Abstract
Adoptive T-cell immunotherapy is a rapidly growing field and is shifting the paradigm of clinical cancer treatment. Axicabtagene ciloleucel (axi-cel) is an anti-CD19 chimeric antigen receptor T-cell therapy that was initially developed at the National Cancer Institute and has recently been commercially approved by the US Food and Drug Administration for relapsed or refractory aggressive non-Hodgkin’s lymphomas including diffuse large B-cell lymphoma and its variants. The ZUMA-1 Phase I and II clinical trials formed the basis of the US Food and Drug Administration approval of this product, and we discuss the particulars of the clinical trials and the pharmacology of axi-cel. In addition, we review the CD19 chimeric antigen receptor T-specific toxicities of cytokine release syndrome and neurotoxicity, which remain the challenges to the safe delivery of this important therapy for aggressive B-cell lymphomas with poor prognosis.
Epidemiology of aggressive B-cell lymphomas
Aggressive B-cell lymphoma is a subtype of B-cell non-Hodgkin’s lymphomas (NHLs) that encompasses a clinically and molecularly heterogeneous group. In this group, diffuse large B-cell lymphoma (DLBCL) is the most common with ~22,000 cases a year and it represents 30%–35% of all lymphomas.Citation1 Within DLBCL itself, there is additional heterogeneity and this is reflected in the World Health Organization 2016 classification that further subtypes of DLBCL to include high-risk histologies such as high-grade B-cell lymphomas not otherwise specified (NOS) and high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements, known as double (DHL) or triple hit lymphomas (THL).Citation2 Primary mediastinal B-cell lymphoma (PMBCL) and transformed follicular lymphoma (TFL) are less frequent, but important variant subtypes that are also described.
Clinical indicators of prognosis in DLBCL
The prognosis of aggressive B-cell lymphomas is based on clinical, molecular and genetic factors as well as responsiveness to induction chemotherapy. The International Prognostic Index (IPI), based on clinical features found at initial diagnosis (age, stage, extranodal disease, performance status and lactate dehydrogenase elevation) is historically the most widely used and is a powerful prognostic system.Citation3 The revised version of the IPI score extended the predictive value of the IPI into the rituximab era (ie, when the anti-CD20 rituximab became widely added to chemotherapy due to overall survival [OS] benefits).Citation4 An enhanced scoring system, the National Comprehensive Cancer Network-International Pronostic Index (NCCN-IPI) score, stratifies newly diagnosed DLBCL patients in four risk groups based on the same prognostic factors used in the IPI score, but with better discrimination of the low- and high-risk subgroups. Patients in the high-risk subgroup category per the revised version of the IPI score and the NCCN-IPI score have a 5-year OS of 54% and 33%, respectively.Citation5,Citation6 Overall, the prognostic scores highlight that a proportion of patients do not have successful outcomes with standard DLBCL treatment.
Molecular and genetic factors predictive of poor prognosis in DLBCL
Molecular profiling studies and next-generation sequencing have improved the understanding of DLBCL and its heterogeneity, and may prospectively identify cases with high-risk features.Citation7,Citation8 Based on the cell of origin using gene expression profiling studies, DLBCL can be classified as activated B-cell (ABC) subtype and germinal center B-cell (GCB) subtype (with 10% of cases being unclassifiable). ABC and GCB have distinct phenotypes and clinical characteristics, and with upfront chemotherapy, the ABC–DLBCL subtype has inferior outcomes after standard R-CHOP.Citation9–Citation11 Widespread use of gene expression profiling or next-generation sequencing remains limited due to cost and complexity, while immunohistochemistry methods are more practical for most pathology laboratories. By immunohistochemistry, the Hans classifier uses three markers (CD10, BCL6 and MUM1/IRF4) and classifies DLBCL as GCB or non-GCB, and seems to be a useful tool to differentiate DLBCL by the cell of origin.Citation12,Citation13 That said, RNA-based diagnostic testing to discriminate ABC and GCB subtypes appears to have higher accuracy, and recent advances allow testing in standard formalin-fixed paraffin-embedded biopsies rather than requiring fresh or frozen material.Citation14
Another high-risk subgroup of DLBCL patients is char-acterized by the chromosomal translocation of the proto-oncogene c-MYC that promotes uncontrolled cell growth, unregulated cell division and extranodal dissemination of lymphomas.Citation15 IgH-MYC rearrangements detected by fluorescent in situ hybridization or cytogenetics can be present in 3%–17% of all DLBCL cases and have particularly poor outcomes when coupled with BCL2 and/or BCL6 rearrangements (DHL or THL).Citation16–Citation18 Clinically, DHL and THL are char-acterized by advanced disease stage, highly elevated lactate dehydrogenase and central nervous system involvement.Citation19,Citation20 The optimal treatment of DHL has not been well defined, but it appears that frontline intensive regimens (such as dose-adjusted EPOCH-R with central nervous system prophylaxis) are associated with better outcomes.Citation20–Citation22 Given its poor outcomes with standard chemotherapy, DLBCL patients with c-MYC rearrangements represent another unmet need.
