Abstract
Background
Major abdominal surgery (MAS) is associated with increased morbidity and mortality. The main objective of our study was to evaluate the predictive value of heart-rate variability (HRV) concerning development of postoperative complications in patients undergoing MAS. The secondary objectives were to identify the relationship of HRV and use of vasoactive drugs during anesthesia, intensive care unit length of stay (ICU-LOS), and hospital length of stay (H-LOS).
Patients and methods
Sixty-five patients scheduled for elective MAS were enrolled in a prospective, single-center, observational study. HRV was measured by spectral analysis (SA) preoperatively during orthostatic load. Patients were divided according to cardiac autonomic reactivity (CAR; n=23) and non-cardiac autonomic reactivity (NCAR; n=30).
Results
The final analysis included 53 patients. No significant difference was observed between the two groups regarding type of surgery, use of minimally invasive techniques or epidural catheter, duration of surgery and anesthesia, or the amount of fluid administered intraoperatively. The NCAR group had significantly greater intraoperative blood loss than the CAR group (541.7±541.9 mL vs 269.6±174.3 mL, p<0.05). In the NCAR group, vasoactive drugs were used during anesthesia more frequently (n=21 vs n=4; p<0.001), and more patients had at least one postoperative complication compared to the CAR group (n=19 vs n=4; p<0.01). Furthermore, the NCAR group had more serious complications (Clavien–Dindo ≥ Grade III n=6 vs n=0; p<0.05) and a greater number of complications than the CAR group (n=57 vs n=5; p<0.001). Significant differences were found for two specific subgroups of complications: hypotension requiring vasoactive drugs (NCAR: n=10 vs CAR: n=0; p<0.01) and ileus (NCAR: n=11 vs CAR: n=2; p<0.05). Moreover, significant differences were found in the ICU-LOS (NCAR: 5.7±3.5 days vs CAR: 2.6±0.7 days; p<0.0001) and H-LOS (NCAR: 12.2±5.6 days vs CAR: 7.2±1.7 days; p<0.0001).
Conclusion
Preoperative HRV assessment during orthostatic load is objective and useful for identifying patients with low autonomic physiological reserves and high risk of poor post-operative course.
Introduction
A fundamental feature of a healthy organism is continuous communication between vital organs through the autonomic nervous system (ANS).Citation1 The ANS is composed of the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) and plays a crucial role in maintaining homeostasis in response to internal and external stimuli.Citation2 Surgical trauma leads to tissue injury and activates inflammatory pathways.Citation3 The organism responds with complex reactions, leading to structural and functional repair of injured tissues. However, dysregulation of the inflammatory response may aggravate local tissue damage, leading to shock, multiple organ dysfunction syndrome (MODS), and death.Citation4 Under physiological conditions, the pro-inflammatory response is balanced by an anti-inflammatory response, including the cholinergic anti-inflammatory pathway of the vagus nerve.Citation5 In addition, anesthetics influence cardiovascular homeostasis, and preexisting autonomic dysfunction (AD) can lead to hemodynamic instability during anesthesia.Citation6 Preserved autonomic regulation represents adequate physiological reserves and better reactivity to various insults during the perioperative course.Citation7
Analysis of heart-rate variability (HRV) – the oscillation of intervals between consecutive heart beats – is an accepted noninvasive method of evaluating the autonomic influence on heart activity.Citation8,Citation9 The human organism is a nonlinear biological system characterized by so-called oscillation phenomena. The oscillations originate from a neurally mediated organ interaction. The disturbance in variations of oscillations may show the beginning of, or advanced, deterioration of organ systems. HRV is an objective method for possibly evaluating this phenomena.Citation1,Citation8 In our study, we used spectral analysis of HRV, which is a linear method, that simplified the biosignal of HRV. However, some autonomic challenges, such as orthostatic load or the Valsalva maneuver, can improve the sensitivity of ANS assessment and better reflect the real autonomic physiological reserves of patients.Citation10,Citation11
AD represented by reduced HRV is widely accepted as an independent adverse prognostic factor in various pathological conditions (eg, myocardial infarction, coronary artery disease, congestive heart failure, diabetes mellitus, MODS).Citation12–Citation16 AD may negatively influence the perioperative course due to hemodynamic instability during anesthesia, thus increasing postoperative morbidity and mortality.Citation17 The link between low HRV and worse surgical outcome has been confirmed by several studies.Citation18–Citation27 Nevertheless, routine assessment of HRV by anesthesiologists is still rare.Citation28
The main goal of our study was to investigate the relationship between spectral analysis of HRV during orthostatic load with postsurgical outcome in patients scheduled for elective major abdominal surgery (MAS). The primary endpoint was the occurrence of postoperative complications, according to the Clavien–Dindo classification.Citation29,Citation30 Secondary endpoints were evaluation of HRV as a predictor of the need for vasoactive drugs during anesthesia, intensive care length of stay (ICU-LOS), and hospital length of stay (H-LOS).
