Abstract
Approximately 10%–12% of patients in chronic-phase chronic myeloid leukemia (CP-CML) have additional chromosomal aberrations at diagnosis; moreover, CML occurs in up to 10% of pregnancy-associated leukemias, with an annual incidence of 1 per 100,000 pregnancies. In this report we describe the case of a 36-year-old female with CP-CML diagnosed in the 18th week of pregnancy and with a new complex variant translocation t(4;9;22;21)(q24;q34;q11;q22) and an additional chromosomal aberration t(1;20)(p36;p11). In consideration of her pregnancy, the patient strictly monitored her blood cell count without any specific treatment. At 32 weeks of pregnancy, the patient delivered via cesarean section a healthy baby girl. After 10 days from childbirth, dasatinib was started at a standard dosage of 100 mg/day and 3 months later complete cytogenetic response and major molecular response were obtained, with the achievement of an optimal response according to European Leukemia Net recommendations and showing efficacy of this tyrosine kinase inhibitor (TKI) in the presence of a complex karyotype.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the presence of the Philadelphia chromosome (Ph) resulting from the reciprocal translocation t(9;22)(q34;q11).Citation1 Approximately 10%–12% of patients in chronic-phase CML (CP-CML) present with additional chromosomal aberrations (ACAs) at diagnosis.Citation2 Recently different researchers have studied this subgroup, including European Leukemia Net (ELN)Citation3 and have classified CML ACAs into “major” and “minor” route changes.Citation4 The major route ACAs are the most common chromosomal abnormalities (>10% of cases with ACAs) and include trisomy 8, an extra Ph (1der(22)t(9;22) (q34;q11)), isochromosome 17(i(17)(q10)), trisomy 19 and ider(22)(q10)t(9;22) (q34;q11). Other less common ACAs belong to minor route ACAs. Moreover, microdeletions can be present too and the occurrence of genomic microdeletions proximally to ABL1 or distally to BCR have been reported in CML cases with variant translocations with a greater frequency (30%–40%) than in cases with classic t(9;22) (10%–18%).Citation5 When the chromosome changes are submicroscopic, the translocation can be masked and revealed only by fluorescence in situ hybridization (FISH) or by molecular analysis.Citation6 Chromosome 9 deletions and variant translocations have no value for prognosisCitation2,Citation7,Citation8 whereas ACA/Ph1 have been reported to have an adverse prognostic value, particularly in the case of the major route abnormalities, including, as described previously, trisomy 8, an extra Ph (1der(22)t(9;22)(q34;q11)), isochromosome 17 (i(17)(q10)), trisomy 19, and ider(22)(q10)t(9;22)(q34;q11).Citation2,Citation9 Major route ACA/Ph1 at diagnosis do not mandate different initial treatments but represent a warning: this implies that the characteristics of the disease and the response to treatment require more frequent monitoring to permit timely changes in therapy in case of treatment failure.Citation3 Major route ACA/Ph1 developing during treatment were confirmed to be a signal of acceleration.Citation10–Citation12 CML occurs in up to 10% of pregnancy-associated leukemias, with an annual incidence of 1 per 100,000 pregnancies.Citation13 In this report we describe the case of a 36-year-old female diagnosed in her 18th week of pregnancy with CP-CML and presenting with a new complex variant translocation t(4;9;22;21)(q24;q34;q11;q22) and an ACA t(1;20)(p36;p11). The rearrangement was analyzed by cytogenetic and FISH tests. To the best of our knowledge, this translocation has not been described in CML previously and specifically in a pregnant patient.
Case report
Patient presentation
A 36-year-old female, 18 weeks pregnant, presented in October 2015 with abnormal blood cell counts (white blood count 29.87×10*9/L, hemoglobin 11.5 g/dL and platelets 592×10*9/L); evaluation of the peripheral blood smear revealed basophils (2%), myelocytes (7%), metamyelocytes (13%), and blasts (1%). Bone marrow aspiration was performed and its examination revealed a slightly hypercellular marrow with granulocytic hyperplasia. Cytogenetic analysis revealed the presence of a complex karyotype in all examined metaphases: 46 XX, t(1;20)(p36;p11), t(4;9;22;21) (q24;q34;q11;q22). Reverse transcriptase-polymerase chain reaction (RT-PCR) done on peripheral blood and bone marrow showed a b3a2 BCR/ABL fusion gene. The patient was diagnosed with Ph+ CML (low risk Sokal score). In consideration of her pregnancy and leukocyte and platelet values she was advised to strictly monitor her blood cell count, with no other treatment apart from a low dose of aspirin. At 32 weeks (January 2016), the patient delivered via cesarean section a healthy baby girl (weight 2,120 g, height 43 cm; APGAR 9). At the time of childbirth, the hematological analysis revealed a white blood cell count of 73.51 × 10*9/L, a hemoglobin level of 10 g/dL, a platelet count of 494 × 10*9/L. After 10 days from childbirth, the patient started dasatinib at a standard dosage of 100 mg/day. After 3 months on dasat-inib the patient obtained complete cytogenetic response and major molecular response (MMR), achieving an optimal response according to the ELN recommendations.
