Abstract
Background
Left ventricular ejection fraction (LVEF) is the most widely used parameter to evaluate the cardiac function in patients with heart failure (HF). However, the association between LVEF and contrast-induced nephropathy (CIN) is still controversial. Therefore, the aim of this study is to evaluate the association of LVEF with CIN and long-term mortality following coronary angiography (CAG) or intervention in patients with HF.
Methods
We analyzed 1,647 patients with HF (New York Heart Association [NYHA] or Killip class >1) undergoing CAG or intervention, including 207 (12.57%) patients with reduced LVEF (HFrEF), 238 (14.45%) with mid-range LVEF (HFmrEF) and 1,202 (72.98%) with preserved LVEF (HFpEF). CIN was defined as an absolute increase of ≥0.5 mg/dL or a relative increase of ≥25% from baseline serum creatinine within 48–72 h after contrast medium exposure. Multivariable logistic regression and Cox proportional hazards regression analyses were performed to identify the association between LVEF, CIN and long-term mortality, respectively.
Results
Overall, 225 patients (13.7%) developed CIN. Individuals with lower LVEF were more likely to develop CIN (HFrEF, HFmrEF and HFpEF: 18.4%, 21.8% and 11.2%, respectively; P<0.001), but without a significant trend after adjusting for the confounding factors (HFrEF vs HFpEF: odds ratio [OR] =1.01; HFmrEF vs HFpEF: OR =1.31; all P>0.05). However, advanced HF (NYHA class >2 or Killip class >1) was an independent predictor of CIN (adjusted OR =1.54, 95% confidence interval [CI], 1.07–2.22; P=0.019). During the mean follow-up of 2.3 years, reduced LVEF (HFrEF group) was significantly associated with increased mortality (HFrEF vs HFpEF: adjusted hazard ratio =2.88, 95% CI, 1.77–4.69; P<0.001).
Conclusion
In patients with HF undergoing CAG or intervention, not worsened LVEF but advanced HF was associated with an increased risk of CIN. In addition, reduced LVEF was an independent predictor of long-term mortality following cardiac catheterization.
Introduction
With the development of interventional technology and medication strategies, the number of cardiac catheterization procedures being performed continues to grow rapidly.Citation1 Simultaneously, the incidence of contrast-induced nephropathy (CIN), a common and well-known complication which occurs following coronary angiography (CAG) or percutaneous coronary intervention (PCI) and is significantly associated with renal and cardiovascular adverse events and long-term mortality, has also increased gradually.Citation2,Citation3 Since the effective treatment measures for CIN are unknown, risk identification is important for ensuring that high-risk patients receive appropriate prophylactic measures and postoperative monitoring.Citation4
Heart failure (HF) is a common and deteriorating condition, which has a high prevalence of ischemic origin.Citation5 With the advancement of HF or cardiac impairment, adverse hemodynamic state results in inadequate renal perfusion and accelerates the renal impairment after contrast medium (CM) administration.Citation6,Citation7 Previous studies indicated that HF is one of the critical factors influencing the development of CIN.Citation8,Citation9 Left ventricular ejection fraction (LVEF) is another parameter that reflects the cardiac function and a useful term to categorize the type of HF, such as HF with reduced ejection fraction (HFrEF; EF <40%), HF with mid-range ejection fraction (HFmrEF; EF 40%–49%) and HF with preserved ejection fraction (HFpEF; EF ≥50%).Citation10 However, the association between LVEF and the risk of CIN is still controversial.Citation11–Citation14 Therefore, the purpose of our study was to analyze the association of LVEF with CIN and long-term mortality following CAG/PCI in patients with HF.
Methods
Study population
This prospective observational study was conducted at the Guangdong General Hospital from April 2009 to December 2013. We included patients aged >18 years who had HF, defined as New York Heart Association (NYHA) or Killip class >1, and were undergoing PCI/CAG. Based on the protocol, exclusion criteria included pregnancy, malignancy, cardiovascular surgery or endovascular repair, end-stage renal disease or renal replacement, treatment with nephroprotective (eg, N-acetylcysteine) or nephrotoxic (eg, glucocorticoids, aminoglycosides) drugs and exposure to CM within the previous 7 days. In addition, patients who had missing preoperative or postoperative creatinine values (n=87) and LVEF (n=448) were excluded.
