers. Male patients with C allele had 110% higher risk than male patients with G allele. The extremely high OR values among males might be partly attributed to the insufficient sample size or selection bias. In addition, some of the extracted ORs with their 95% CIs originated from multivariate analyses and were adjusted for potential confounders.Citation4,Citation5,Citation12,Citation16 On the other hand, some ORs were given from the univariate model.Citation6,Citation10,Citation18 Therefore, the aggregation of these heterogeneous ORs might distort the truth. Finally, the controversial association of OA with ADAM12 haplotype frequency was not investigated. It was mainly attributed to lack of evidence, different haplotypes, and different outcomes of interest.
In conclusion, ADAM12 rs3740199 polymorphism was likely to be associated with OA vulnerability among male patients, other than the general population, based on limited evidence. More studies are needed to further investigate this proposal. In addition, the biological mechanism by which ADAM12 gene and protein link with OA occurrence and progression needs to be elucidated.
Disclosure
The authors report no conflicts of interest in this work.
References
- Glyn-JonesSPalmerAJAgricolaROsteoarthritisLancet2015386999137638725748615
- LoeserRFCollinsJADiekmanBOAgeing and the pathogenesis of osteoarthritisNat Rev Rheumatol201612741242027192932
- RamosYFMeulenbeltIImplementation of functional genomics for bench-to-bedside transition in osteoarthritisCurr Rheumatolo Rep201517853
- LouSZhaoZQianJZhaoKWangRAssociation of single nucleotide polymorphisms in ADAM12 gene with susceptibility to knee osteoarthritis: a case–control study in a Chinese Han populationInt J Clin Exp Pathol2014785154515925197389
- WangLGuoLTianFHaoRYangTAnalysis of single nucleotide polymorphisms within ADAM12 and risk of knee osteoarthritis in a Chinese Han populationBiomed Res Int2015201551864325667922
- PoonpetTTammachoteRTammachoteNKanitnateSHonsawekSAssociation between ADAM12 polymorphism and knee osteoarthritis in Thai populationKnee201623335736126875044
- GiebelerNZigrinoPA Disintegrin and metalloprotease (ADAM): historical overview of their functionsToxins (Basel)20168412227120619
- GilpinBJLoechelFMatteiMGEngvallEAlbrechtsenRWewerUMA novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivoJ Biol Chem199827311571669417060
- ValdesAMHartDJJonesKAAssociation study of candidate genes for the prevalence and progression of knee osteoarthritisArthritis Rheum20045082497250715334463
- KernaIKisandKTammAELintropMVeskeKTammAOMissense single nucleotide polymorphism of the ADAM12 gene is associated with radiographic knee osteoarthritis in middle-aged Estonian cohortOsteoarthritis Cartilage20091781093109819268722
- Rodriguez-LopezJPombo-SuarezMLoughlinJAssociation of a nsSNP in ADAMTS14 to some osteoarthritis phenotypesOsteoarthritis Cartilage200917332132718790654
- ShinMHLeeSJKeeSJGenetic association analysis of GDF5 and ADAM12 for knee osteoarthritisJoint Bone Spine201279548849122284607
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- LimerKLToshKBujacSRAttempt to replicate published genetic associations in a large, well-defined osteoarthritis case–control population (the GOAL study)Osteoarthritis Cartilage200917678278919036616
- SandellLJEtiology of osteoarthritis: genetics and synovial joint developmentNat Rev Rheumatol201282778922231237
- ValdesAMVan OeneMHartDJReproducible genetic associations between candidate genes and clinical knee osteoarthritis in men and womenArthritis Rheum200654253353916453284
- KernaIKisandKTammAEKummJTammAOTwo single-nucleotide polymorphisms in ADAM12 gene are associated with early and late radiographic knee osteoarthritis in Estonian populationArthritis2013201387812623606964
- OkadaAMochizukiSYatabeTADAM-12 (meltrin alpha) is involved in chondrocyte proliferation via cleavage of insulin-like growth factor binding protein 5 in osteoarthritic cartilageArthritis Rheum200858377878918311789
- KernaIKisandKSuutreSThe ADAM12 is upregulated in synovitis and postinflammatory fibrosis of the synovial membrane in patients with early radiographic osteoarthritisJoint Bone Spine2014811515623578941
- KernaIKisandKLaitinenPAssociation of ADAM12-S protein with radiographic features of knee osteoarthritis and bone and cartilage markersRheumatol Int201232251952321258805
- KernaIKisandKLaitinenPTammATammAAssociation of metallopeptidase Domain 12 (Adam12) gene polymorphisms and Adam12 protein with the development of knee osteoarthritisOsteoarthritis Cartilage201018S170
- LoechelFGilpinBJEngvallEAlbrechtsenRWewerUMHuman ADAM 12 (meltrin alpha) is an active metalloproteaseJ Biol Chem19982732716993169979642263
- RoyRWewerUMZurakowskiDPoriesSEMosesMAADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stageJ Biol Chem200427949513235133015381692
- KveiborgMAlbrechtsenRRudkjaerLWenGDamgaard-PedersenKWewerUMADAM12-S stimulates bone growth in transgenic mice by modulating chondrocyte proliferation and maturationJ Bone Miner Res20062181288129616869727
- NagaosaYMateusMHassanBDevelopment of logically devised line drawing atlas for grading of knee osteoarthritisAnn Rheum Dis20005958759510913052
- HartDJSpectorTDThe relationship of obesity, fat distribution and osteoarthritis in the general population: the Chingford StudyJ Rheumatol1993203313358474072
- ZhangWRobertsonJDohertySLiuJJMaciewiczRAMuirKRIndex to ring finger length ratio and the risk of osteoarthritisArthritis Rheum2008581137e4418163515