Cytogenetic analysis
Cytogenetic analysis was performed on bone marrow culture using a standard technique.Citation14 In total, 20 GTG banded bone marrow metaphase cells were analyzed. The karyotypes were named according to the International System for Human Cytogenetic Nomenclature.Citation15 The cytogenetic analysis performed on 20 metaphase cells identified the presence of a complex, four-way (4;9;22;21)(q24;q34;q11;q22) Ph chromosome translocation () and an additional chromosomal aberration t(1;20)(p36;p11). In the present case, the Philadelphia translocation is likely to be the first event, followed by the further rearrangement involving chromosomes 4q and 21q, in a step by step sequence. The additional t(1;20) (p36;p11) can be considered an independent event.
Figure 1 Cytogenetic analysis.
FISH analysis
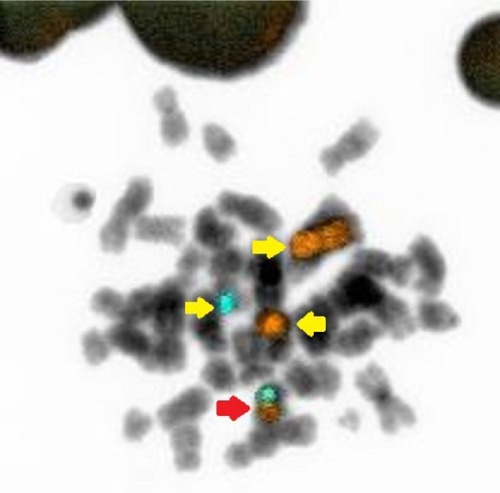
FISH analysis using a BCR/ABL dual color dual fusion probe (), showed the presence of a variant BCR/ABL translocation in approximately 80% of interphase nuclei with a single fusion signal; two BCR and two ABL signals showing the splitting of one of the two expected fusion signals, thus suggested the involvement of additional chromosomes and, therefore, we extended the analysis to metaphase spreads. Painting probes for chromosomes 4 and 22 confirmed the presence of a translocation involving chromosomes 4 and 22, generating two derivative chromosomes, one normal chromosome 4 and one normal 22 ().
Figure 2 FISH analyses with Bcr and Abl probes.
Abbreviation: FISH, fluorescence in situ hybridization.
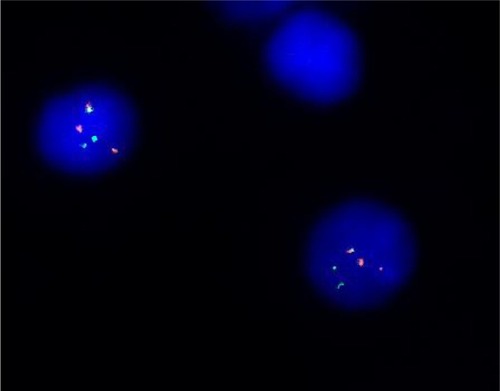
Figure 3 FISH analyses with chromosomes 4 and 22 probes.
Abbreviation: FISH, fluorescence in situ hybridization.
Molecular testing
At diagnosis, nested RT-PCR was done on peripheral blood and bone marrow showing a b3a2 BCR/ABL fusion gene; at 3 months real-time quantitative RT-PCR was perfomed using TaqMan system and a BCR-ABL1 transcripts’ level <10% (0.0829%) according to the International Scale was found (MMR).
Cytological analysis
Examination of aspirated bone marrow revealed a slightly hypercellular marrow with granulocytic hyperplasia. Histological examination of the placenta revealed the presence of chorionic villus with characters of development corresponding to gestational age (32 weeks), with intervillous space characterized by the presence of increased lymphomonocytic and granulocytic elements. Membranes and umbilical cord were without significant histological alterations.
