Abstract
Background
In January 2014, the European Medicines Agency issued a marketing authorization for dolutegravir (DTG), a second-generation integrase strand transfer inhibitor for HIV treatment. The study aimed at determining the incremental cost-effectiveness ratio (ICER) of the use of DTG+backbone compared with raltegravir (RAL)+backbone, darunavir (DRV)+ritonavir(r)+backbone and efavirenz/tenofovir/emtricitabine (EFV/TDF/FTC) in HIV-positive treatment-naïve patients and compared with RAL+backbone in treatment-experienced patients, from the Italian National Health Service’s point of view.
Materials and methods
A published Monte Carlo Individual Simulation Model (ARAMIS-DTG model) was used to perform the analysis. Patients pass through mutually exclusive health states (defined in terms of diagnosis of HIV with or without opportunistic infections [OIs] and cardiovascular disease [CVD]) and successive lines of therapy. The model considers costs (2014) and quality of life per monthly cycle in a lifetime horizon. Costs and quality-adjusted life years (QALYs) are dependent on OI, CVD, AIDS events, adverse events and antiretroviral therapies.
Results
In treatment-naïve patients, DTG dominates RAL; compared with DRV/r, the ICER obtained is of 38,586 €/QALY (6,170 €/QALY in patients with high viral load) and over EFV/TDF/FTC, DTG generates an ICER of 33,664 €/QALY. In treatment-experienced patients, DTG compared to RAL leads to an ICER of 12,074 €/QALY.
Conclusion
The use of DTG+backbone may be cost effective in treatment-naïve and treatment-experienced patients compared with RAL+backbone and in treatment-naïve patients compared with DRV/r+backbone and EFV/TDF/FTC considering a threshold of 40,000 €/QALY.
Background
In the last decade, several new antiretroviral drugs for the treatment of HIV have been approved by drug regulatory agencies in Europe, US, Canada and Australia (ie, darunavir [DRV], raltegravir [RAL], elvitegravir, rilpivirine, maraviroc). Most of these marketing authorizations are based on non-inferiority Phase III studies that demonstrated the new drug to be non-inferior compared with the comparators taken into consideration.
Antiretroviral therapy (ART) usually consists of a backbone composed of two nucleoside reverse transcriptase inhibitors (NRTIs) and of a third agent, either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), a CCR5 receptor antagonist or an integrase strand transfer inhibitor (INSTI). The first-line treatment recommended by the Italian Guidelines On The Use Of Antiretroviral Drugs is a backbone plus an INSTI or a backbone plus an NNRTI or, in particular conditions, a backbone plus a PI.Citation1
The most recent data available on the Italian National Health Service (NHS) expenditure for ARTs show a total of 672.7 million €, of which 336.9 million € for drug combinations, 161.8 million € for PIs (alone or in one pill combination), 52.4 million € for NRTIs, 33.3 million € for NNRTIs and 88.3 million € for other antiretroviral drugs.Citation2
In January 2014, the European Medicines Agency issued a marketing authorization for dolutegravir (DTG), a second-generation INSTI for the treatment of HIV on the European market.Citation3
DTG is the only antiretroviral drug recommended with both abacavir/lamivudine (ABC/3TC) and tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) backbones by the Department of Health and Human Services’ Panel Guidelines.Citation4 It proved to be superior for viral suppression in treatment-naïve patients to DRV plus ritonavir (DRV+r) at 48 and 96 weeksCitation5,Citation6 and to efavirenz/TDF/FTC (EFV/TDF/FTC).Citation7 Compared to RAL, it proved to be non-inferior in treatment-naïve patients at 48 and 96 weeksCitation8,Citation9 and to be superior in treatment-experienced patients at 48 weeks.Citation10
Within the aforementioned studies, the analyses conducted considering as target population a high viral load patients subgroup (HIV RNA >100,000 copies/mL), provided only assuming DRV+r and RAL+backbone as comparators, showed an increased difference in terms of effectiveness due to the use of DTG+backbone vs the comparator compared with the total population.Citation5,Citation6,Citation8,Citation9
In Italy, HIV clinical pathways at a regional level introduced the concept of cost-effectiveness as a parameter to be taken into consideration when selecting ART to be administered.Citation11–Citation13 Therefore, the investigation of the value for money of ARTs is increasingly needed within the Italian context.