Response to therapy as a prognostic indicator
The addition of rituximab to CHOP improved the OS of patients with DLBCL; however, despite this advancement, ~30%–40% of patients relapse or are refractory to this regimen.Citation23 In general, patients with refractory disease (less than partial response [PR] to initial treatment) or early relapse (relapse within a year from diagnosis or 6 months after the end of treatment) or progression within 2 years (event free survival 24) have poorer prognosis than those with late relapses.Citation24,Citation25 The current standard for relapsed DLBCL consists of salvage chemotherapy followed by autologous stem cell transplantation (ASCT) with a proportion of patients achieving cure.Citation24,Citation26 Approximately 50% of these patients who completed ASCT will ultimately relapse with minimal hope of cure despite international efforts at clinical trials and drug development in DLBCL.Citation24,Citation27,Citation28 Indeed, the prognosis of relapsed DLBCL after ASCT relapse is poor with a median OS of 10 months, and is particularly worse for those relapsing within 6 months of transplantation with a median OS of 5.7 months.Citation27,Citation29
A retrospective international multicenter study (SCHOLAR-1) was carried out using data from Phase III randomized clinical trials (the European trial CORAL and the Canadian Clinical Trials Group LY.12 trial) and two observational studies (MD Anderson Cancer Center and University of Iowa/Mayo Clinic) that evaluated patients who achieved stable disease (SD) or progressive disease as the best response at any point during chemotherapy, or if relapse occurred within 12 months of ASCT. The results of this pooled analysis showed an overall response rate of 26% with a median OS of 6.3 months.Citation30 Another large retrospective study of 15 US academic institutions assessed the risk factors of refractory DLBCL. Three subgroups were identified to have poor outcomes: primary progression (progressive disease with or within 6 months of chemoimmunotherapy [CIT]), residual disease (PR or SD after completion of CIT) and early relapse (relapse within 6 months of CIT and having achieved a complete response [CR]), with a 2-year OS of 18.5%, 30.6% and 45.5%, respectively.Citation31 Multivariate analysis demonstrated that ultra-high risk (UHR) features in this cohort were primary progression, the presence of MYC rearrangement and intermediate-high or high NCCN-IPI score at the time of progression. The 2-year OS was very poor at 13.9% if any of these UHR factors were present (versus 57.6% in those without UHR features).Citation31 These poor outcomes represent a significant unmet need in which novel approaches are urgently needed. It is in the landscape of relapsed/refractory and UHR DLBCL that CD19 chimeric antigen receptor (CAR) T cells have been studied to date.
Pharmacology and mode of action of axicabtagene ciloleucel (axi-cel)
One mechanism by which the immune system is able to recognize and fight cancer cells is through activation of T-lymphocytes that use T-cell receptors to recognize tumor peptides presented on major histocompatibility complexes.Citation32 Once the T cells are activated, proliferation of T cells and cytotoxic granule secretion ensues, which leads to antitumor activity and lysis of tumor cells. However, tumors perform immune evasion by several mechanisms including increased expression of immune checkpoints (that limit T-cell activation and cause T-cell exhaustion) and effects of the tumor microenvironment. The design of CAR T cells is intended to enhance T-cell responses against tumor cells. The CARs are composed of an antibody-derived single-chain variable fragment linked to CD28 and CD3 zeta signaling domains. The combination of the specificity of a monoclonal antibody with the activation domain of T cells allows the CARs to deliver activated T cells with potent cytotoxicity against tumor-specific antigens and to target tumors independently of the major histocompatibility complex. Overall, CAR T engagement of CD19-expressing cancer cells results in T-cell activation, proliferation and secretion of inflammatory cytokines and chemokines resulting in tumor cell lysis.Citation33,Citation34
First-generation CARs utilized a signaling domain composed of CD3 zeta only and demonstrated weak proliferation ability and short survival, with short-lived antitumor activity. Co-stimulatory molecules, such as CD28, were introduced in second-generation CARs, and they proved to significantly improve signaling strength, expansion and persistence of CAR T cells.Citation35 There are other co-stimulatory domains that are effective in improving T-cell signaling; however, CD28 and 4-1BB are currently the most widely used in CAR T-cell clinical trials. The optimal co-stimulatory domain is currently unknown. In general, the CD28-based CAR T-cell construct exhibits a greater peak expansion, whereas CAR T cells using 4-1BB co-stimulation show greater longevity (persistence).Citation36 Further data are needed to confirm these findings and to find out whether the co-stimulatory domain used impacts clinical outcomes.
