Abstract
Purpose
The development of emergence agitation (EA) is associated with several factors including age, preoperative anxiety, postoperative pain, anesthesia method, and surgery type. No studies have investigated whether the withdrawal reaction following rocuronium injection can predict the occurrence of EA. Therefore, we investigated this relationship in preschool-aged children undergoing inguinal herniorrhaphy, and which grade of withdrawal reaction is appropriate for identifying patients at risk of experiencing EA.
Methods
A total of 40 patients were enrolled in this study. During anesthesia induction, the withdrawal reaction after loss of consciousness following rocuronium injection was assessed using a 4-point scale. After surgery, EA was assessed using the Watcha scale.
Results
There was a correlation between withdrawal reaction and EA on admission to the postanesthesia care unit (PACU). Patients with a severe withdrawal reaction (grade 3) showed a significantly higher incidence of severe EA requiring medication on admission to the PACU.
Conclusion
The findings of this preliminary exploratory observational study suggest that it is possible for withdrawal movement following rocuronium injection during anesthesia induction to reflect pain sensitivity of pediatric patients, which in turn may be useful in identifying those at risk of severe EA on admission to the PACU among preschool children undergoing inguinal herniorrhaphy. Further studies with a larger sample size are required to validate these findings. The exact correlation between pain reaction following rocuronium injection and postoperative pain or pain-related phenomenon should be elucidated.
Introduction
Postoperative emergence agitation (EA) is a clinical condition, marked by crying, agitation, thrashing behavior, and inconsolability during recovery from general anesthesia.Citation1,Citation2 It increases the risk of self-injury, burden for caregivers, and dissatisfaction of parents.Citation3,Citation4 To date, various preventive strategies have been primarily focused on reducing the overall frequency and severity of EA.Citation5,Citation6 There is considerable individual variation in the level of EA.Citation7,Citation8 Thus, the identification of patients at risk of severe EA may help to further optimize individualized prevention strategies.
The exact mechanism of EA after general anesthesia remains unclear, but several factors are thought to be involved. They include age, preoperative anxiety, postoperative pain, the use of halogenated anesthetics, and surgery type.Citation1,Citation9 Under the same anesthetic and surgery conditions in a similar age group, a previous study found no significant predictors of EA other than anxiety. Pain was thought to be the major cause of EA, but more recent studies have found that EA frequently occurs after nonpainful procedures, suggesting that there must be other mechanisms involved.Citation4,Citation10 Nonetheless, considerable pain is a potential risk factor for EA because uncomfortable stimuli could be distressing in partially awake or dissociated patients.Citation10,Citation11 If so, a pain-sensitive temperament might also affect the incidence or severity of EA. Pain sensitivity was reported to be mutually independent of anxiety for predicting postoperative pain.Citation12 Thus, pain sensitivity itself might be useful as another predictor of EA. For the assessment of pain sensitivity, we focused on clinical pain, specifically the reaction to pain from a rocuronium injection during anesthesia induction. It is well known that rocuronium injections elicit an intense burning pain, and this often appears as a withdrawal movement during anesthesia induction.Citation13,Citation14 The withdrawal reaction has a wide spectrum of reactions from no response to generalized movement.Citation14
The aim of this study was to evaluate if the withdrawal reaction due to rocuronium injection during anesthesia induction correlates with clinical EA in the postanesthesia care unit (PACU) and which grade of withdrawal reaction is appropriate for identifying patients at risk of experiencing EA preoperatively in order to improve clinical management and implement preventative strategies.
Methods
After receiving approval from the institutional review board of Ajou University Hospital (Suwon, South Korea), this study was registered with ClinicalTrials.gov (NCT no: NCT02646722), and written informed consent was obtained from the parents of all patients. We recruited patients from Ajou University Hospital, Suwon, South Korea, from January 2016 to May 2016. Patients with an American Society of Anesthesiologists physical status I or II who were 1–5 years of age undergoing inguinal herniorrhaphy were enrolled in the study. Patients with a history of neurological problems and medications including sedatives and analgesics within 24 h of surgery were excluded. Patients who had a venous line in the forearm that was not 24 gauge were also excluded.