Materials and methods
This prospective observational study was approved by the Institutional Ethics Committee of the University Hospital Ostrava (ref no 606/2014) and registered at ClinicalTrials.gov (Identifier NCT02375412). Written informed consent was signed by all enrolled patients. The study was conducted between February 2015 and December 2016 at University Hospital Ostrava, Czech Republic. We enrolled 65 patients scheduled for elective MAS. The exclusion criteria were: emergency or acute surgery, absence of sinus rhythm, American Society of Anesthesiologists (ASA) physical status I, and age <18 years. Standard baseline demographic and clinical data were collected before surgery.
HRV was measured in a quiet, mildly illuminated room, at room temperature 22°C–24°C, between 14:00 and 17:00 the day before the surgery. Short-term spectral analysis of HRV was conducted according to Task Force recommendations using a DiANS PF8 diagnostic system (Olomouc, Czech Republic).Citation31 The R-R intervals on the electrocardiogram were recorded with a sampling rate of 1,000 Hz, and the acquired data were automatically filtered in order to eliminate ectopic beats or artifacts using the recognition algorithm, as verified manually. The intervals were analyzed using modified fast Fourier transformation (FFT). Recordings were obtained in three consecutive positions (supine-1, standing, and supine-2), each lasting 300 seconds. The first supine position (supine-1) was an adaptive one, the active standing position (standing) represents autonomic load, and another supine position (supine-2) is the final position for an overall evaluation of the spectral parameters of HRV.Citation9,Citation32 The final results were displayed in the form of a three-dimensional chart of HRV patterns. This HRV analysis allowed a visual overview of HRV dynamics and improved the evaluation of cardiac autonomic reactivity.
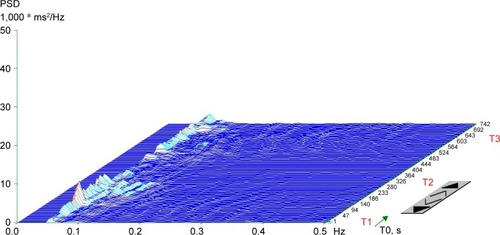
In addition to the visual representation, the following parameters of frequency (spectral) domain were calculated in every position: (1) spectral power of low frequency (LF; 0.05–0.15 Hz), reflecting a combination of sympathetic and parasympathetic effects on cardiac autonomic tone;Citation33 (2) spectral power of high frequency (HF; 0.15–0.4 Hz) corresponding exclusively to parasympathetic tone;Citation34 (3) total spectral power (TP; 0.05–0.4 Hz), representing the main outcome variables in both frequency bands (LF + HF); and (4) the LF to HF ratio (LF/HF), representing the index of sympathovagal interaction.Citation35 Spectral indexes were expressed both in absolute values (ms2) and natural logarithm (ln). For the final evaluation of HRV, we used the TP from the supine-2 position. Patients were divided into two groups: cardiac autonomic reactivity (CAR), characterized by a calculated TP (supine-2) ≥200 ms2 and a chart with peaks of spectral energy in a particular frequency band (), and non-cardiac autonomic reactivity (NCAR), characterized by TP <200 ms2 and a plain chart due to a reduction in overall spectral power ().