CD19 is a transmembrane glycoprotein that is expressed at all stages of differentiation of normal B cells. As a target, CD19 is expressed in over 95% of B-cell malignancies including chronic lymphocytic leukemia, B-cell NHL and acute lymphoblastic leukemia. Thus, CD19 is an attractive target for immunotherapeutic approaches with several companies and academic institutions developing pivotal trials with anti-CD19 CAR T cells.Citation37,Citation38
The development of axi-cel began with preclinical and clinical work at the National Cancer Institute (NCI). Initial studies demonstrated the proof of principle that anti-CD19 CAR T cells derived from human T cells can eradicate CD19-expressing malignant B cells, co-stimulation is required for CAR T-cell persistence and efficacy and finally CD19 CAR T cells can be clinically useful.Citation34,Citation39 The NCI CD19 CAR T cells were then developed by Kite Pharma (now owned by Gilead), leading to axi-cel, an US Food and Drug Administration-approved CD19-directed cellular immunotherapy of autologous T cells genetically engineered to express CARs.Citation39
Pharmacokinetics
As a cytoreductive agent targeting rapidly dividing cells, chemotherapy has the greatest impact immediately following initiation of therapy. The decrease in tumor burden is initially dramatic, but short-lived, resulting in a return to the pretreatment growth rate and necessitating multiple cycles of chemotherapy to provide a long-term antineoplastic effect.Citation40
Axi-cel offers a unique mechanism of action and pharmacokinetic profile in the treatment of NHL. In contrast to cytoreductive therapy, the decrease in tumor burden is not as rapid, but sustained after a single dose infusion. Although CAR T cells have the potential to cause serious toxicities such as cytokine release syndrome and neurotoxicity, the targeted action of axi-cel against CD19 limits additional adverse effects related to the damage of healthy cells to on-target off-tumor effects. Due to the expression of CD19 on all nonmalignant B-cell lymphocytes, on-target prolonged suppression of B cells (B-cell aplasia) is one of the complications of CD19 CAR T-cell therapy.Citation41
Axi-cel is a single-dose infusion containing a suspension of 2×106 CAR-positive viable T cells per kilogram body weight (maximum of 2×108 CAR-positive viable T cells) iñ68 mL.Citation42 Following administration, axi-cel exhibits a rapid expansion, with peak CAR T-cell levels occurring within 7–14 days. In the pivotal Phase II trial, ZUMA-1, of axi-cel in large B-cell lymphoma, CAR T cells remained detectable in most patients at 180 days. The median CAR T area under the curve (AUC), defined as the cumulative level of CAR-positive cells/µL of blood over the first 28 days following axi-cel, was 462.3 cells/µL (range 5.1–14,329.3). Notably, CAR T expansion and peak cellular concentration within 28 days of infusion demonstrated a positive correlation with objective clinical response. The CAR T AUC was 5.4 times higher in patients with an objective response rate (ORR) versus patients without a response. Of the patients obtaining a CAR T AUC exceeding the median AUC, the ORR was 96%.Citation43,Citation44 These results further emphasize the importance of CAR T expansion post-infusion, as observed in previous studies.Citation45,Citation46 Peak expansion was also associated with grade 3 or higher neurologic events, but was not correlated with cytokine release syndromeCitation43 ().
Figure 1 Comparative serum concentrations of chemotherapy and CAR T-cell expansion.
Abbreviations: CAR, chimeric antigen receptor; LOD, limit of detection.
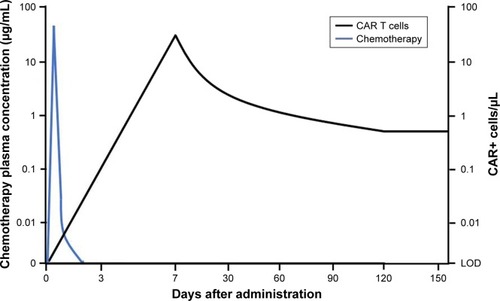
In contrast to cell expansion, the significance of cell persistence is unclear. CAR T-cell persistence and B-cell aplasia have been reported with axi-cel.Citation47,Citation48 In ZUMA-1, three patients with an ongoing complete remission at 24 months still had detectable blood levels of CAR T cells.Citation43 However, recent data from earlier studies of NCI showed that long-term complete remissions are documented with limited persistence and recovery of normal B cells.Citation48 This is in contrast to patients with acute lymphoblastic leukemia in whom recovery of normal B cells may be associated with poor prognosis.Citation49,Citation50 Long-term persistence allows for continued disease assessment, however, at the cost of long-term B-cell aplasia.Citation51 The effect of CAR T-cell persistence is unknown with limited and conflicting data; determining the role and impact on disease response is an area of ongoing research.
Manufacturing
The general manufacturing processes of CAR T cells consist of T-cell collection (harvesting), T-cell selection (through magnetic beads) and activation (with an anti-CD3 antibody in the presence of interleukin [IL]-2), introduction of the CAR gene into the activated T cell through a vector (lentivirus or retrovirus), CAR T-cell expansion and CAR T-cell product formulation.Citation52,Citation53 In order to streamline the process and improve the support for large global trials (or even widespread clinical use), some key steps were taken for the manufacturing of axi-cel, such as removal of human serum from the culture media, thus reducing the infection risk, and excluding the use of magnetic beads for T-cell selection. In addition, there was a decrease in the duration of ex vivo expansion, thus minimizing the risk of T-cell exhaustion.Citation52,Citation54 This process led to the production of predominantly CD3+ T cells with CD8+ T cells in 57% and CD4+ T cells in 43%, with the majority being effector T cells and central memory T cells (TCM).Citation54 The process was highly successful and the expected time of axi-cel production was <2 weeks.Citation43,Citation54
Efficacy of axi-cel
Lymphoma response criteria and trial design
Results of axi-cel in DLBCL and variants have mainly been reported in terms of patients who received infusion of the CAR T cells, although an intent-to-treat analysis is also reported.Citation43,Citation44 Although the lymphoma response criteria have been updated, in ZUMA-1, the Cheson 2007 criteria were used, which allows a comparison with prior clinical trials.Citation55,Citation56 By these criteria, the ORR is defined as the rate of patients attaining a CR (disappearance of measurable disease on computed tomography scan or residual masses that are positron emission tomography negative) or a PR (50% decrease in tumor burden with ongoing positron emission tomography avidity). However, for the purposes of clinical outcome in aggressive lymphoma, attaining CR is the most important and it is further important that these CRs are durable and that relapse does not occur.