No children received premedication. They had a 24-gauge intravenous catheter inserted in the back of the hand in the ward or preoperative waiting room before being transferred to the operating room. A parent was allowed to accompany a patient in the operating room. The patients underwent basic monitoring including electrocardiogram, pulse oximetry, and noninvasive blood pressure measurement. Anesthetic induction was performed with 5 mg/kg thiopental sodium. Immediately after the loss of eyelash reflex, rocuronium was injected (0.3 mg/kg) over 5 s, along with mask ventilation with 6 L/min of 100% oxygen for breathing assistance. Then, any withdrawal movement was recorded. Following this, 5 vol% sevoflurane in 6 L/min of 100% oxygen was inhaled. In all, 2 min after the rocuronium injection, the trachea was intubated and anesthesia was maintained with 2%–3% sevoflurane and a 50% oxygen and air mixture. No analgesic was provided before or during anesthesia, and rescue analgesics were administered as required in the PACU. This design was the commonly used perioperative analgesic method for children undergoing very short surgery in our institution, and moreover, children’s postoperative pain was reported to be similarly effectively managed by using the “as required” method (pro re nata [PRN]) or fixed scheduled method.Citation15
At the end of surgery, the residual neuromuscular blocking agent was reversed with pyridostigmine 0.2 mg/kg and glycopyrrolate 0.008 mg/kg. After extubation, patients were transferred to the PACU. After arrival in the PACU, EA was recorded at 10-min intervals by nurses who were blinded to the presence or absence of a previous withdrawal movement in the patient. If the severity of EA was ≥3 points (Watcha scale, detailed subsequently), 0.5 µg/kg fentanyl was administered. Postoperative adverse events such as nausea, vomiting, bradycardia, and respiratory depression were recorded.
The withdrawal movement was graded using a 4-point scale: 0= no response, 1= movement at the wrist only, 2= movement/withdrawal involving the arm only (elbow/shoulder), and 3= generalized response, movement/withdrawal in more than one extremity.Citation16 EA was assessed using the Watcha scale: 1= calm; 2= crying, but can be consoled; 3= crying, cannot be consoled; and 4= agitated and thrashing around.Citation17
The primary outcome measure of the present study was the EA on admission to the PACU. Because the nature of the study was exploratory, an exact formal calculation of the sample size could not be made. Instead, it was based on a similar survey reported in the literature.Citation18 Secondary end points included the EA scores at 10, 20, and 30 min after PACU admission and at discharge from the PACU. Statistical analysis was performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The data were tested for normality using the Shapiro–Wilk test. Data are presented as mean and standard deviation if normally distributed, median and interquartile range if not normally distributed, and percentage as appropriate. To examine the relationship between the severity of withdrawal movement and EA, the Kendall’s tau correlation test and Eta correlation coefficient were used. We determined the optimal cutoff scores for a mild (grade 0–2) and severe (grade 3) withdrawal reaction based on the highest accuracy using a binary classification of the withdrawal group to identify patients at risk of experiencing EA who required medication.Citation19 For comparison between the mild and severe withdrawal groups as differentiated by the cutoff score, an analysis was conducted using an independent samples t-test if normally distributed and a Mann–Whitney U-test if not normally distributed to compare continuous variables. A chi-square test and Fisher’s exact test were used when appropriate to compare the categorical variables. A P-value of <0.05 was considered as statistically significant.
Results
A total of 40 patients were enrolled in this study. The demographic data are presented in . There was no significant difference in demographic data between the two groups when separated by withdrawal reaction.
Table 1 Patients’ demographic data
There was a significant correlation between the severity of withdrawal movement and EA on admission to the PACU (Kendall’s tau =0.29, P=0.04). There were no other significant differences for EA scores at other time points, ie, at 10, 20, and 30 min after PACU admission and at discharge from the PACU (Kendell’s tau =−0.02, P=0.988; Kendell’s tau =−0.07, P=0.625; Kendell’s tau =−0.09, P=0.533; and Kendell’s tau =−0.01, P=0.959, respectively). A Kendall’s tau value of <0.3 indicates a weak positive linear correlation. and show the association between withdrawal movement and EA. As withdrawal movement increased in patients, so did severe EA.
Figure 1 Correlation between the severity of withdrawal movement following rocuronium injection and severity of EA on admission to the PACU.
Abbreviations: EA, emergence agitation; PACU, postanesthesia care unit.