Starting from the efficacy results presented earlier, the study aimed at determining the incremental cost-effectiveness ratio (ICER) of the use of DTG+backbone compared with RAL+backbone, DRV+r+backbone and EFV/TDF/FTC in HIV-positive treatment-naïve patients and compared with RAL+backbone in treatment-experienced patients, adopting the point of view of the Italian National Health Service.
Further analyses were conducted considering a subgroup of patients with high viral load (HIV RNA >100,000 copies/mL) at baseline, where such subgroup was considered in clinical trials (vs DRV+r and RAL in treatment-naïve patients).
Materials and methods
Model structure
The analysis was performed using a Monte Carlo Individual Simulation Model, the ARAMIS-DTG model (an updated version of the ARAMIS model used to evaluate the cost-effectiveness of maraviroc+optimized background therapy [OBT] vs OBT).Citation14
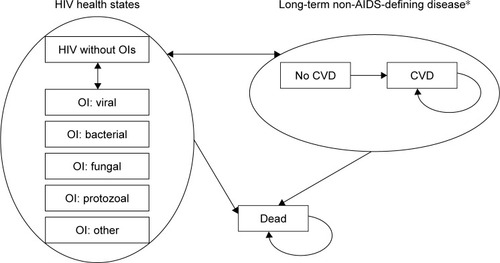
This microsimulation model allows patients to pass through successive lines of therapy considering costs and quality of life per monthly cycle in a lifetime horizon. Patients pass through mutually exclusive health states, defined in terms of diagnosis of HIV with or without opportunistic infections (OI) and cardiovascular disease (CVD).
Each patient within the model is assigned to one of 12 mutually exclusive health states: without OIs or with one of five possible OIs combined with the possibility of being affected by CVD. Patients can transition between any of the HIV health states into the CVD health state (if so, they remain within this state until death) and can experience acute OIs. Patients remain in a given health state for at least one cycle (1 month) and are at risk of changes in CD4+ cell count, consequences of the disease (OIs, AIDS-related morbidity), CVD, adverse events (AEs) and treatment failure due to viral rebound.
shows the model structure in terms of transitions among health states.
Figure 1 Model structure – health states transitions.
Abbreviations: CVD, cardiovascular disease; OI, opportunistic infection.
For the first 48 weeks in which a patient is assigned to a therapy, the model considers the 48 weeks probability to remain virologically controlled (as reported in ). After this period, the probability of a viral rebound becomes monthly, being estimated by dividing the difference in viral load suppression at 48 and 96 weeksCitation5–Citation10,Citation15 by the viral load suppression at 48 weeks. After 96 weeks, the monthly probability of a viral rebound is derived from the study by Rockstroh et al.Citation16
Table 1 Base case analyses effectiveness values (percentage of patients with HIV RNA of <50 copies/mL at week 48)
Patients enter the model with a CD4+ level as observed in the cohort considered for each ART and experience a cell recovery based on the mean CD4+ cell count increase (with a cap of 1,500 cells/μL) of the treatment in use for 24 months, after which the pace of CD4+ cells’ recovery becomes non-treatment-related. Patients failing ART maintain the CD4+ level, since they are switched to the subsequent ART regimen defined within the algorithm implemented. Twelve months after failing the last ART, a decline of CD4+ cell count is observed, as explained later (“Clinical parameters” section).
CD4+ cell count, along with the time since ART started, affects the monthly probability to experience OIs (viral, bacterial, protozoal, fungal, and others), and they were calculated from the study by D’Arminio et al.Citation17 Monthly CVD risk is estimated considering the coronary heart disease’s Framingham score plus equation for stroke. The parameters tracked in the model and used to estimate the score are the total cholesterol, the high-density lipoprotein cholesterol and age of patients. All other parameters derived from clinical trials remain constant. AIDS diagnosis is defined by CD4+ cell count equal to or lower than 200 cells/μL.
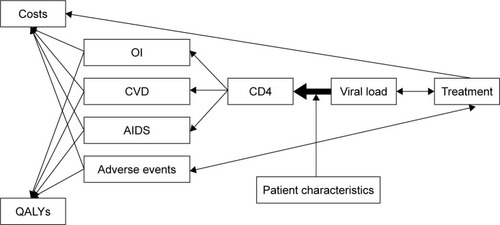
AEs’ monthly probability rate is influenced by the ART in use derived from the incidence of AEs emerged from the clinical trials considered.Citation5,Citation7,Citation8,Citation10 The costs and quality-adjusted life years (QALYs) are dependent on OI, CVD, AIDS, AEs and ART (the latter influencing only costs), as explained in the respective paragraphs. The occurrence of specific events is recorded for each patient from entry into the model until death, using tracker variables.