Biochemical investigations
Serum creatinine (SCr) concentrations were measured at admission and within 24, 48 and 72 h after CM administration. Other biochemical indicators were measured in the morning prior to the procedure. The Modification of Diet in Renal Disease equation was used to calculate the estimated glomerular filtration rate (eGFR),Citation15 and the echocardiography examination was used to evaluate the LVEF. A baseline eGFR <60 mL/min/1.73 m2 was defined as renal insufficiency.Citation16 Furthermore, NYHA class >2 or Killip class >1 was defined as advanced HF.Citation17,Citation18
Cardiac catheterization
Cardiac catheterization was performed according to the standard clinical practice, by experienced interventional cardiologists. Non-ionic, low-osmolality CM was used for all patients. The type of stents was selected by the interventional cardiologists according to operative requirements. All patients received intravenous infusion of normal saline 2–12 h before and 6–24 h after the procedure at a speed of 0.5–1.0 mL/kg/h. The hydration time and speed and the clinical medication were chosen based on the patient condition.
Clinical end points and follow-up
The primary end point of this study was the development of CIN, defined as an absolute increase of ≥0.5 mg/dL or a relative increase of ≥25% from baseline SCr level within 48–72 h after CM exposure (CIN0.5 or 25%).Citation19 Additional end point included another criteria of CIN, defined as an absolute increase of ≥0.3 mg/dL or a relative increase of ≥50% (CIN0.3 or 50%) and an absolute increase of ≥0.5 mg/dL (CIN0.5),Citation20 and all-cause mortality.
All patients included in this study were followed up by telephone or office visits at 1, 6, 12, 24 and 36 months after discharge. Adverse events were recorded on the case report form.
This study was performed according to the Declaration of Helsinki, and the ethics committee of the Guangdong General Hospital approved the study protocol. Written informed consent was obtained from the patients involved in the study.
Statistical analysis
Patients were divided into three groups based on the level of LVEF according to the 2016 European Society of Cardiology guideline for HF.Citation10 For continuous variables, ANOVA was used for normally distributed data (described as mean ± standard deviation), and Wilcoxon rank-sum test was conducted for non-normal distributions (described as interquartile range). For categorical variables, χ2 test or Fisher’s exact test was used (described as absolute values and percentages). Multivariable logistic regression and Cox proportional hazards regression analyses were performed to identify the association of LVEF with CIN and long-term mortality, respectively. HFpEF was considered as the reference group. The effect of HFmrEF and HFrEF on outcomes was estimated and was compared with the reference group. Kaplan–Meier method was used to describe the all-cause mortality by log-rank tests. All statistical analyses were performed with SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA) and R software (version 3.1.2; R Core Team, Vienna, Austria). A two-tailed P<0.05 was considered statistically significant.
Results
Baseline demographics and characteristics
A total of 1,647 patients with HF undergoing CAG/PCI were analyzed, including 207 (12.57%) patients with HFrEF, 238 (14.45%) with HFmrEF and 1,202 (72.98%) with HFpEF. The baseline demographics and characteristics of patients are listed in .
Table 1 Baseline characteristics according to the left ventricular ejection fraction group
Compared to the patients with HFpEF, patients with HFrEF were more likely to have advanced HF, renal insufficiency and prior myocardial fraction. Furthermore, those in the HFrEF group had lower systolic blood pressure on admission and were less likely to have a history of hyper-tension. However, age, gender, smoking, hyperlipidemia and history of coronary artery bypass grafting were similar among the three groups.
On admission, patients with HFrEF had higher SCr and N-terminal pro-brain natriuretic peptide concentrations, but lower eGFR and LVEF level. In addition, those patients were more likely to be on diuretics and less likely to be on β-blockers and stains than the other two groups. Furthermore, the prevalence of emergency PCI and the volume of CM were highest in the patients with HFmrEF.
Incidence of CIN and in-hospital outcomes
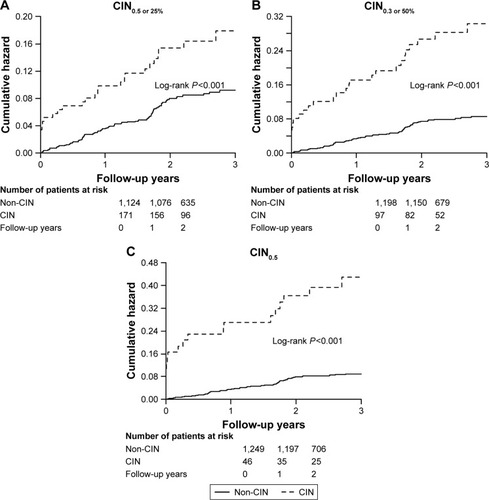
Overall, 225 patients (13.7%) developed CIN, and the incidence of CIN0.5 or 25% was different among the LVEF groups (HFrEF, HFmrEF and HFpEF: 18.4%, 21.8% and 11.2%, respectively; P<0.001). Similar trend was observed in the incidence of CIN0.3 or 50% or CIN0.5 ( and ).