Axi-cell was initially tested outside of the NCI in the Phase I ZUMA-1 study; this was the first multicenter study of CAR T-cell therapy in refractory aggressive NHL including DLBCL, TFL and PMBCL. This study included seven patients and showed durable responses with axi-cel with an ORR and CR rate of 71% and 57%, respectively.Citation47 These encouraging results led to the multicenter Phase II portion of ZUMA-1. Key eligibility criteria for this study were aggressive B-cell lymphomas (DLBCL, PMBCL and TFL), refractory disease with no response to last therapy or relapse within 12 months of autologous hematopoietic stem cell transplantation, prior anti-CD20 monoclonal antibody and anthracycline and an Eastern Cooperative Oncology Group (ECOG) of 0–1. The primary endpoint was ORR in patients with >6 months follow-up post-axi-cel infusion. The secondary endpoints were duration of response, OS, safety and levels of CAR T cells and cytokines. The CAR manufacturing success was 99%. The conditioning regimen consisted of cyclophosphamide 500 mg/m2 and fludarabine 30 mg/m2 ×3 days followed by infusion of KTE-C19 or axi-cel at a dose of 1–2×106 CART cells/kg. The conditioning regimen and doses were determined in previous studies.Citation57
Efficacy of axi-cel on ZUMA-1
Administration of axi-cel was highly efficacious in 101 patients with refractory/relapsed DLBCL, who were considered to have a poor prognosis with a median OS of ~6 months and no available standard therapies.Citation30 The median time to response was 1 month, which is the timing of the first response assessment in the trial protocol of ZUMA-1. However, the range was 0.8–6.0 months, which indicates that patients not showing response at the 1-month assessment could convert and be seen to respond in subsequent months. Indeed, 11 of 35 patients in PR and 12 of 25 patients with SD at 1 month subsequently converted to CR. That said, patients who never attained CR (maximum response of PR) had a median duration of response of 1.9 months only, consistent with the usual situation in aggressive lymphoma where PR is not an adequate depth of responseCitation44 ().
Table 1 Highlights of the ZUMA-1 trial of axicabtagene ciloleucel, a CD19 CAR T-cell product, in DLBCL and variants
Including all variants of DLBCL enrolled, the ORR of axi-cel was 82% with a CR rate of 54%. The expected ORR of conditioning doses of fludarabine and cyclophosphamide alone without CAR T cells is not known. In general, fludarabine-based regimes are not considered to have significant antitumor activity in aggressive lymphomas.Citation58–Citation60 Therefore, these results suggest that axi-cel was active in the majority of patients due to the anti-lymphoma effects of the cellular product.
Although no statistical test was applied, the CR rate of the variants of DLBCL (TFL and PMBCL) was numerically higher than that for de novo DLBCL (DLBCL, CR rate 49%; TFL/PMBCL, CR rate 71%).Citation43,Citation44 Beyond this, the subgroup analysis did not identify clinical or disease-related risk factors that predicted response. The caveat of the subgroup analysis is that it is underpowered and may not identify true differences. Nonetheless, typical DLBCL risk factors such as age, stage, IPI risk score or bulky disease >10 cm did not obviously predict CAR T failure per multivariate analysis.Citation43
The earliest patients treated with the CD19 CAR T construct at the NCI (the same construct as axi-cel) have attained long-term durable CRs up to 56 months.Citation48 In ZUMA-1, the updated analysis was conducted after a minimum follow-up of 1 year, with 42% of patients still in CR. In general, very few relapsed occurred if patients remained in CR at 6 months post-CAR T infusion, but long-term follow-up will be required to determine the curative potential of this therapy.
Efficacy comparison of axi-cel to other CD19 CAR T products in DLBCL
When comparing the efficacy of axi-cel to other CD19 CAR T-cell products including tisagenleucel (tis-gen) and lisocabtagene maraceucel (liso-cel), it is important to note some key differences in trial design. For example, tis-gen was tested in both DLBCL and follicular lymphoma, and not all DLBCL patients (12 patients – 86%) met the criteria for refractory disease.Citation61 Conversely, the variants TFL and PMBCL (with high CR rates for axi-cel) were not included. The efficacy results of tis-gen were similar to those of axi-cel with 6 out of 14 DLBCL patients (43%) attaining CR.Citation61 Similarly, liso-cel (at the optimized dose level) showed a 6-month CR rate of 50%, in 14 patients with DLBCL or TFL only.Citation62 While cross-trial comparisons should be viewed with great caution for methodological reasons, overall, it seems that the efficacy of axi-cel is similar to that of the other two CD19 CAR T-cell products. This is despite substantial design and manufacturing differences among the three products (reviewed in Jain and DavilaCitation63).