Both outcomes (QALYs) and costs were discounted at an annual rate of 3%.Citation18
illustrates the inter-relationship between the different components of the model.
Figure 2 Inter-relationships between the different components of the model.
Death may occur due to acute OI, HIV-related disease (including OI), CVD and background mortality (Italian-specific life tables).
Each cost utility analysis has been conducted with a lifetime horizon.
Intervention and comparator
The comparators considered in the analysis are RAL+ backbone, the main competitor within the same drug class as DTG (INSTI); DRV+r+backbone, which, along with atazanavir+r, is the PI recommended by international HIV guidelines in first-line therapies;Citation1,Citation4 and EFV/TDF/FTC, which represented the gold standard therapy for years, before the development of protease inhibitors, and is among the cheapest ART options on the market.
Population
Each cost utility analysis was conducted considering a theoretical cohort of 1 million patients.
The cohort characteristics in terms of age, gender, CD4+ cell count, viral load at baseline, hepatitis B and C infection and Framingham score are based on the clinical trials mentioned earlier,Citation5–Citation10 as well as the effectiveness values of the therapies considered.
For naïve patients, the aforementioned studies considered a population with median age between 34 and 37 years, of which males were between 83% and 86%. In Italy, newly diagnosed patients, as reported in the most recent publication by the Italian National Health Institute,Citation19 were 77.4% males with a median age of 39 years for males and 36 years for females. Caucasians represent between 68% and 86% of the cohorts of the studies considered. In Italy, the proportion of Caucasians is estimated to be >80% based on pub-lished dataCitation19 and experts’ opinion. Median baseline HIV-1 RNA copies/mL and median CD4 cells/μL were between 4.48 log10 and 4.68 log10 and between 338 and 400 cells/μL, respectively, in clinical trials. Unpublished data of the Italian Cohort of Antiretroviral Naïve patients show higher HIV-1 RNA, while CD4 cells copies’ levels are in line with the aforementioned data. This seems coherent considering that clinical trials’ cohorts are selected, presenting lower viral load compared to the general HIV-positive population. Finally, a higher hepatitis B virus (HBV) and hepatitis C virus (HCV) proportion of co-infected patients are observed in Italy, which is due to the higher prevalence of hepatitis virus in Italy, compared with central and north European countries and with United States and Canada.
Model input
Treatment algorithm
Following an algorithm defined by opinion leaders for each arm of the model, based on Italian HIV/AIDS guidelines,Citation20 patients are assigned to a first-line antiretroviral therapy (ART), DTG+backbone or one of the comparators considered, and to subsequent ARTs (the treatment algorithm is reported as Supplementary materials). The treatment algorithm was defined by four directors of hospital Infectious Diseases departments, among which the Medical Director of the Italian National Institute for Infectious Diseases; the Director of Infectious Diseases Division, the Scientific Director for Clinical Research of a clinical research university hospital and the Director of Infectious Diseases Department of a clinical research university hospital. Consensus was reached through discussions within advisory boards. The choice of subsequent lines of treatment depends on the reason of the switch: tolerability failure with no resistance, virologic failure with no resistance or virologic failure with resistance.
Clinical parameters
The therapy in use influences the viral load of each patient, as observed in the clinical trials reported earlier,Citation5–Citation10 affecting the decline of CD4+ cell countCitation21 (also influenced by the patient’s characteristics). The effectiveness value of each therapy (percentage of patients with HIV RNA of <50 copies/mL at week 48) is reported in .
Mortality ratios for CVD, HIV and background mortality are taken from the Italian Statistics National Institute database,Citation22 and a standardized mortality ratio is considered based on CD4+ cell count, as in the study by Lewden et al.Citation23
Utility
The utility values related to CD4+ cell count, CVD, acute and post-acute OIs and the AEs considered within the model are presented in . Changes in the quality of life are associated with the following parameters: six CD4+ cell count categories (0–50, 50–100, 100–200, 200–350, 350–500 and >500 cells/μL), acute OIs according to five categories (bacterial, protozoal, fungal, viral, other), long-term non-AIDS-defining diseases and acute AEs leading to ART discontinuation.