Figure 1 Incidence of CIN in different definitions between left ventricular ejection fraction groups.
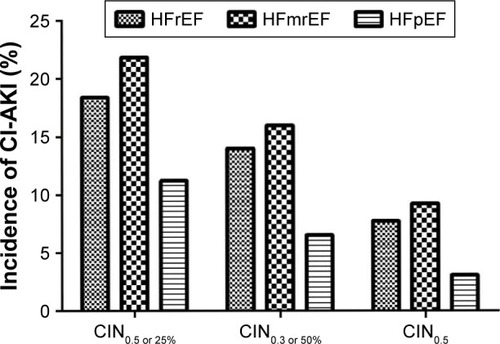
Table 2 Incidence of CIN and in-hospital outcomes between left ventricular ejection fraction groups
Furthermore, patients with HFrEF were more likely to experience death (HFrEF, HFmrEF and HFpEF: 5.8%, 5.5% and 1.0%, respectively; P<0.001) and hypotension (HFrEF, HFmrEF and HFpEF: 11.1%, 9.2% and 2.1%, respectively; P<0.001). In addition, patients with lower LVEF had a significantly higher rate of requirement of intra-aortic balloon pump (IABP) (HFrEF, HFmrEF and HFpEF: 14.0%, 12.2% and 2.9%, respectively; P<0.001) and renal replacement therapy (HFrEF, HFmrEF and HFpEF: 3.4%, 2.5% and 0.7%, respectively; P=0.002) ().
Association of LVEF with CIN
After adjusting for the confounders, including age >75 years, hypertension, diabetes mellitus, renal insufficiency, advanced HF, prior myocardial infarction, emergency PCI, CM volume >100 mL, hypotension and use of stains, diuretics and IABP, multivariate logistic regression results revealed that individuals with lower LVEF were not at significantly increased risk of CIN compared with the highest LVEF group (HFrEF vs HFpEF: odds ratio [OR] =1.01, 95% confidence interval [CI], 0.69–1.74; P=0.700; HFmrEF vs HFpEF: OR =1.31, 95% CI, 0.87–1.96; P=0.194). Similar results were demonstrated for CIN0.3 or 50% or CIN0.5. However, age >75 years, advanced HF, emergency PCI and use of IABP were the significantly independent risk factors for CIN in different criteria ().
Table 3 Association of left ventricular ejection fraction with CIN in different definition
Association between LVEF, CIN and long-term mortality
The mean follow-up period was 2.30±0.93 years. Log-rank analyses indicated that patients with lower LVEF were associated with higher mortality rate (log-rank, P<0.001). The Kaplan–Meier curve is shown in . After adjusting for the confounders which were associated with long-term mortality, multivariate Cox regression showed that HFrEF was an independent predictor of mortality (HFrEF vs HFpEF: adjusted hazard ratio [HR] =2.88, 95% CI, 1.77–4.69; P<0.001; HFmrEF vs HFpEF: HR =1.55, 95% CI, 0.95–2.53; P=0.079) ().
Figure 2 Cumulative rate of all-cause mortality during the follow-up in patients with HFrEF, HFmrEF and HFpEF.
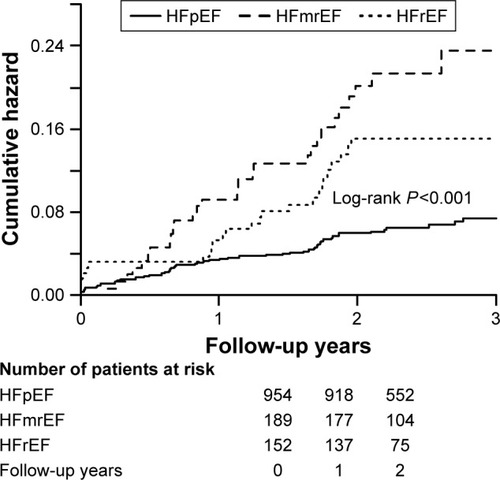
Table 4 Association between left ventricular ejection fraction and long-term mortality
Moreover, patients who developed CIN0.5 or 25% had higher rate of all-cause mortality than those without during the follow-up. Similar results were found in those who developed CIN0.3 or 50% or CIN0.5 ().