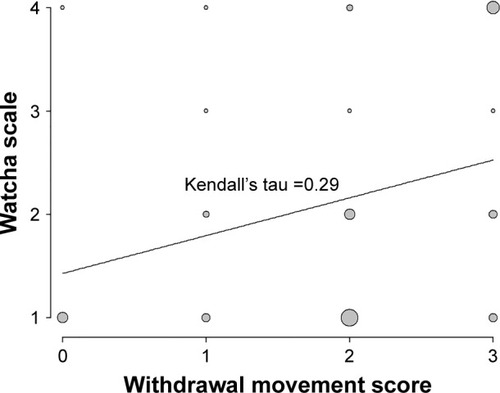
Table 2 Severity of withdrawal movement following rocuronium injection during anesthesia induction and severity of EA on admission to the PACU
In all, 27 patients exhibited mild withdrawal (grade 0–2) and 13 patients showed severe withdrawal (3 points). This classification was performed according to the optimal cutoff score to preoperatively identify patients at risk for experiencing EA requiring medication (EA score ≥3). A comparison between the mild and severe withdrawal movement groups is presented in . The incidence of EA requiring medication (EA score ≥3) on admission to the PACU in the severe withdrawal group was significantly higher than that in the mild withdrawal group. The time of initial fentanyl administration from the end of surgery was significantly shorter in the severe withdrawal group compared to the mild withdrawal group. However, there was no significant difference in the length of stay and fentanyl consumption in the PACU between the two groups.
Table 3 EA according to mild and severe withdrawal reactions
Discussion
The results of this study suggest that, in pediatric patients, the withdrawal movement following rocuronium injection during anesthesia induction could be used to predict the occurrence of severe EA on admission to the PACU after recovery from general anesthesia. Patients who demonstrated severe withdrawal movement had a significantly higher incidence of severe EA on admission to the PACU, requiring active intervention.
EA is a common clinical condition in which the emergence from general anesthesia is accompanied by confusion and psychomotor agitation.Citation20 The exact mechanism of EA after general anesthesia still remains poorly understood.Citation21–Citation23 In pediatric patients, it may be related to differences in neurodevelopmental characteristics and the variable effect of anesthetics.Citation24 Nevertheless, pain is still reported to be a potential risk factor affecting the incidence of EA.Citation10,Citation11 Even though pain and EA might not have a causal relationship, uncomfortable stimuli may be distressing in partially awake or dissociated patients, ie, pain may aggravate the EA phenomenon. Several other risk factors of EA have been proposed, such as preoperative anxiety, preschool age, type of surgery, and anesthetics.Citation25 Among them, anxiety has yielded conflicting results.Citation24 A previous study found that pain sensitivity and anxiety were mutually independent predictors of postoperative pain in the same patient population, with pain sensitivity having a stronger correlation with postoperative pain than anxiety.Citation12 Based on this finding, we speculated that pain sensitivity might predict the development and severity of EA, and conducted the present study, which is the first to investigate the association between pain sensitivity and EA. The findings of the current study indicate that patients with higher pain sensitivity are more likely to experience severe EA immediately after surgery. This may imply that the individual responses to postoperative pain in the PACU affect the severity of EA.
Among the methods to assess pain sensitivity, quantitative sensory testing is reported to have a higher predictive strength than demographic and psychological factors, which may predict up to 54% of the variance in postoperative pain experience.Citation26 Moreover, it has been demonstrated that an increased pain score for supra-threshold noxious stimulation has a better predictive value than pain thresholds.Citation26,Citation27 Furthermore, authentic pain stimuli reflect pain tolerance more closely than pain threshold,Citation28 suggesting that they can be utilized as a representative of supra-threshold noxious stimuli. In human beings, the peripheral veins are innervated by polymodal nociceptors, which mediate the signal transmission for the injection pain of certain agents.Citation29 It is unclear which levels of pain processing, ranging from the nociceptors to the brain, contribute to the individual differences in pain perception and sensitivity.Citation30 However, a recent study reported that propofol injection pain was a predictor for postoperative pain levels.Citation31 In this study, we used withdrawal movement, which occurs when rocuronium is injected intravenously during anesthesia induction. Rocuronium injection is associated with a strong burning pain, which elicits brisk flexion of the elbow and wrist, even after loss of consciousness.Citation16,Citation32 In this study, the rocuronium injection took place at the beginning of manual ventilation immediately after the loss of eyelash reflex, so psychological and cognitive effects on the pain reaction might have been depressed. Thus, it is unclear whether the observed movement accurately measured the individual responses to standardized pain stimuli measured in the conscious state.Citation33 However, a previous study suggested that the patient’s response to pain after anesthesia induction has a significant relationship with preoperative pain tolerance.Citation28 These findings support our assumption that the withdrawal reaction is associated with the patients’ pain-related symptoms and EA in this study.