Table 2 Utility values and utility decrements considered in the analysis
Once an acute OI is experienced, the related utility is applied and after 3 months post-OI utilities are applied for the remainder of time that the individual is alive. Within the model, the lowest utility is applied for health states considering CD4+ cell count, OIs and CVD, while utilities decrement are subtracted from the base utility when patients experience one of the AEs considered.
Costs
The costs considered within the model are direct health costs related to ART, OI prophylaxis, treatment of OIs, routine care (hospitalization, outpatient activity, stratified by CD4+ cell count), costs of therapy switch, costs due to acute AEs and cost of death.
The cost of OIs, OIs’ prophylaxis, CVD and AEs were calculated on the basis of interviews submitted to directors of Infectious Diseases departments of Italian hospitals, to assess the resources consumption in real clinical practice for the diagnosis and care of the aforementioned events. These costs were approximated considering the reimbursement tariffs used within the Italian NHS for DRG and outpatient activities.
The costs, referred to year 2014, are reported in .
Table 3 Costs considered in the analysis
ART costs were considered based on Lombardy Region diagnostic clinical pathway.Citation11 INSTI- and PI-based first-line therapies’ cost (INSTI+backbone and PI+backbone) were considered to be the sum of third drug’s cost (INSTI or PI) and of a weighted mean of the cost of the two most relevant backbones for Italian clinical practice on the basis of experts’ opinion (40% cost of ABC/3TC and 60% of emtricitabine/tenofovir).
The price of drugs in Italy is negotiated at a national level by the Italian Medicines Agency (AIFA). The ex-factory price is published in the Official Gazette of the Italian Republic and is then reduced by applying mandatory discounts defined at a national level. Regional prices are then subject to regional tenders; however, limited differences are observed for antiretroviral drugs. The prices used in the analysis, published in the Lombardy Region diagnostic clinical pathway,Citation11 are comparable (with limited differences of <1 €) with those published within the most recent Italian HIV National Guidelines,Citation1 excluding the drugs whose price was changed after 2014. The different prices (related to only three drugs) are, however, at the same level of clinical pathways and publications concerning other regions, as the clinical pathway of VenetoCitation24,Citation25 and that of Calabria,Citation26 with differences of <0.5 €. Therefore, the prices used in the analysis are considered by the authors as representative at a national level.
Sensitivity analysis
Univariate sensitivity analyses were performed to address the uncertainty of the main parameters of the model. The range of each parameter considered is presented in . A probabilistic sensitivity analysis was not performed due to its estimated running time being, as reported by Pialoux et al,Citation27 ~417 days per each comparator.
Table 4 Ranges and values of the parameters modified within the sensitivity analysis
Based on experts’ opinion, we tested the effects of a decrease of 10% of DTG cost (an increase of the drug cost was considered highly unlikely), of a change of ±10% of the cost of subsequent therapies and salvage therapies (due to the possible decrease of therapies’ costs and increase due to new available drugs), of not considering any discount rate both for costs and QALYs, of a decrease of 2.5% of DTG effectiveness and of the use of alternative utility values associated with each CD4+ cell count level.
Results
Base case results
The results of the analyses conducted are reported in .
Table 5 Results of the analysis performed
DTG dominates RAL in treatment-naïve patients, leading to lower costs for the Italian National Health Service and being more effective, resulting in a higher number of QALYs per patient. The same result is observed in treatment-naïve patients with high viral load.
Compared with DRV+r, DTG leads to an increase of costs and an increase of QALYs, with an ICER of 38,586 €/QALY in treatment-naïve patients and of 6,170 €/QALY in treatment-naïve patients with high viral load. The same scenario is observed in the comparison of DTG+backbone vs EFV/TDF/FTC, with an ICER of 33,664 €/QALY.
Considering treatment-experienced patients, the use of DTG compared with RAL leads to an increase of costs and to an increase of QALYs with an ICER of 12,074 €/QALY.
Sensitivity analysis results
The results of sensitivity analysis are presented in .
Table 6 Results of the sensitivity analysis
In treatment-naïve patients, the sensitivity analysis results confirmed the dominance of DTG-based therapies compared with RAL-based therapies; confirmed DTG-based therapies to be cost effective compared with DRV+r-based therapies and with EFV/TDF/FTC considering a 40,000 €/QALY threshold, excluding the analyses in which the effectiveness of DTG is lowered by 2.5%, the cost of subsequent therapies is lowered by 10% and the cost of salvage therapies is raised by 10%; confirmed the dominance of DTG-based therapies compared with RAL-based therapies in high viral load patients and showed ICERs <12,500 €/QALY for DTG-based therapies vs DRV-based therapies in patients with high viral load.