Figure 1 Typical three-dimensional chart of a spectral analysis of heart-rate variability during orthostatic load in the cardiac autonomic reactivity group.
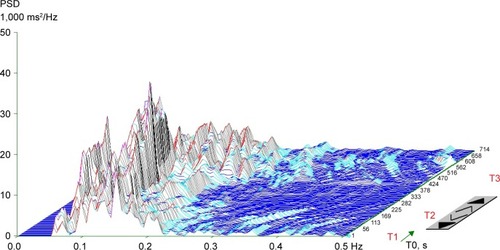
Figure 2 Typical plain three-dimensional chart of a spectral analysis of heart-rate variability during orthostatic load in the non-cardiac autonomic reactivity group.
Anesthetic and surgical techniques were carried out in a standardized manner. Surgical procedures were undertaken under general anesthesia (GA) or a combination of general and epidural anesthesia. GA was induced by the administration of sufentanil, propofol, and cis-atracurium. Sevoflurane in an oxygen/air mixture was added to maintain balanced anesthesia. Mechanical ventilation was standardized (6–8 mL/kg predicted body weight, PEEP 5–7 cm H2O). Anesthesia management (ie, use of balanced crystalloids, blood products, and vasoactive drugs) was left to the discretion of the anesthesiologist. Intraoperative data such as the duration of the procedure, fluid intake, output, and the use of vasoactive drugs were recorded. All patients were transferred to the ICU after the surgical procedure.
Complications were defined as any postoperative process that was not part of normal recovery during the postoperative period. These complications were classified according to the Clavien–Dindo classification. Grades I and II are defined as any deviation from the normal postoperative course without need for surgical, endoscopic, or radiological intervention (Grade I), with Grade II including a need for pharmacological treatment; Grade III requires a surgical, endoscopic, or radiological intervention without (Grade IIIa) or with GA (Grade IIIb); Grade IV includes single-organ failure requiring ICU care (Grade IVa) or multiple organ failure (Grade IVb); and Grade V is characterized by the patient’s death.Citation29 We recorded the most severe grade of complications for each patient according to recommendations.Citation30 Moreover, the complications were divided into two subgroups: minor (Grade I and Grade II) and major (Grade ≥ III). All complications encountered in our study were recorded.
Statistical analysis
For numerical data, the arithmetic mean ± standard deviation (SD) was computed. Categorical data were summarized by absolute count and relative frequency (%). Differences between the CAR and NCAR were assessed using the t-test (numerical data) or Fisher exact test (categorical data). All statistical analyses, figures, and tables were created by a certified statistician (co-author MB) using R software (version 3.3.3). The level of significance was defined as p≤0.05.
Results
Sixty-five patients were screened for the study. We excluded 12 patients from further analysis due to cancellation of the operative procedures (n=2), technically inadequate recordings (artifacts or excessive ectopic beats, n=7), and missing data during the postoperative period (n=3). Therefore, the final study population comprised 53 patients; see the CONSORT flow diagram (). No significant differences were found between groups with regard to demographic characteristics. We found some significant differences in medical history between the CAR and NCAR (myocardial infarction: n=0 vs n=6, p<0.05; diabetes mellitus: n=1 vs n=11, p<0.01; and hyperlipidemia: n=4 vs n=18, p<0.01, respectively). These differences in comorbidities were reflected by differences in chronic medications between the CAR and NCAR (per oral antidiabetics: n=0 vs n=7, p<0.05; statins: n=3 vs n=14, p<0.05, respectively). We also found that a significantly greater proportion of patients in the CAR had ASA II than the NCAR (78% vs 47%, p<0.05; ).
Figure 3 CONSORT flow diagram.