Discussion
To our knowledge, this is the first study to describe the clinical characteristics and investigate the association of LVEF with CIN and long-term mortality following CAG/PCI in patients with HF. Our data showed that patients with lower LVEF were more likely to have comorbidities and develop CIN. However, advanced HF was significantly associated with an increased risk of CIN. In addition, age >75 years, emergency PCI and use of IABP were the independent risk factors for CIN. It is noteworthy that reduced LVEF was an independent predictor of long-term mortality following CAG/PCI.
In recent years, the proportion of patients with HFpEF has increased significantly,Citation21 with a prevalence of 71%–74% being reported in large-cohort studies from Western and Asians countries.Citation22–Citation24 Additionally, myocardial ischemia has been demonstrated as the major etiology of HF.Citation25,Citation26 However, the incidence of HFpEF among these patients following CAG/PCI has not been analyzed. As observed in our analysis, the incidence of HFpEF was highest in the study population (72.98%), which was similar to the prior analyses. The high prevalence of HFpEF suggests that it should be given high priority in risk assessment.
Characteristics of HFmrEF were demonstrated to be intermediate between those of HFrEF and HFpEF.Citation27 Similar results were found in the patients with HF following CAG/PCI. Our present study indicated that HFmrEF patients were closer to the HFrEF patients in terms of use of diuretics and IABP and presence of comorbidities, such as advanced HF, renal insufficiency and hypotension, but closer to the HFpEF in terms of use of statins, all of which have been demonstrated as contributing factors for CIN.Citation19,Citation28 Moreover, patients with HFmrEF were more likely to undergo emergency PCI than other groups. Based on those characteristics, the incidence of CIN was highest in this particular population. In recent years, CIN has been reported as the third most common cause of hospital-acquired renal failure.Citation3 Therefore, effective pre-procedural identification of patients at high risk of CIN is vital.
LVEF is the most widely used parameter to evaluate cardiac functions associated with hemodynamic instability, and consequently causes inadequate renal perfusion. However, the association between LVEF and CIN still remains controversial. An observational study by Shacham et alCitation11 included 386 patients undergoing PCI and found that patients with worsened LVEF had significantly higher rate of CIN compared with those with LVEF ≥45% (14.4% vs 5.7%; P=0.02). Moreover, worsened LVEF was an independent predictor of CIN. Similar results were found in another extensive cohort study, and a risk score of CIN was named AGEF, including advanced age, depressed LVEF and reduced eGFR.Citation12,Citation29 However, studies conducted by Kurtul et alCitation13 and Barbieri et alCitation14 showed an opposite effect after adjusting for several confounders. As observed in all the above-mentioned studies, only a small number of patients with HF were included, and consequently, those studies were unable to analyze the association between LVEF and CIN. Furthermore, HF, as an important risk factor of CIN,Citation8,Citation19 was not included in the multivariate analysis. In contrast, our study included sufficient patients with HF and adjusted for the potential confounders to investigate the association of LVEF with CIN following CAG/PCI.
Previous studies indicated that the incidence of CIN in those with segment elevation myocardial infarction after PCI to be ranged from 10% to 20%. The potential factors such as impaired hemodynamic stability, large CM dose and insufficient prophylactic hydration led to higher risk of CIN in this particular group. In addition, inflammatory response and neurohumoral factors were also involved in this progress.Citation30 Therefore, emergency PCI was significantly and independently related to the risk of CIN.Citation31,Citation32 Recently, Duan et alCitation33 developed a simple model for early prediction of CIN, which indicated that emergency PCI was a significant influencing factor in this model. Similarly, emergency PCI increased the risk of CIN in our analysis. Therefore, more prophylactic measures and attention should be paid in this particular population.
The physiopathology of CIN remains poorly understood. Nevertheless, hemodynamic deterioration plays a significant role in the process. Worsened cardiac function contributes to the hemodynamic instability, which reduces effective renal blood flow, consequently trigging renin–angiotensin, activating sympathetic nervous system and increasing inflammatory factors and oxygen radical levels, all of which contribute to the development of CIN.Citation34 Therefore, among the eight variables from a classical risk assessment model for CIN, three (hypotension, advanced HF and use of IABP) are directly reflecting worsened cardiac function.Citation8 In addition, a high NYHA class reflects not only advanced HF but also adverse hemodynamic parametersCitation35 which accelerate the renal hypoperfusion and potentiate CIN. Therefore, it is likely that advanced HF plays an important role in the development of CIN in patients with HF.