In treatment-experienced patients, the sensitivity analysis confirmed DTG-based therapies to be cost-effective compared with RAL-based therapies considering a threshold of 40,000 €/QALY in all scenarios.
Discussion
The results of the analysis performed show how the use of DTG+backbone may be cost effective in treatment-naïve patients compared with RAL+backbone (being dominant, leading to lower costs and to a higher number of QALYs), DRV/r+backbone and EFV/TDF/FTC, considering the threshold of 40,000 €/QALY identified by the Italian Health Economics Association.Citation18
DTG-based therapies showed a better cost-effectiveness profile compared with RAL+backbone and DRV/r+backbone in treatment-naïve patients subgroup with high viral load.
In 2015, Despiégel et alCitation14 published an article presenting the results of a cost-effectiveness analysis in which, using the ARAMIS-DTG model, they compared the use of DTG in treatment-naïve patients with EFV, RAL, DRV+r, rilpivirine, elvitegravir/cobicistat, atazanavir+r and lopinavir/r and in treatment-experienced patients with RAL. DTG resulted to be dominant (leading to a decrease of costs for the Canadian NHS and to an increase of QALYs) in all the comparisons. These results are consistent with our analysis considering RAL as a comparator in treatment-naïve patients. When DRV+r and EFV/TDF/FTC are assumed as comparators in treatment-naïve patients and RAL is assumed as comparator in treatment-experienced patients, the Italian results showed increased QALYs and costs due to the use of DTG, while in the analysis performed in Canada, DTG led to higher QALYs and lower costs.
In Europe, a similar analysis was conducted in France by Pialoux et al.Citation27 The authors performed a cost-effectiveness analysis, through the use of the ARAMIS-DTG model, comparing the use of DTG vs RAL in treatment-experienced and INSTI-naïve adults with at least two-class resistance. DTG led to an increase of QALYs (+0.35) and of costs (+7,266 €), with a cost per QALY of 21,048 €, leading the authors to consider DTG cost effective compared to RAL in the target population. The results obtained by Pialoux et al are consistent with those obtained in the analysis presented.
A further study concerning the health economics evaluation of DTG is that of Peng et al,Citation28 in which DTG+ABC/3TC was compared with EFV/TDF/FTC as first-line treatment of HIV-1 patients in the United States. The study shows a life-time better result for DTG in terms of QALY gained (+0.12 per person) and higher costs (+58,188 US$ per person), with an ICER of 482,717 US$/QALY. Even if the analysis shows a higher effectiveness and higher costs for DTG+backbone compared with EFV/TDF/FTC as in the study performed in Italy, it is difficult to compare the results of analyses performed in two health systems with different characteristics as those of Italy (universalistic public system) and of the United States (market-based health insurance system).
The analysis performed is the first to assess the cost-effectiveness of DTG vs its main comparators within the Italian context. The main limitation of our analysis is related to the generalizability of the results, being based on Italian costs and on Italian HIV/AIDS guidelines. The prices of HIV drugs are subject to continuous modifications due to pharmaceutical companies’ market strategies; therefore, the analysis should be updated whenever changes in drugs prices are observed. To estimate the cost of OIs’ management, OIs’ prophylaxis, CVD, AEs and laboratory tests, we considered the reimbursement tariffs of the Italian NHS. This is consistent with the point of view assumed but might underestimate the costs for the Italian NHS. In fact, if the cost to perform a health care service for a public provider is higher than the tariff with which it is reimbursed by the NHS, at the end of the fiscal year, the NHS might have to provide further economic resources to the aforementioned provider. Moreover, concerning the clinical effectiveness of the therapies considered, the analysis presented is based on efficacy data coming from published trials, while future analyses should focus on effectiveness data collected within the Italian health service (not available at present) to ensure the reliability of the results. Furthermore, specific studies on the cost of AEs and complications in Italy in HIV-positive patients’ cohorts should be performed to provide reference data for future analyses. Limitations related to the model used for the analysis are related to the absence of a probabilistic sensitivity analysis and to a possible underestimation of CVD incidence, due to the fact that the model estimates this risk through Framingham equation, not considering the increased risk associated with HIV infection.