When the scores for withdrawal movement were divided into two categories based on our analysis of clinical feasibility, the optimal cutoff grade was 3, rather than the median. This might be because the scale for withdrawal movement was an ordinal scale with irregular intervals. Our findings suggest that, when using withdrawal movement to predict EA, cases with a severe withdrawal reaction following rocuronium injection must be considered for administration of medications that may ameliorate or prevent EA on admission to the PACU. However, clinical usefulness may be limited because its prediction was confined to EA on admission to the PACU and there was no significant difference in fentanyl consumption in the PACU.
The findings of this study should be interpreted in the context of the following limitations. First, postoperative pain using a pediatric pain scale was not evaluated. Hence, this study has a limitation in that the actual relationship of withdrawal movement with EA after excluding the effect of postoperative pain on EA could not be investigated. Second, only a simple 4-point scale (Watcha scale) was used for assessing the EA instead of the Pediatric Anesthesia Emergence Delirium scale, which is the most commonly used validated scale for quantification of EA.Citation34 Third, the results were obtained from a small number of patients. The nature of this study was exploratory, and therefore, an exact power analysis was not performed. Although we did find a significant difference in the incidence of EA requiring medication (EA score ≥3) in the severe withdrawal group compared to the mild withdrawal group, as well as a correlation between withdrawal movement and EA, the study lacks sufficient power. Fourth, EA may have been affected by other confounding factors. For example, anxiety is known to be associated with pain sensitivity and independently can predict postoperative pain.Citation12 The age-related differences in characteristics of blood vessels may have affected the results, although we did not observe statistically significant differences between age groups. Furthermore, we did not verify that the anesthetic depth after the injection of thiopental sodium was the same between patients, which could also represent a confounding factor. Fifth, the clinical usefulness of withdrawal movement may be limited because its prediction was confined to EA on admission to the PACU and there was no significant difference in fentanyl consumption in the PACU.
Conclusion
The findings from this preliminary exploratory study suggest that it is possible for withdrawal movement following rocuronium injection during anesthesia induction to reflect pain sensitivity of pediatric patients, which in turn may be useful in identifying those at risk of severe EA on admission to the PACU among preschool-aged patients undergoing inguinal herniorrhaphy. Further studies with a larger sample size are required to validate these findings. The exact correlation between pain reaction following rocuronium injection and postoperative pain or pain-related phenomenon should be elucidated.
Disclosure
The authors report no conflicts of interest in this work.
References
- AouadMTNasrVGEmergence agitation in children: an updateCurr Opin Anaesthesiol200518661461916534301
- DahmaniSDelivetHHillyJEmergence delirium in children: an updateCurr Opin Anaesthesiol201427330931524784918
- KanayaAEmergence agitation in children: risk factors, prevention, and treatmentJ Anesth201630226126726601849
- VlajkovicGPSindjelicRPEmergence delirium in children: many questions, few answersAnesth Analg20071041849117179249
- ChenJLiWHuXWangDEmergence agitation after cataract surgery in children: a comparison of midazolam, propofol and ketaminePaediatr Anaesth201020987387920716081
- ThomasDLagooJKilpadiKEmergence agitation in children after sevoflurane anaesthesia: a comparative evaluation of ketamine and varying doses of fentanylSri Lankan J Anesthesiol20152311016
- CurriePUnderstanding and treating emergence deliriumNurse Anesthesia Capstones201520154
- SuryMCrazy kids in recovery. Faculty Manuscript for SPAPaper presented at: SPA/APA Joint Annual MeetingOctober 12; 2007San Francisco, CA