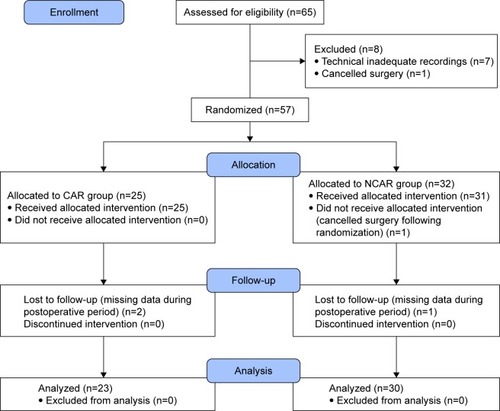
Table 1 Patient characteristics
Cardiac autonomic reactivity during orthostatic load was observed in 23 patients (CAR) and a reduced autonomic response was observed in 30 patients (NCAR). Significant differences were found between study groups in all calculated spectral power indices of HRV expressed in absolute values (ms2) and natural logarithm (LN) values in all three measurement positions during orthostatic load (p<0.0001). The LF/HF ratio was significantly different in only supine-1 (p<0.05; ).
Table 2 Intergroup comparison of parameters in a frequency domain analysis of heart-rate variability during orthostatic load (supine-1-standing-supine-2)
With regarding intraoperative characteristics, no difference was observed between groups for the type of surgery, use of minimally invasive techniques, use of an epidural catheter, amount of intraoperative fluids, and duration of surgery and anesthesia. However, we found significant differences in blood loss (269.6±174.3 mL vs 541.7±541.9 mL, p<0.05) and the use of at least one vasoactive drug (n=4 vs n=21, p<0.001, ) between the CAR and NCAR, respectively.
Table 3 Intraoperative characteristics
The number of patients with at least one complication was significantly lower in the CAR than the NCAR (n=4 vs n=19, p=0.001) group. In addition, significant differences were found in Clavien–Dindo classification Grade II (CAR, n=2 vs NCAR, n=12, p<0.05) and Grade ≥ III (CAR, n=0 vs NCAR, n=6; p<0.05; ). Analyzing the frequency of particular types of complications, we found a significant difference in the occurrence of hypotension requiring vasoactive drugs (CAR, n=0 vs NCAR, n=10, p<0.05) as well as ileus (CAR, n=2 vs NCAR, n=11, p<0.05). Finally, the overall number of complications was significantly lower in the CAR than the NCAR group (n= 5 vs n= 57, p<0.001, ).
Table 4 Postoperative complications according to the Clavien–Dindo classification and length of stay
Table 5 Complications encountered in the study
Concerning outcomes, ICU-LOS and H-LOS were significantly lower in the CAR than the NCAR group (2.6±0.7 days vs 5.7±3.5 days [p<0.0001] and 7.2±1.7 days vs 12.2±5.6 [p<0.0001], respectively; ).
Discussion
Our study used a dynamic autonomic test (orthostatic load) for preoperative assessment of HRV to identify patients with reduced autonomic physiological reserves undergoing elective MAS. Our findings clearly show a higher occurrence of postoperative complications, higher hemodynamic instability during anesthesia, and prolonged ICU-LOS and H-LOS in patients with low autonomic reserves (NCAR). Therefore, we confirmed that patients with AD are at risk of an adverse intraoperative and postoperative course.
The NCAR patients had a plain spectral analysis for HRV without reactivity during orthostatic load, and all of their calculated parameters were reduced. The reduction in HRV parameters was similar to the identification of diabetic cardiovascular autonomic neuropathy (CAN).Citation36 CAN is an accepted risk factor for intraoperative hemodynamic instability and increased occurrence of postoperative cardiovascular events, including sudden death.Citation37,Citation38 CAN is a complication not only of diabetes mellitus, but also of other comorbidities, including chronic coronary disease, chronic heart failure, hypertension, and chronic obstructive pulmonary disease. Neurological and psychiatric disorders have some degree of AD.Citation6–Citation8,Citation12–Citation17 In our study, the NCAR group comprised patients with more chronic comorbidities than in the CAR group. We found that AD reflected both the number and severity of illnesses in the NCAR group. These findings were confirmed by an assessment of ASA; the NCAR group comprised more patients with ASA III and IV than the CAR group, which comprised predominantly ASA II patients.