Biomarkers of efficacy
After infusion into the patient, CAR T cells duplicate inside the patient. On ZUMA-1, CAR T-cell numbers rapidly increased, peaking at day +7 after the infusion.Citation64 The peak number of cells (known as peak expansion) was fourfold higher in responding patients compared to that in patients who did not respond (p=0.002), highlighting that expansion is important to obtain a response. An identified determinant of CAR T expansion was the composition of the T-cell subsets that made up the manufactured axi-cel product.Citation65 Axi-cel product that had a higher proportion of naïve and TCM led to superior expansion after infusion compared to the product that had more effector and effector memory T cells. It is not well understood which clinical, biologic or manufacturing factors promote naïve and TCM retention in manufactured CAR T-cell products.
Safety and tolerability
While anti-CD19 CAR T-cell therapy offers a new therapeutic strategy for aggressive B-cell lymphomas, its growing use requires further education/training and prompt recognition and management of these unique toxicities. In the primary analysis of ZUMA-1, all 101 patients who received axi-cel encountered adverse events, with 95% having grade 3 or higher related adverse events.Citation43 Two of the most common toxicities observed are cytokine release syndrome (CRS) and CAR T-cell–related encephalopathy syndrome (CRES). Close monitoring parameters, early identification and intensive supportive management minimize the life-threatening risks caused by this potentially curative therapy.
Cytokine release syndrome
CRS is the most common and well-described toxicity associated with CAR T-cell therapy, occurring in over 90% of patients at any grade, as reported in the ZUMA-1 clinical trial.Citation43 The activation of T cells upon recognition of tumor antigens by CARs results in the release of inflammatory cytokines and chemokines including IL-6, soluble IL-6R, IL-2, soluble IL-2Rα, interferon-γ, tumor necrosis factor α, IL-8, and IL-10, granulocyte-macrophage colony-stimulating factor and activated immune effector cells.Citation66
Given the nature of the systemic inflammatory response, any organ system can be affected, including cardiovascular, pulmonary, gastrointestinal, hepatic, renal, nervous, integumentary, musculoskeletal and hematologic systems. The most common symptoms of CRS include pyrexia, hypoxia and hypotension, and the onset of CRS symptoms typically occurs within the first week after CAR T-cell infusion with a median onset at 2 days in the ZUMA-1 trial. However, CRS sometimes persists, with symptoms resolving by a median time of 8 days.Citation41,Citation43 As the use of CAR T-cell therapy becomes widespread, more experience and knowledge with clinical use will lead to more detailed assessment systems, algorithms, preventive measures and supportive interventions.
Lee et al proposed the current consensus criteria for CRS grading as seen in , and organ toxicity assessment was graded as per the Common Terminology Criteria for Adverse Events (v4.0).Citation67 For management of grade 1 and 2 CRS, there should be assessment for infection and vigilant supportive care including intravenous fluids, antipyretics and analgesics as needed, with a close monitoring of cardiac complications and other organ function. Tocilizumab is an anti-IL-6 monoclonal antibody that can be considered in patients with identified CRS, and tocilizumab can result in rapid resolution of CRS toxicities without loss of CAR T-cell expansion or efficacy. The timing for tocilizumab administration has evolved in the last few years, especially since more experience has been gained with high-risk immunotherapy trials. It was initially recommended for grade 3 CRS, but the current consensus recommendation is to use it with grade 2 CRS and higher.Citation43,Citation44 Tocilizumab doses of 4–8 mg/kg (maximum dose 800 mg) can be repeated as needed for patients with persistent signs/symptoms of CRS. However, if there is no improvement despite anti-IL-6 therapy with ongoing severe CRS grade 3 or higher, high-dose corticosteroids could be considered. The role of steroids is fundamental in the treatment of CRS (and neurotoxicity) and should be considered for severe cases after tocilizumab therapy failure or even concomitantly to anti-IL-6 therapy in special situations.Citation66,Citation67 Although there is a theoretical risk of subsequent lymphotoxic effect and CAR T-cell suppression with steroid therapy, this has not been demonstrated in the ZUMA-1 study.Citation43,Citation68 In the ZUMA-1 trial, tocilizumab and steroids (at various doses) were administered to 43% and 27% of patients, respectively, for the treatment of CRS and/or neurotoxicityCitation43 ().
Table 2 Grading of CRS
CAR T-cell–related neurotoxicity
Grading scales for neurologic events in the ZUMA-1 trial followed the NCI Common Terminology Criteria for Adverse Events, version 4.03, and the grading of common neurotoxicities seen is displayed in . In this trial, 64% of patients experienced a neurologic event at any grade, with 28% having grade 3 or higher neurotoxicity.Citation43,Citation44 The best described is CRES, which occurred in 34% of patients to any grade and 21% of patients at grade 3. Other grade 3 neurologic events frequently observed were confusional state, aphasia and somnolence, and early neurologic signs included word-finding difficulties, inattention, disorientation, agitation or seizures. Median onset of neurotoxicity occurred at day 5 post-CAR T-cell infusion, with the median time of resolution of neurologic symptoms being day 17. All patients who developed neurologic symptoms had complete resolution, except for four patients who died from either progressive disease or unrelated adverse eventsCitation43 ().