- KainZNCaldwell-AndrewsAAMaranetsIPreoperative anxiety and emergence delirium and postoperative maladaptive behaviorsAnesth Analg20049961648165415562048
- YuDChaiWSunXYaoLEmergence agitation in adults: risk factors in 2,000 patientsCan J Anaesth201057984384820526708
- KimHJKimDKKimHYKimJKChoiSWRisk factors of emergence agitation in adults undergoing general anesthesia for nasal surgeryClin Exp Otorhinolaryngol201581465125729495
- KilHKKimWOChungWYKimGHSeoHHongJYPreoperative anxiety and pain sensitivity are independent predictors of propofol and sevoflurane requirements in general anaesthesiaBr J Anaesth2012108111912522084330
- ChoiGJLeeSLeeJHParkSGKangHPharmacological and non-pharmacological intervention for rocuronium-induced withdrawal movement in the Korean population: a meta-analysis of 41 studies including 4,742 subjectsKorean J Anesthesiol201466641943225006365
- PrabhakarHSinghGPAliZKalaivaniMSmithMAPharmacological and non-pharmacological interventions for reducing rocuronium bromide induced pain on injection in children and adultsCochrane Database Syst Rev20162CD00934626871982
- HobsonAWiffenPJConlonJAAs required versus fixed schedule analgesic administration for postoperative pain in childrenCochrane Database Syst Rev20152CD01140425719451
- ShevchenkoYJocsonJCMcRaeVAThe use of lidocaine for preventing the withdrawal associated with the injection of rocuronium in children and adolescentsAnesth Analg199988474674810195516
- WatchaMFRamirez-RuizMWhitePFJonesMBLagueruelaRGTerkondaRPPerioperative effects of oral ketorolac and acetaminophen in children undergoing bilateral myringotomyCan J Anaesth19923976496541394752
- HsuYWSommaJHungYCTsaiPSYangCHChenCCPredicting postoperative pain by preoperative pressure pain assessmentAnesthesiology2005103361361816129988
- López-RatónMRodríguez-ÁlvarezMXCadarso-SuárezCGude-SampedroFOptimalCutpoints: an R package for selecting optimal cutpoints in diagnostic testsJ Stat Softw2014618136
- Voepel-LewisTBurkeCDifferentiating pain and delirium is only part of assessing the agitated childJ Perianesth Nurs2004195298299 author reply 29915472877
- WongDDBaileyCREmergence delirium in childrenAnaesthesia201570438338725764401
- YasuiYMasakiEKatoFSevoflurane directly excites locus coeruleus neurons of ratsAnesthesiology20071076992100218043068
- LimBGShenFYKimYBPossible role of GABAergic depolarization in neocortical neurons in generating hyperexcitatory behaviors during emergence from sevoflurane anesthesia in the ratASN Neuro201462e0014124597723
- LermanJ webpage on the InternetEmergence delirium and agitation in children2017 [updated March 30, 2017; cited June 5, 2017]. Available from: https://www.uptodate.com/contents/emergence-delirium-and-agitation-in-childrenAccessed June 5, 2017
- KurataniNOiYGreater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trialsAnesthesiology2008109222523218648231
- WernerMUMjoboHNNielsenPRRudinAPrediction of postoperative pain: a systematic review of predictive experimental pain studiesAnesthesiology201011261494150220460988
- GranotMCan we predict persistent postoperative pain by testing preoperative experimental pain?Curr Opin Anaesthesiol200922342543019352173
- WangHCaiYLiuJDongYLaiJPain sensitivity: a feasible way to predict the intensity of stress reaction caused by endotracheal intubation and skin incision?J Anesth201529690491126187542
- ArndtJOKlementWPain evoked by polymodal stimulation of hand veins in humansJ Physiol19914404674781804973
- NielsenCSStaudRPriceDDIndividual differences in pain sensitivity: measurement, causation, and consequencesJ Pain200910323123719185545
- PerssonAKPetterssonFDDyrehagLEAkesonJPrediction of postoperative pain from assessment of pain induced by venous cannulation and propofol infusionActa Anaesthesiol Scand201660216617626373922
- BorgeatAKwiatkowskiDSpontaneous movements associated with rocuronium: is pain on injection the cause?Br J Anaesth19977933823839389860
- LundILundebergTKowalskiJSandbergLBudhCNSvenssonEEvaluation of variations in sensory and pain threshold assessments by electrocutaneous stimulationPhysiother Theory Pract2005212819216392461
- SomainiMEngelhardtTFumagalliRIngelmoPMEmergence delirium or pain after anaesthesia – how to distinguish between the two in young children: a retrospective analysis of observational studiesBr J Anaesth2016116337738326865130