During anesthesia, the NCAR group had a significantly greater need for vasoactive drugs to adequately maintain blood pressure. NCAR and CAR were similar in regard to intraoperative characteristics (ie, amount of administered fluids, type of surgery, use of minimally invasive technique, and duration of surgery and anesthesia). However, NCAR patients had greater blood loss, although it was not significant enough to necessitate the use of vasoactive drugs. AD decreases the cardiovascular compensatory response to the administration of anesthetics and surgical stress, facilitating hemodynamic instability. This finding is consistent with studies considering low HRV as a sensitive method for identifying blood pressure instability during GA.Citation39,Citation40 Huang et al reported that low TP may serve as an independent predictor of hypotension during GA, showing a significantly greater incidence of hypotension during GA in patients with diabetes mellitus. Interestingly, only a few of these patients had a diagnosis of CAN based on traditional autonomic tests (expiration:inspiration ratio, the Valsalva ratio, and standing 30:15 ratio) and suggested that spectral analysis of HRV is a sensitive method for detecting hemodynamic instability during GA.Citation39 Hanss et al confirmed that low TP has high sensitivity and specificity for predicting hypotension as well as bradycardia after the induction of GA in high-risk cardiovascular patients undergoing elective vascular or abdominal surgery. Moreover, they found a TP density cut-off value of <500 ms2 Hz−1 for the prediction hemodynamic instability,Citation40 but this value means extremely reduced HRV and is rarely used. Importantly, HRV is a useful method for identifying early stages of AD which cannot be diagnosed by traditional autonomic tests and when clinical symptoms of AD (ie, tachycardia at rest, orthostatic hypotension, constipation, neurological bladder, heat intolerance, etc.) may not be evident during routine preoperative assessment.Citation38
We observed a significantly higher occurrence of post-operative complications in NCAR patients, including complications related to autonomic regulation (hypotension requiring vasoactive drugs and ileus). Increased use of vasoactive drugs in NCAR patients during and after GA confirmed one of the main problems of AD – an inability to maintain blood pressure during the perioperative period. The crucial mechanism for maintaining blood pressure stability is baroreflex. It is a feedback system comprising sensors (the baroreceptors), a processing unit (the central nervous system), and an output unit (autonomic nervous fibers). Baroreflex adjusts heart rate via both the vagal and sympathetic action, thus minimize short-term fluctuations in pressure. Baroreflex is involved in the modulation of spectral components (LF and HF) of HRV. Impaired baroreflex (measurement by baroreflex sensitivity – BRS) is closely associated with low HRV.Citation8,Citation41 Both decreased HRV and depressed BRS were confirmed as independent predictors of postoperative ischemia, myocardial infarction, cardiac arrest, and overall morbidity.Citation20,Citation21,Citation25–Citation27,Citation42 Various measurements to evaluate ANS (triangular index, fractal scaling exponent α1, BRS) were used, but all studies confirmed the significant influence of ANS on cardiovascular homeostasis.
Postoperative ileus increases postoperative morbidity and is a cause of prolonged hospital stay after MAS. The complex pathophysiology of postoperative ileus involves several factors, including the degree of surgical trauma. However, AD is also an important factor in its development.Citation43 In our study, NCAR patients had extremely reduced HF power (dominantly parasympathetic vagal activity) and LF power (sympathetic and parasympathetic influence). The reduced parasympathetic and sympathetic parts of the ANS represent serious AD, the main reason for impaired gastro-intestinal motility in NCAR.