Table 3 Grading of the common neurotoxicity symptoms
In a review of 133 adults who received lymphodepletion chemotherapy followed by infusion of CD19 CAR T cells, 53 (40%) developed one or more grade 1 or higher neurologic adverse events. Grade ≥3 neurotoxicity was associated with more severe CRS, disseminated intravascular coagulation with subsequent coagulopathy, earlier peak of IL-6 concentration and evidence of endothelial activation with capillary leak.Citation69 Patients who developed severe neurotoxicity also showed increased cerebrospinal fluid protein levels, increased cells including CAR T cells, and inflammatory cytokines such as IL-6, interferon-γ and tumor necrosis factor α in the cerebrospinal fluid.Citation66,Citation69 This suggests increased blood–brain barrier permeability associated with the pathophysiology of neurotoxicity after CAR T-cell infusion.
Monitoring and grading of CRES involves close neuro-logic assessments with ongoing evaluation for signs or symptoms of further complications including increased intracranial pressure and seizures. Grade 1 CRES involves supportive care with consideration of electroencephalogram, magnetic resonance imaging, funduscopic exam and neurology consultation, and grade 2 CRES adds to this management with anti-IL6 therapy, particularly if concurrent CRS occurs. More severe CRES grades 3 and 4 require intensive care unit admission, corticosteroids for symptoms not responding to anti-IL-6 and medical management of status epilepticus and increased intracranial pressure with consideration of neurosurgical evaluation if indicated.Citation66
Other safety and supportive care considerations
Other commonly encountered and potential risks include cytopenias, B-cell aplasia, consumptive coagulopathies, neutropenic fever, infections and tumor lysis syndrome. Patients enduring B-cell aplasia may require intravenous immunoglobulin infusions for prolonged period of time.Citation66 Ongoing investigations are looking into preventive measures to limit toxicity experienced by patients receiving CAR T-cell therapy. Efforts include identifying baseline and treatment-related risk factors for development of CRS, which have been associated with higher marrow tumor burden, fludarabine/cyclophosphamide lymphodepletion and higher CAR T-cell dose.Citation70
Prophylactic supportive care considerations have previously consisted of seizure prophylaxis with levetiracetam 750 mg orally every 12 hours for 30 days starting on the day of CAR T infusion, tumor lysis precautions per institutional guidelines and other institution-dependent algorithms for monitoring and symptom management.Citation66,Citation67 As clinical utilization and experience grows, further standardized assessments, grading and adverse event management protocols can be developed for improved early recognition and expedited treatment.
Patient-focused perspectives
The results of ZUMA-1 (and other multicenter studies in CAR T-cell therapy for refractory lymphomas) met the primary endpoint of improving objective responses in a patient population with refractory aggressive B-cell lymphomas with otherwise no options of cure.Citation30,Citation43,Citation48 Thus, these data suggest that there is a significant patient benefit with CAR T-cell therapy. It is most likely to be accepted as a viable alternative for the disease with an otherwise very poor prognosis, despite the potential associated toxicity.
The toxicities of CAR T-cell therapy are unique; thus, strategies for recognition and management of CRS and CRES are of paramount importance in order to minimize and/or speed up the recovery of patients receiving this type of high-risk immunotherapy. Conversely, patient education will become essential not only for understanding the efficacy and toxicity of CAR T-cell therapy, but also for the recognition of early toxicity signs. The development of guidelines and harmonization of the management of CAR T-cell therapy–related toxicities have been the focus of investigators recently.Citation66,Citation67 In addition, the development of institutional guidelines and specialized immunotherapy centers with multidisciplinary support from different specialties and allied health providers may ensure the success of utilizing this therapy in a large scale for further benefiting patients.Citation71
With the approval of axi-cel for the treatment of refractory DLBCL and since its clinical benefit will likely outweigh the risk associated with this therapy, a Risk Evaluation and Mitigation Strategies program has been established in order to mitigate the side effects related to CAR T-cell therapy. The Risk Evaluation and Mitigation Strategies program ensures that hospitals or cancer centers are adequately trained for the management of the toxicity and have the essential elements to treat toxicity (ie, availability of tocilizumab).
As this therapy will become widely available as the standard of care for refractory aggressive B-cell lymphomas, it is important to recognize that not all patients will have a favorable clinical condition/comorbidities that will make them candidates to receive this therapy. Honest discussion of the pros and cons of CAR T-cell therapy should occur and alternatives should be offered, including quality of life recommendations.
Conclusion
The development of CAR T-cell therapy represents the paradigm of personalized medicine and cancer immunotherapy. With the imminent widespread use (given the US Food and Drug Administration approval), one of the main focuses has been the optimization of CAR T-cell construct technology and improving the manufacturing process in order to increase its efficiency without affecting its activity and potency.Citation72 The understanding and management of CAR T-related toxicities represent another challenge and focus of research.
The dramatic responses seen with CAR T-cell therapy in B-cell lymphomas (and other hematologic malignancies) will likely shift the treatment paradigm of these conditions. This may include the use of this therapy in earlier stage of the disease or even as frontline therapy. However, the expected widespread availability CAR T-cell therapy will also come with a significant economic burden due to the high cost of the therapy and the care of patients developing related toxicities.
Several hurdles and setbacks have marked the path of CAR T-cell therapy from its initial preclinical and case reports to multicenter studies with promising results.Citation73 There is still much to do in the field, and it will depend on the collaboration, hope and vision of investigators, patients and supporters.
Disclosure
Julio C Chavez received fees from Kite Pharma (advisory board, speakers’ bureau) and Novartis (advisory board). The other authors report no conflicts of interest in this work.