Discussion
In this report we describe a unique case of a 36-year-old female with CP-CML diagnosed at 18 weeks of pregnancy, characterized by a new complex variant translocation t(4;9;22;21)(q24;q34;q11;q22) and t(1;20)(p36;p11) as ACA. The rearrangement was analyzed by cytogenetic and FISH tests. To the best of our knowledge, this translocation has not been observed in CML previously and particularly in a pregnant patient. The management of CML diagnosed during pregnancy is very challenging due to the physiological changes, including those in hematological parameters which accompany the pregnancy, that may mask the symptoms. Previously, it seemed that CML diagnosed during pregnancy was associated with low birth weight and preterm birth, but this is no longer described in more recent reports. Reassuringly, the course of the disease does not appear to be affected by pregnancy.Citation16 The prothrombotic potential of a normal pregnancy is well recognized as a result of a physiological increase in hemostatic factors and prothrombotic proteins in addition to the physical obstruction of venous blood flow. As a result, thrombosis continues to be the most common cause of maternal morbidity and this may be compounded in the myeloproliferative diseases where there is an associated elevation in the platelet count. Therapeutic approaches for CML diagnosed in pregnancy include supportive care in the form of leukapheresis and platelet pheresis and interferon-a (IFN-a), while the use of tyrosine kinase inhibitors (TKIs) is controversial.Citation17 Leukapheresis and platelet pheresis allow avoidance of potentially teratogenic drugs but are not easily available and tolerable. The frequency of these procedures depends on white cell and platelet count. IFN-a, which has a high molecular weight, does not cross the placenta and does not inhibit DNA synthesis; for these reasons it is considered safe in pregnancy. Among TKIs, imatinib is the most studied. It does not cross the placenta but various congenital abnormalities have been described after exposure to imatinib in the first trimester, when placenta formation is not completed, probably due to platelet derived growth factor receptor alpha inhibition. Thus, imatinib and generally all TKIs should be avoided in the first trimester and during organogenesis. Dasatinib crosses the placenta and leads to considerable levels in fetal plasma. It should be avoided in all pregnant patients since it is responsible for fetal hydrops and severe fetal bicytopeniaCitation18 even if normal pregnancies during exposure have been reported.Citation19–Citation21 Nilotinib does not cross the placenta in a significative concentration and does not seem to be teratogenic, but data are limited. A recent review summarized reported cases and provided recommendations of management of pregnancy in CML; so far no case of pregnancy in a CML patient associated with bosutinib and ponatinib therapy has been described.Citation22 However, as in our case, treatment is not always mandatory; it is necessary if white cell count exceeds 100×109/L and platelet count exceeds 500×109/L, as reported by Milojkovic and Apperley.Citation23 Low-molecular-weight heparin, as well as aspirin, can be used. Regarding the complex karyotype of our patient, Philadelphia translocation is likely to be the first event, followed by the further rearrangement involving chromosomes 4q and 21q, in a step by step sequence. The additional t(1;20)(p36;p11) can be considered an independent event. t(1;20) has been reported associated with a high rate of recurrent first trimester abortions in a large family;Citation24 in our case, the mother did not spontaneously abort her child during the pregnancy despite the presence of this aberration. Mkrtchyan et alCitation25 suggested two possible mechanisms which may be involved in the formation of variant or complex translocations. The first is a single event in which rearrangement due the simultaneous breakage of several chromosomes is by mismatched joining. The second is a multi-step mechanism in which a classical Ph translocation is followed by further translocation events involving chromosomes 9 and 22, plus a second, third, and subsequent event leading to a multiple-way translocation. Concerning treatment, after strict observation, low dose aspirin and a safe delivery, our patient was treated with dasatinib, a second-generation TKI, at a dosage of 100 mg per day, considering early age and the complex, never described before, translocation of uncertain prognostic significance. Dasatinib is a multi-targeted kinase inhibitor of BCR/ABL, SRK, c-KIT, ephrin receptors, and PDGFRB and from 2012 it can be used also in first line treatment of CML. Treatment was well tolerated with no significant side effects. After 3 months the patient showed a complete cytogenetic response and MMR achieving an optimal response according to ELN recommendations, proving dasatinib’s efficacy also in the case of complex karyotype.
Conclusion
We report a novel case of CP-CML with a new complex variant translocation t(4;9;22;21)(q24;q34;q11;q22) and an ACA t(1;20)(p36;p11). Notably, the patient concerned showed a good tolerability and a good response to dasatinib also, in the presence of a complex karyotype.