We found significantly longer ICU-LOS and H-LOS in NCAR patients. These results are related to higher grade complications according to the Clavien–Dindo classification (grade ≥ III) in NCAR – that is, the number of reoperations and organ failures. This finding is consistent with another study in which decreased HRV was found to be a predictor of prolonged ICU stay in patients after coronary artery bypass grafting (CABG) surgery and abdominal aortic surgery.Citation18,Citation19,Citation24,Citation25 However, variable parameters used for HRV assessment and time of own measurement (fractal scaling exponent α1, very low frequency [VLF] measurement during 24 h, preoperative and postoperative measurements) were also used in these studies.
HRV was identified as a powerful predictor of long-term mortality, with LF/HF ratio <2 and postoperative elevation of serum troponin I levels being independent predictors of 1-year mortality in patients undergoing major non-cardiac surgery.Citation22 The same authors investigated 2-year mortality and found that LF/HF ratio <2 remained an independent predictor of mortality together with a history of heart congestion and age >70 years.Citation23 These findings suggest that HRV measurement reflects physiological reserves necessary for recovery after hospital discharge. We evaluated LF/HF in the present study, but our values were different and we did not find significant differences in the LF/HF ratio between our study groups (except the supine-1 adaptive position). These findings may be due to different measurement methods. Our LF/HF ratios were calculated from measurements made during orthostatic load, which influenced the particular frequency band depending on the specific position. Moreover, our measurements were performed the day before surgery. Filipovic et al used LF/HF from a supine position immediately before induction of GA. Measurement in only one static position and psychological stress before surgery could have significantly influenced the LF and HF values.Citation22,Citation23
In clinical research of HRV, various methods of analysis (time-domain analysis, geometric analysis, frequency-domain analysis, and entropy or fractal analysis), indexes, and units (absolute, logarithmic, standard units) have been used. Moreover, differences are present in the time and duration of measurements. No normative HRV values are currently, generally, accepted.Citation44,Citation45 As HRV is a very sensitive biosignal, high interindividual (age, gender, psychological stress during measurement, etc.) and intraindividual (circadian rhythm, food intake, smoking, etc.) variability can be obtained.Citation46–Citation48 Most chronic diseases manifest some degree of autonomic dysfunction.Citation8,Citation12–Citation15 In addition, HRV measurements are influenced by the use of chronic medication.Citation6–Citation8,Citation17 The use of some autonomic challenge (ie, the Valsalva maneuver or orthostatic load) can improve HRV measurement and better reflect the real degree of autonomic physiological reserves, in contrast to measurements under static conditions.Citation10,Citation11 In our study, we used orthostatic load which can help us identify patients without autonomic reactivity (NCAR) among elderly patients with comorbidities scheduled for MAS. NCAR was characterized by a plain chart of HRV patterns without reactivity in particular positions and low TP in the supine-2 position (TP <200 ms2). We used TP because it represents overall HRV, and we used TP from the supine-2 position because it showed the real status of autonomic regulation, especially due to uncovered vagal activity, which can be suppressed by psychological stress during evaluation in the supine-1 position. Moreover, vagal activity is reflexively augmented after laying down from standing to the supine-2 position. As a global parameter of HRV in the supine-2 position, TP can be considered acceptable for quick determination of the real autonomic physiological reserves of patients.
This study has a few possible limitations: the small sample size and its being performed at a single center.
Conclusion
Preoperative assessment of autonomic physiological reserves based on HRV is important for the early identification of patients at high risk for the development of intraoperative and postoperative complications. This has the potential to influence perioperative clinical decision-making and eventually improve outcome. We suggest that standardized measurement of HRV during autonomic challenge be performed by anesthesiologists as routine perioperative practice. However, further research in the field is needed.
Acknowledgments
The authors thank Dr Rudolf Metelka and Professor Jaroslav Opavský from University Hospital Olomouc for advice on HRV measurement.
Michal Burda acknowledges partial support by the NPU II project LQ1602 “IT4Innovations excellence in science” provided by the Ministry of Education Youth and Sport, Czech Republic.