References
- HowladerNNooneAKrapchoMSEER Cancer Statistics Review, 1975–2012, Based On November 2014 SEER Data Submission, Posted to the SEER Web Site, April 2015Bethesda, MD, USANational Cancer Institute2015 Forrás: Available from: http://seercancergov/csr/1975_2010/(2015 június 10 17: 41)Accessed November 1, 2017
- SwerdlowSHCampoEPileriSAThe 2016 revision of the World Health Organization classification of lymphoid neoplasmsBlood2016127202375239026980727
- International Non-Hodgkin’s Lymphoma Prognostic Factors ProjectA predictive model for aggressive non-Hodgkin’s lymphomaN Engl J Med1993329149879948141877
- SehnLHBerryBChhanabhaiMThe revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOPBlood200710951857186117105812
- ZhouZSehnLHRademakerAWAn enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab eraBlood2014123683784224264230
- El-GalalyTCVillaDAlzahraniMOutcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: a Danish-Canadian study of 443 patients with diffuse-large B-cell lymphomaAm J Hematol201590111041104626260224
- PasqualucciLTrifonovVFabbriGAnalysis of the coding genome of diffuse large B-cell lymphomaNat Genet201143983083721804550
- ZhangJGruborVLoveCLGenetic heterogeneity of diffuse large B-cell lymphomaProc Natl Acad Sci U S A201311041398140323292937
- RosenwaldAWrightGChanWCLeukemia Molecular Profiling ProjectThe use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphomaN Engl J Med2002346251937194712075054
- AlizadehAAEisenMBDavisREDistinct types of diffuse large B-cell lymphoma identified by gene expression profilingNature2000403676950351110676951
- LenzGWrightGDaveSSLymphoma/Leukemia Molecular Profiling ProjectStromal gene signatures in large-B-cell lymphomasN Engl J Med2008359222313232319038878
- HansCPWeisenburgerDDGreinerTCConfirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarrayBlood2004103127528214504078
- ViscoCLiYXu-MonetteZYComprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program StudyLeukemia20122692103211322437443
- ScottDWMottokAEnnishiDPrognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsiesJ Clin Oncol201533262848285626240231
- AdhikarySEilersMTranscriptional regulation and transformation by Myc proteinsNat Rev Mol Cell Biol20056863564516064138
- SavageKJJohnsonNABen-NeriahSMYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapyBlood2009114173533353719704118
- BarransSCrouchSSmithARearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximabJ Clin Oncol201028203360336520498406
- HornHZiepertMBecherCGerman High-Grade Non-Hodgkin Lymphoma Study GroupMYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphomaBlood2013121122253226323335369
- JohnsonNASlackGWSavageKJConcurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisoneJ Clin Oncol201230283452345922851565
- PetrichAMGandhiMJovanovicBImpact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysisBlood2014124152354236125161267
- OkiYNooraniMLinPDouble hit lymphoma: the MD Anderson Cancer Center clinical experienceBr J Haematol2014166689190124943107
- HowlettCSnedecorSJLandsburgDJFront-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysisBr J Haematol2015170450451425907897
- CoiffierBThieblemontCVan Den NesteELong-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’AdulteBlood20101161220402045 French20548096
- GisselbrechtCGlassBMounierNSalvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab eraJ Clin Oncol201028274184419020660832
- MaurerMJGhesquieresHJaisJPEvent-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapyJ Clin Oncol201432101066107324550425
- CrumpMKuruvillaJCoubanSRandomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12J Clin Oncol201432313490349625267740
- Van Den NesteESchmitzNMounierNOutcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL studyBone Marrow Transplant201752221622127643872
- Van Den NesteESchmitzNMounierNOutcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL studyBone Marrow Transplant2016511515726367239
- NagleSJWooKSchusterSJOutcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab eraAm J Hematol2013881089089423813874
- CrumpMNeelapuSSFarooqUOutcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 studyBlood2017130161800180828774879
- CostaLJMaddocksKEpperlaNDiffuse large B-cell lymphoma with primary treatment failure: ultra-high risk features and benchmarking for experimental therapiesAm J Hematol201792216117027880984
- YangYCancer immunotherapy: harnessing the immune system to battle cancerJ Clin Invest201512593335333726325031
- KochenderferJNWilsonWHJanikJEEradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19Blood2010116204099410220668228
- KochenderferJNFeldmanSAZhaoYConstruction and preclinical evaluation of an anti-CD19 chimeric antigen receptorJ Immunother200932768970219561539
- SavoldoBRamosCALiuECD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patientsJ Clin Invest201112151822182621540550
- van der StegenSJHamiehMSadelainMThe pharmacology of second-generation chimeric antigen receptorsNat Rev Drug Discov201514749950926129802
- ScheuermannRHRacilaECD19 antigen in leukemia and lymphoma diagnosis and immunotherapyLeuk Lymphoma1995185–63853978528044
- BrentjensRJLatoucheJBSantosEEradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15Nat Med20039327928612579196