Furthermore, previous studies suggested that patients with HFrEF experienced higher mortality compared to those with HFpEF, whereas others have indicated similar outcomes among the groups.Citation36–Citation38 The marked disparity in long-term prognosis may contribute to the different inclusion criteria and various cut-offs of LVEF to define the type of HF. According to the classification of HF from guideline,Citation10 our data demonstrated that HFrEF in patients increased the risk of all-cause mortality. Therefore, early identification of patients at high risk of mortality may assist in directing treatment.
Limitations
There are several limitations in this study. First, this was a prospective, observational and a single-center study. Therefore, the risk of bias cannot be ruled out, although we attempted to adjust for the confounding factors. Therefore, large-scale multicenter clinical trials are needed before these conclusions can be applied elsewhere. Second, variation in measurement times may lead to missed post-procedure peak levels of creatinine and may underestimate the true incidence of contrast-induced acute kidney injury. Third, as the study was limited to patients with HF, we were unable to extend the results to patients without HF. Fourth, the diagnosis of HF was based on the clinical evaluation, which has limited reliability.
Conclusion
Our data indicated that in patients with HF, not worsened LVEF but advanced HF was significantly associated with an increased risk of CIN following CAG/PCI. In addition, the reduced LVEF (HFrEF group) was an independent predictor of long-term mortality. The predictive value of worsened LVEF and advanced HF for CIN and mortality following cardiac catheterization needs to be investigated in patients with HF in large multicenter clinical trials.
Author contributions
KW, NT and YL conceived and designed the study and helped to draft the manuscript. KW, HLL and WJB carried out the database search, and SQC performed the statistical analysis. SMSI revised the manuscript critically. JYC performed the data collection and extraction and arrangement. NT and YL approved the final version of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by Guangdong Provincial Cardiovascular Clinical Medicine Research Fund (grant number: 2009X41, awarded to Yong Liu and Ning Tan), Science and Technology Planning Project of Guangdong Province (PRECOMIN study by Yong Liu in 2011; and study grant number 2008A030201002, awarded to Ji-yan Chen) and Guangdong Cardiovascular Institute. This study was also supported by Progress of Science and Technology Project in Guangdong Province (grant numbers: 2013b031800025, 2016b020215130) and Cardiovascular Research Foundation Project of Chinese Medical Doctor Association (grant number: SCRFCMDA201216).
Disclosure
The authors report no conflicts of interest in this work.
References
- RoffiMPatronoCColletJPManagement of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)Eur Heart J201637326731526320110
- TsaiTTPatelUDChangTIContemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registryJACC Cardiovasc Interv2014711924456715
- AurelioADuranteAContrast-induced nephropathy in percutaneous coronary interventions: pathogenesis, risk factors, outcome, prevention and treatmentCardiology20141281627224557146
- StaculFReducing the risks for contrast-induced nephropathyCardiovasc Intervent Radiol200528Suppl 2S12S1816419278
- ReyesEBHaJWFirdausIHeart failure across Asia: same healthcare burden but differences in organization of careInt J Cardiol201622316316727541646
- RoncoCMcCulloughPAnkerSDAcute Dialysis Quality Initiative (ADQI) consensus groupCardio-renal syndromes: report from the consensus conference of the Acute Dialysis Quality InitiativeEur Heart J201031670371120037146
- RosenstockJLGillesEGellerABImpact of heart failure on the incidence of contrast-induced nephropathy in patients with chronic kidney diseaseInt Urol Nephrol20104241049105420602168
- MehranRAymongEDNikolskyEA simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validationJ Am Coll Cardiol20044471393139915464318
- RihalCSTextorSCGrillDEIncidence and prognostic importance of acute renal failure after percutaneous coronary interventionCirculation2002105192259226412010907
- PonikowskiPVoorsAAAnkerSDAuthors/Task Force Members2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESCEur Heart J201637272129220027206819
- ShachamYLeshem-RubinowEGal-OzAAssociation of left ventricular function and acute kidney injury among ST-elevation myocardial infarction patients treated by primary percutaneous interventionAm J Cardiol2015115329329725476561
- FlintNKaufmanNGal-OzAEchocardiographic correlates of left ventricular filling pressures and acute cardio-renal syndrome in ST segment elevation myocardial infarction patientsClin Res Cardiol2017106212012627550512