Disclosure
The authors report no conflicts of interest in this work.
References
- GodinPJBuchmanTGUncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndromeCrit Care Med1996247110711168674321
- KenneyMJGantaCKAutonomic nervous system and immune system interactionsCompr Physiol2014431177120024944034
- MácaJBuršaFŠevčíkPSklienkaPBurdaMHolubMAlarmins and clinical outcomes after major abdominal surgery – a prospective studyJ Invest Surg201730315216127689623
- PavlovVAWangHCzuraCJFriedmanSGTraceyKJThe cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulationMol Med200395–812513414571320
- TraceyKJThe inflammatory reflexNature2002420691785385912490958
- MustafaHIFesselJPBarwiseJDysautonomia: perioperative implicationsAnesthesiology2012116120521522143168
- McGraneSAtriaNPBarwiseJAPerioperative implications of the patient with autonomic dysfunctionCurr Opin Anaesthesiol201427336537024722004
- ErnstGHeart Rate Variability1st edLondonSpringer2014
- MetelkaRHeart rate variability – current diagnosis of the cardiac autonomic neuropathy. A reviewBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2014158332733825004914
- HoworkaKPumprlaJJirkovskaALacigovaSNolanJModified orthostatic load for spectral analysis of short-term heart rate variability improves the sensitivity of autonomic dysfunction assessmentJ Diabetes Complications2010241485419062311
- DeschampsADenaultARochonACoganJPagéPD’AntonoBEvaluation of autonomic reserves in cardiac surgery patientsJ Cardiothorac Vasc Anesth201327348549323036623
- KleigerREMillerJPBiggerJTJrMossAJDecreased heart rate variability and its association with increased mortality after acute myocardial infarctionAm J Cardiol19875942562623812275
- MaierPToepferMDambacherMTheisenKRoskammHFreyAWHeart rate variability and its relation to ventricular tachycardia in patients with coronary artery diseaseClin Sci (Lond)199691678813831
- PiepoliMCoatsAJAutonomic abnormality in chronic heart failure evaluated by heart rate variabilityClin Sci (Lond)19969184868813837
- VinikAIZieglerDDiabetic cardiovascular autonomic neuropathyCirculation2007115338739717242296
- SchmidtHHoyerDWilhelmJThe alteration of autonomic function in multiple organ dysfunction syndromeCrit Care Clin200824114916318241783
- MazzeoATLa MonacaEDi LeoRVitaGSantamariaLBHeart rate variability: a diagnostic and prognostic tool in anesthesia and intensive careActa Anaesthesiol Scand201155779781121658013
- LaitioTTHuikuriHVKentalaESCorrelation properties and complexity of perioperative RR-interval dynamics in coronary artery bypass surgery patientsAnesthesiology2000931698010861148
- SteinPKSchmiegREJrEl-FoulyADomitrovichPPBuchmanTGAssociation between heart rate variability recorded day 1 and length of stay in abdominal aortic surgery patientsCrit Care Med20012991738174311546974
- MamodeNDochertyGLoweGDThe role of myocardial perfusion scanning, heart rate variability and D-dimers in predicting the risk of perioperative cardiac complications after peripheral vascular surgeryEur J Vasc Endovasc Surg200122649950811735198
- LaitioTTHuikuriHVMakikallioTHThe breakdown of fractal heart rate dynamics predicts prolonged postoperative myocardial ischemiaAnesth Analg20049851239124415105194
- FilipovicMJegerRProbstCHeart rate variability and cardiac troponin I are incremental and independent predictor of one-year all-cause mortality after major noncardiac surgery in patients at risk of coronary artery diseaseJ Am Coll Cardiol200342101767177614642686
- FilipovicMJegerRVGirardTPredictors of long-term mortality and cardiac events in patients with known or suspected coronary artery disease who survive major non-cardiac surgeryAnaesthesia200560151115601265
- WuZKVikmanSLaurikkaJNonlinear heart rate variability in CABG patients and the preconditioning effectEur J Cardiothorac Surg200528110911315982594