Conclusion
The use of DTG+backbone may be cost effective in treatment-naïve and treatment-experienced patients compared with RAL+backbone and in treatment-naïve patients compared with DRV/r+backbone and EFV/TDF/FTC considering the threshold of 40,000 €/QALY identified by AIES.
Acknowledgments
The study has been sponsored by ViiV Healthcare Srl.
Disclosure
UR declares honoraria for conference talks by Janssen-Cilag. GR declares honoraria for conference talks and advisory board participations (Abbvie, BMS, MSD, Gilead, ViiV, Janssen-Cilag). AA declares grants, personal fees and non-financial support from Gilead Sciences, personal fees from Merck, grants and personal fees from Bristol Myers Squibb, personal fees and non-financial support from Abbvie, grants, personal fees and non-financial support from ViiV Healthcare and grants and personal fees from Janssen-Cilag, all outside the submitted work. AL declares honoraria for conference talks and advisory board participations: Abbvie, BMS, MSD, Gilead, ViiV and Jannsen-Cilag. MB declares no conflicts of interest. PB declares honoraria for conference talks and advisory board participations: Abbvie, BMS, MSD, Gilead, ViiV and Jannsen-Cilag. DC declares honoraria for advisory board participations by MSD, Abbvie and Baxter. The authors report no other conflicts of interest in this work.
References
- HIV/AIDS Italian Expert PanelLinee Guida Italiane sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-12016 Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2545_allegato.pdf Accessed November 22, 2016
- The Medicines Utilisation Monitoring CentreNational Report on Medicines Use in ItalyRomeItalian Medicines Agency2015 Available from: http://www.agenziafarmaco.gov.it/sites/default/files/Rap-porto_OsMed_2015__AIFA.pdfAccessed May 19, 2017
- European Medicines AgencyEPAR Summary for the Public – Tivicay Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002753/WC500160681.pdfAccessed May 19, 2017
- Panel on Antiretroviral Guidelines for Adults and AdolescentsGuidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and AdolescentsDepartment of Health and Human Services Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdfAccessed May 19, 2017
- ClotetBFeinbergJvan LunzenJING114915 Study TeamOnce-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b studyLancet201438399362222223124698485
- MolinaJMClotetBvan LunzenJOnce-daily dolutegravir is superior to once-daily darunavir/ritonavir in treatment-naïve HIV-1-positive individuals: 96 week results from FLAMINGOJ Int AIDS Soc2014174 suppl 31949025393999
- WalmsleySLAntelaAClumeckNSINGLE InvestigatorsDolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infectionN Engl J Med2013369191807181824195548
- RaffiFRachlisAStellbrinkHJSPRING-2 Study GroupOnce-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 studyLancet2013381986873574323306000
- RaffiFJaegerHQuiros-RoldanEExtended SPRING-2 Study GroupOnce-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trialLancet Infect Dis2013131192793524074642
- CahnPPozniakALMingroneHExtended SAILING Study TeamDolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING studyLancet2013382989370070823830355
- Directorate General for Health of Lombardy RegionD.d.g. 22 December 2014 – n. 12515 – Approvazione del documento avente ad oggetto “Percorso diagnostico terapeutico (PDT) del paziente affetto da malattia HIV/AIDS – anno 2105” Available from: http://omceomi.it/docs/default-source/leggi-e-norme/percorso-diagnostico-terapeutico-paziente-affetto-da-malattia-hiv-aids-anno-2015-burl-30-12-2014.pdf?sfvrsn=0Accessed May 15, 2015
- Regione LazioDecreto del Commissario ad acta n. U00002 del 13 Gennaio 2014 – Allegato A “Percorso Diagnostico Terapeutico (POT) Regionale sulla Terapia Antiretrovirale” Available from: http://www.regione.lazio.it/binary/rl_sanita/tbl_normativa/Decr_U00002_13_1_13_aggiornamento_terapia_HIV.pdfAccessed November 24, 2015
- Decreto del Segretario della Segreteria Regionale per la Sanità n. 148 del 02 Dicembre 2013 – Allegato A “Percorso Diagnostico Terapeutico Assistenziale (PDTA) del paziente adulto affetto da infezione da HIV/AIDS nella Regione Veneto” Available from: http://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=263808Accessed November 24, 2015