Acknowledgments
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
- RowleyJDA new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa stainingNature19732432902934126434
- FabariusALeitnerAHochhausAImpact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IVBlood2011118266760676822039253
- BaccaraniMDeiningerMWRostiGEuropean LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013Blood2013122687288423803709
- FioretosTJohanssonBChronic myeloid anchor leukemiaHeimSMitelmanFCancer CytogeneticsHoboken, NJWiley-Blackwell2009179207
- HuntlyBJReidAGBenchAJDeletions of the derivative chromosome 9 occur at the time of the Philadelphia translocation and provide a powerful and independent prognostic indicator in chronic myeloid leukemiaBlood2001981732173811535505
- Quinta’s-CardamaACortesJMolecular biology of bcr-abl1-positive chronic myeloid leukemiaBlood200911381619163018827185
- CastagnettiFTestoniNLuattiSDeletions of the derivative chromosome 9 do not influence the response and the outcome of chronic myeloid leukemia in early chronic phase treated with imatinib mesylate: GIMEMA CML Working Party analysisJ Clin Oncol201028162748275420439635
- MarzocchiGCastagnettiFLuattiSVariant Philadelphia translocations: molecular-cytogenetic characterization and prognostic influence on frontline imatinib therapy, a GIMEMA Working Party on CML analysisBlood2011117256793680021447834
- LuattiSCastagnettiFMarzocchiGAdditional chromosomal abnormalities in Philadelphia-positive clone: adverse prognostic influence on frontline imatinib therapy: a GIMEMA Working Party on CML analysisBlood2012120476176722692507
- DeiningerMWCortesJPaquetteRThe prognosis for patients with chronic myeloid leukemia who have clonal cytogenetic abnormalities in philadelphia chromosome-negative cellsCancer200711071509151917702093
- VermaDKantarjianHShanJSurvival outcomes for clonal evolution in chronic myeloid leukemia patients on second generation tyrosine kinase inhibitor therapyCancer2010116112673268120499401
- LeeSEChoiSYBangJHThe long-term clinical implications of clonal chromosomal abnormalities in newly diagnosed chronic phase chronic myeloid leukemia patients treated with imatinib mesylateCancer Genet20122051156357123111092
- LichtmanMAAcute myelogenous leukemiaWilliams Hematology10476th edNew York, NYMcGraw-Hill2001
- ShafferLGSlovakMLCambellLJISCN 2009: An International System for Human Cytogenetic NomenclatureBaselKarger20094044
- BrothmanARPersonsDLShafferLGNomenclature evolution: Changes in the ISCN from the 2005 to the 2009 editionCytogenet Genome Res200912711420110655
- ApperleyJCML in pregnancy and childhoodBest Pract Res Clin Haematol200922345547419959094
- MilojkovicDApperleyJState-of-the-art in the treatment of chronic myeloid leukaemiaCurr Opin Oncol200820111212118043265
- CortesJEAbruzzeseEChelyshevaEGuhaMWallisNApperleyJFThe impact of dasatinib on pregnancy outcomesAm J Hematol201590121111111526348106
- KrollTAmesMBPruettJAFenskeTSSuccessful management of pregnancy occurring in a patient with chronic myeloid leukemia on dasatinibLeuk Lymphoma20105191751175320629520
- ConchonMSanabaniSSSerpaMSuccessful pregnancy and delivery in a patient with chronic myeloid leukaemia while on dasat-inib therapyAdv Hematol2010201013625220224653
- BayraktarSMorencyBEscalónMPSuccessful pregnancy in a patient with chronic myeloid leukaemia exposed to dasatinib during the first trimesterBMJ Case Rep20102010 pii:bcr0520102975
- AbruzzeseETrawinskaMMde FabritiisPBaccaraniMManagement of pregnant chronic myeloid leukemia patientsExpert Rev Hematol20169878179127352939
- MilojkovicDApperleyJFHow I treat leukemia during pregnancyBlood2014123797498424269956
- MadanKKleinhoutJFirst trimester abortions associated with a translocation t(1;20)(p36;p11)Hum Genet19877611093570298
- MkrtchyanHGhazaryanSAvetisyanGNovel complex t(V;9;22) rearrangements in three cases with chronic myeloid leukemia and a rare translocation in a case with classical Philadelphia chromosomeOncol Rep20082019910418575724