- LaitioTJalonenJKuuselaTScheininHThe role of heart rate variability in risk stratification for adverse postoperative cardiac eventsAnesth Analg200710561548156018042846
- UshiyamaTNakatsuTYamaneSHeart rate variability for evaluating surgical stress and development of postoperative complicationsClin Exp Hypertens2008301455518214733
- SchefflerPMuccioSEgizianoGHeart rate variability exhibits complication-dependent changes postsurgeryAngiology201364859760323091271
- ReimerPAdamusMSklienkaPŠevčíkPPreoperative examination of the autonomic nervous system by measurement of heart rate variability for prediction of the periopeartive courseAnest Intenziv Med2015263137144
- DindoDDemartinesNClavienPAClassification of surgical complications: a new proposal with evaluation in cohort of 6336 patients and results of a surveyAnn Surg2004240220521315273542
- ClavienPABarkunJde OliveiraMLThe Clavien-Dindo classification of surgical complications: five-year experienceAnn Surg2009250218719619638912
- Task Force of the European Society of Cardiology and the North American Society of Pacing and ElectrophysiologyHeart rate variability. standard of measurement, physiological interpretation, and clinical useEur Heart J19961733543818737210
- PumprlaJHoworkaKGrovesDChesterMNolanJFunctional assessment of heart rate variability: physiological basis and practical applicationsInt J Cardiol200284111412104056
- EckbergDLSympathovagal balance: a critical appraisalCirculation1997969322432329386196
- HayanoJSakakibaraYYamadaAAccuracy of assessment of cardiac vagal tone by heart rate variability in normal subjectsAm J Cardiol19916721992041987723
- MallianiAPaganiMLombardiFPhysiology and clinical implications of variability of cardiovascular parameters with focus on heart rate and blood pressureAm J Cardiol199473103C9C
- HoworkaKPumprlaJSchabmannAOptimal parameters of short-term heart rate spectrogram for routine evaluation of diabetic cardiovascular autonomic neuropathyJ Auton Nerv Syst1998692–31641729696273
- BurgosLGEbertTJAsiddaoCIncreased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathyAnesthesiology19897045915972929996
- OakleyIEmondLDiabetic cardiac autonomic neuropathy and anesthetic management: review of the literatureAANA J201179647347922400413
- HuangCJKuokCHKuoTBHsuYWTsaiPSPre-operative measurement of heart rate variability predicts hypotension during general anesthesiaActa Anaesthesiol Scand200650554254816643221
- HanssRRennerJIliesCDoes heart rate variability predict hypotension and bradycardia after induction of general anaesthesia in high risk cardiovascular patients?Anaesthesia200863212913518211442
- WehrweinEAJoynerMJRegulation of blood pressure by the arterial baroreflex and autonomic nervous systemHandb Clin Neurol20131178910224095118
- TonerAJenkinsNAcklandGLPOM-O Study InvestigatorsBaroreflex impairment and morbidity after major surgeryBr J Anaesth2016117332433127543527
- HolteKKehletHPostoperative ileus: a preventable eventBr J Surg200087111480149311091234
- SandercockGNormative values, reliability and sample size estimates in heart rate variabilityClin Sci (Lond)2007113312913017451377
- NunanDSandercockGRBrodieDAA quantitative systematic review of normal values for short-term heart rate variability in healthy adultsPacing Clin Electrophysiol201033111407141720663071
- SteinPKKleigerRERottmanJNDiffering effects of age on heart rate variability in man and womanAm J Cardiol19978033023059264423
- DelaneyJPBrodieDAEffects of short-term psychological stress on the time and frequency domains of heart rate variabilityPercept Mot Skills200091251552411065312
- BonnemeierHRichardtGPotratzJCircadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variabilityJ Cardiovasc Electrophysiol200314879179912890036