- KurtulADuranMYarliogluesMAssociation between N-terminal pro-brain natriuretic peptide levels and contrast-induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndromeClin Cardiol201437848549224805995
- BarbieriLVerdoiaMNardinMMarinoPSuryapranataHDe LucaGNovara Atherosclerosis Study Group (NAS)Gender difference in the risk of contrast-induced nephropathy in patients undergoing coronary angiography or percutaneous coronary interventionAngiology201768654254627662891
- LeveyASBoschJPLewisJBGreeneTRogersNRothDA more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study GroupAnn Intern Med1999130646147010075613
- CourtneyAEMaxwellAPFogartyDGUsing estimated glomerular filtration rate (eGFR) to help manage patients with chronic kidney diseaseUlster Med J200776315415617853643
- MetraMPonikowskiPDicksteinKHeart Failure Association of the European Society of CardiologyAdvanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of CardiologyEur J Heart Fail200796–768469417481947
- KillipT3rdKimballJTTreatment of myocardial infarction in a coronary care unit. A two year experience with 250 patientsAm J Cardiol19672044574646059183
- StaculFvan der MolenAJReimerPContrast Media Safety Committee of European Society of Urogenital Radiology (ESUR)Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelinesEur Radiol201121122527254121866433
- ZengXMcMahonGMBrunelliSMBatesDWWaikarSSIncidence, outcomes, and comparisons across definitions of AKI in hospitalized individualsClin J Am Soc Nephrol201491122024178971
- OwanTEHodgeDOHergesRMJacobsenSJRogerVLRedfieldMMTrends in prevalence and outcome of heart failure with preserved ejection fractionN Engl J Med2006355325125916855265
- HoggKSwedbergKMcMurrayJHeart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosisJ Am Coll Cardiol200443331732715013109
- LamCSDonalEKraigher-KrainerEVasanRSEpidemiology and clinical course of heart failure with preserved ejection fractionEur J Heart Fail2011131182820685685
- KanekoHSuzukiSYajimaJClinical characteristics and long-term clinical outcomes of Japanese heart failure patients with preserved versus reduced left ventricular ejection fraction: a prospective cohort of Shinken Database 2004–2011J Cardiol201362210210923731923
- CowieMRWoodDACoatsAJIncidence and aetiology of heart failure; a population-based studyEur Heart J199920642142810213345
- LamCSTengTKTayWTRegional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registryEur Heart J201637413141315327502121
- LamCSSolomonSDThe middle child in heart failure: heart failure with mid-range ejection fraction (40–50%)Eur J Heart Fail201416101049105525210008
- ZhangMMLvQZLiXYDrug effects and clinical investigations for contrast-induced nephropathy after coronary angiography or percutaneous coronary intervention in patients with diabetesAm J Ther Epub2015824
- AndòGMorabitoGde GregorioCTrioOSaporitoFOretoGAge, glomerular filtration rate, ejection fraction, and the AGEF score predict contrast-induced nephropathy in patients with acute myocardial infarction undergoing primary percutaneous coronary interventionCatheter Cardiovasc Interv201382687888523703775
- GuerchicoffAStoneGWMehranRAnalysis of biomarkers for risk of acute kidney injury after primary angioplasty for acute ST-segment elevation myocardial infarction: results of the HORIZONS-AMI trialCatheter Cardiovasc Interv201585333534225130788
- TanNLiuYZhouYLContrast medium volume to creatinine clearance ratio: a predictor of contrast-induced nephropathy in the first 72 hours following percutaneous coronary interventionCatheter Cardiovasc Interv2012791707521990069
- FuNLiXYangSRisk score for the prediction of contrast-induced nephropathy in elderly patients undergoing percutaneous coronary interventionAngiology201364318819423196639
- DuanCCaoYLiuYA new preprocedure risk score for predicting contrast-induced acute kidney injuryCan J Cardiol201733671472328392272
- AzzaliniLSpagnoliVLyHQContrast-induced nephropathy: from pathophysiology to preventive strategiesCan J Cardiol201632224725526277092
- AhmedAAronowWSFlegJLHigher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular functionAm Heart J2006151244445016442912
- MacDonaldMRWeePPCaoYComparison of characteristics and outcomes of heart failure patients with preserved versus reduced ejection fraction in a multiethnic Southeast Asian cohortAm J Cardiol201611881233123827561195
- LundLHDonalEOgerEKaRen InvestigatorsAssociation between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fractionEur J Heart Fail2014169992100125046483
- ColesAHFisherKDarlingCLong-term survival for patients with acute decompensated heart failure according to ejection fraction findingsAm J Cardiol2014114686286825092194