- DespiégelNAngerDMartinMCost-effectiveness of dolutegravir in HIV-1 treatment-naive and treatment-experienced patients in CanadaInfect Dis Ther20154333735326099626
- StellbrinkHJReynesJLazzarinADolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging studyAIDS201327111771177823807273
- RockstrohJKDeJesusESaagMLong-term safety and efficacy of raltegravir (RAL)-based versus efavirenz (EFV)-based combination therapy in treatment-naïve HIV-1 infected patients: final 5-year double-blind results from STARTMRKPresented at: XIX International AIDS ConferenceJuly 22–27; 2012Washington, DC
- D’ArminioMASabinCAPhillipsAThe changing incidence of AIDS events in patients receiving highly active antiretroviral therapyArch Intern Med200516541642315738371
- Italian Health Economics Association (AIES)Proposta di Linee-Guida per la valutazione economica degli interventi sanitariPolitiche Sanitarie20091029199
- Italian National Institute of HealthAIDS Operative CentreNotiziario dell’Istituto Superiore di Sanità2016299 Suppl 1 Available from: http://www.iss.it/binary/ccoa/cont/dic_2015.pdfAccessed May 19, 2017
- HIV/AIDS Italian expert panelLinee Guida Italiane sull’utilizzo dei farmaci antiretrovirali e sulla gestione diagnostico-clinica delle persone con infezione da HIV-12014 Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2261_allegato.pdfAccessed December 18, 2014
- MauskopfJBroganAJTalbirdSEMartinSCost-effectiveness of combination therapy with etravirine in treatment-experienced adults with HIV-1 infectionAIDS201226335536422089378
- Italian Statistics National Institute [homepage on the Internet]I.Stat Database Available from: http://dati.istat.it/Index.aspxAccessed May 19, 2017
- LewdenCCheneGMorlatPAgence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO8 APROCO-COPILOTE Study GroupAgence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO3 AQUITAINE Study GroupHIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general populationJ Acquir Immune Defic Syndr2007461727717621240
- Regione del Veneto [webpage on the Internet]Percorso Diagnostico Terapeutico Assistenziale (PDTA) del paziente adulto affetto da infezione da HIV/AIDS nella Regione Veneto. Allegato A al Decreto n. 148 del 2 Dicembre 2013 Available from: http://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=263808Accessed May 19, 2017
- Regione del VenetoPercorso Diagnostico Terapeutico Assistenziale (PDTA) del paziente adulto affetto da infezione da HIV/AIDS nella Regione Veneto – aggiornamento a febbraio 2016. Allegato A al Decreto n. 55 del 8 Giugno 2016 Available from: https://www.regione.veneto.it/c/document_library/get_file?uuid=3beb4023-6e19-4d71-a73e-48-a4e6161d3d&groupId=10793Accessed May 19, 2017
- Regione CalabriaPercorso Diagnostico Terapeutico Assistenziale (PDTA) regionale del Paziente affetto da malattia da HIV/AIDS Available from: http://www.regione.calabria.it/sanita/allegati/dpgr_2012/allegato_1_dpgr_198_del_20_dicembre_2012.pdfAccessed May 19, 2017
- PialouxGMarcelinAGDespiégelNCost-effectiveness of dolutegravir in HIV-1 treatment-experienced (TE) patients in FrancePLoS One20151012e014588526714188
- PengSTafazzoliADormanERosenblattLVillasis-KeeverASorensenSCost-effectiveness of DTG+ABC/3TC versus EFV/TDF/FTC for first-line treatment of HIV-1 in the United StatesJ Int AIDS Soc2014174 suppl 31960525394109
- KaufTLRoskellNShearerAA predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapyValue Health20081171144115318494750
- SimpsonKNLuoMPChumneyECKingMSBrunSCost effectiveness of lopinavir/ritonavir compared with atazanavir in antiretroviral-naive patients: modelling the combined effects of HIV and heart diseaseClin Drug Investig20072716774
- PaltielADScharfsteinJASeageGR3rdA Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infectionMed Decis Making1998182 supplS93S1059566470
- SchackmanBRGoldieSJFreedbergKALosinaEBrazierJWeinsteinMCComparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDSMed Decis Making2002221273811833663
- RizzardiniGRestelliUBonfantiPCost of human immunodeficiency virus infection in Italy, 2007–2009: effective and expensive, are the new drugs worthwhile?Clinicoecon Outcomes Res2012424525222973114
- RaitanoM“The Impact of Death-Related Costs on Health-Care Expenditure: A Survey”, ENEPRI Research Report No. 1722006
- SimpsonKNLuoMPChumneyESunEBrunSAshrafTCost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infectionHIV Clin Trials20045529430415562370