- KochenderferJNYuZFrasheriDRestifoNPRosenbergSAAdoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cellsBlood2010116193875388620631379
- MadanRAGulleyJLFojoTDahutWLTherapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progressionOncologist201015996997520798195
- BrudnoJNKochenderferJNToxicities of chimeric antigen receptor T cells: recognition and managementBlood2016127263321333027207799
- Yescarta (axicabtagene ciloleucel) [package insert]Santa Monica, CA, USAKite Pharma, Inc;2017
- NeelapuSSLockeFLBartlettNLAxicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphomaN Engl J Med2017377262531254429226797
- NeelapuSSLockeFLBartlettNLLong-term follow-up ZUMA-1: a pivotal trial of Axicabtagene Ciloleucel (Axi-Cel; KTE-C19) in patients with refractory aggressive Non-Hodgkin Lymphoma (NHL)Blood2017130Suppl 1578
- TurtleCJHanafiLABergerCImmunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cellsSci Transl Med20168355355ra116
- KochenderferJNSomervilleRPTLuTLymphoma remissions caused by Anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levelsJ Clin Oncol201735161803181328291388
- LockeFLNeelapuSSBartlettNLPhase 1 results of ZUMA-1: a Multicenter Study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphomaMol Ther201725128529528129122
- KochenderferJNSomervilleRPTLuTLong-duration complete remissions of diffuse large B cell lymphoma after Anti-CD19 chimeric antigen receptor T cell therapyMol Ther201725102245225328803861
- LeeDWKochenderferJNStetler-StevensonMT cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trialLancet2015385996751752825319501
- MaudeSLFreyNShawPAChimeric antigen receptor T cells for sustained remissions in leukemiaN Engl J Med2014371161507151725317870
- MausMVJuneCHMaking better chimeric antigen receptors for adoptive T-cell therapyClin Cancer Res20162281875188427084741
- RobertsZJBetterMBotARobertsMRRibasAAxicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHLLeuk Lymphoma Epub20171023
- WangXRiviereIClinical manufacturing of CAR T cells: foundation of a promising therapyMol Ther Oncolytics201631601527347557
- BetterMChiruvoluVOliverJ287. Production of KTE-C19 (Anti-CD19 CAR T Cells) for ZUMA-1: a Phase 1/2 Multi-Center Study evaluating safety and efficacy in subjects with refractory aggressive Non-Hodgkin Lymphoma (NHL)Mol Ther201624Suppl 1S115
- ChesonBDThe International Harmonization Project for response criteria in lymphoma clinical trialsHematol Oncol Clin North Am200721584185417908623
- ChesonBDFisherRIBarringtonSFRecommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classificationJ Clin Oncol201432273059306825113753
- KochenderferJNDudleyMEKassimSHChemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptorJ Clin Oncol201533654054925154820
- LossosISPaltielOPolliackASalvage chemotherapy using a combination of fludarabine and cyclophosphamide for refractory or relapsing indolent and aggressive non-Hodgkin’s lymphomasLeuk Lymphoma1999331–215516010194133
- ZajaFRogatoARussoDMarinLSilvestriFBaccaraniMCombined therapy with Fludarabine and cyclophosphamide in relapsed/resistant patients with B-cell chronic lymphocytic leukaemia and non-Hodgkin’s lymphomasEur J Haematol19975953273289414645
- RedmanJRCabanillasFVelasquezWSPhase II trial of fludarabine phosphate in lymphoma: an effective new agent in low-grade lymphomaJ Clin Oncol19921057907941373760
- SchusterSJSvobodaJChongEAChimeric antigen receptor T cells in refractory B-cell lymphomasN Engl J Med2017377262545255429226764
- AbramsonJSPalombaMLGordonLIHigh durable CR rates in Relapsed/Refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001): defined composition allows for dose-finding and definition of pivotal cohortBlood201713058128584136
- JainMDDavilaMLConcise review: emerging principles from the clinical application of chimeric antigen receptor T cell therapies for B cell malignanciesStem Cells2018361364429024301
- NeelapuSSLockeFLBartlettNLAxicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphomaN Engl J Med2017377262531254429226797
- LockeFLRossiJNeelapuSSProduct characteristics associated with in vivo expansion of anti-CD19 CAR T cells in patients treated with axicabtagene ciloleucel (axi-cel)J Clin Oncol20173515 Suppl3023
- NeelapuSSTummalaSKebriaeiPChimeric antigen receptor T-cell therapy – assessment and management of toxicitiesNat Rev Clin Oncol2018151476228925994
- LeeDWGardnerRPorterDLCurrent concepts in the diagnosis and management of cytokine release syndromeBlood2014124218819524876563
- DavilaMLRiviereIWangXEfficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemiaSci Transl Med20146224224ra225
- GustJHayKAHanafiLAEndothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cellsCancer Discov20177121404141929025771
- HayKAHanafiLALiDKinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapyBlood2017130212295230628924019
- LockeFLAnasettiCMoffitt Immunotherapy Working Group and the Immune Cell Therapy (ICE-T) ProgramTransplanters drive CARs to the clinic by brewing ICE-T: the Moffitt roadmapJ Immunother Cancer2017515928716155
- LevineBLMiskinJWonnacottKKeirCGlobal manufacturing of CAR T cell therapyMol Ther Methods Clin Dev201749210128344995
- RosenbaumLTragedy, perseverance, and chance – The story of CAR-T therapyN Engl J Med2017377141313131528902570
