Abstract
Purpose
Eradication of chronic hepatitis C virus (HCV) after interferon-based therapy and its association with the reduction of risk of hepatocellular carcinoma (HCC) in HCV-infected patients with advanced fibrosis is controversial. The study is aimed to develop a simple scoring model for HCC prediction among advanced fibrotic chronic hepatitis C (CHC) patients after pegylated interferon (pegIFN) and ribavirin (RBV) therapy.
Patients and methods
We enrolled 271 biopsy-proven CHC patients with advanced fibrosis between 2003 and 2016, and divided them into non-HCC (n=211) and HCC (n=60) groups. The median observation duration was 6.0 years (range: 0.9–12.6 years).
Results
The HCC prevalence after pegIFN and RBV therapy in CHC patients with sustained virologic response (SVR) and without SVR was 14.7% and 32.2%, respectively. Multivariate Cox regression showed age ≥59.5 years old at initiation of therapy (HR: 2.542, 95% CI: 1.390–4.650, P=0.002), pretreatment total bilirubin ≥1.1 mg/dL (HR: 2.630, 95% CI: 1.420–4.871, P=0.002), pretreatment platelet counts <146.5 × 103/μL (HR: 2.751, 95% CI: 1.373–5.511, P=0.004), no achievement of SVR (HR: 2.331, 95% CI: 1.277–4.253, P=0.006), and no diabetes at treatment initiation (HR: 3.085, 95% CI: 1.283–7.418, P=0.012) were significant predictors of HCC development. The scoring model consisted of the five categorical predictors and had an optimal cutoff point of 2.5. The area under receiver operating characteristic (AUROC) of the scoring model was 0.774±0.035 (P<0.001). The sensitivity and specificity of the cutoff value to detect HCC were 81.3% and 57.5%. The 5-year and 10-year cumulative incidence of HCC was 4.9% and 10.0% in patients with simple score ≤2; and 25.9% and 44.6% in patients with simple score ≥3 (P<0.001).
Conclusion
The simple clinical-guided score has high discriminatory power for HCC prediction in advanced fibrotic CHC patients after pegIFN and RBV therapy.
Introduction
Chronic hepatitis C infection is a major global health problem and an important cause of morbidity and mortality from sequelae such as liver cirrhosis and HCC.Citation1–Citation4 It has been estimated that, globally, 27% of cirrhosis and 25% of HCC cases develop in HCV-infected people.Citation5 In patients with HCV-related cirrhosis, the annual incidence rates of developing hepatic decompensation and HCC are 3.9% and 1.4%–8%, respectively.Citation4,Citation6,Citation7 The main goals of therapy in patients with HCV-related cirrhosis are to eradicate HCV, improve liver histologic activity, and reduce fibrosis. In addition, several studies have shown that chronic HCV infected patients with advanced fibrosis achieving SVR after IFN-based therapy have reduced risks of developing liver decompensation, HCC, and liver-related mortality.Citation8–Citation13 Therefore, although these patients are considered to be a difficult-to-treat population with less tolerability and poor therapeutic responses to pegIFN and PBV therapy, they could still benefit from the treatment with a lower risk of HCC, liver disease progression and liver-related complications.Citation14,Citation15 There are several risk factors associated with HCC development in CHC patients with advanced fibrosis, including male gender, older age at treatment initiation, HCV genotype 1b, low serum albumin and high serum total bilirubin levels, low platelet counts, and presence of esophageal varices.Citation2,Citation11,Citation16–Citation18 However, another report demonstrated a controversial result that achievement of SVR after IFN-based therapy did not influence the rate of HCC development.Citation19
Combination therapy with pegIFN plus RBV has greatly improved the rate of SVR compared to conventional IFN with and without RBV and is the standard treatment strategy for chronic HCV infection before the introduction of DAAs.Citation20–Citation22 However, only a few studies have been focused on the influence of the pegIFN and RBV therapy on HCC development in CHC patients with advanced fibrosis.Citation11,Citation18,Citation19 Besides, there was no report regarding the assessment of HCC risk score of those advanced fibrotic CHC patients after pegIFN and RBV therapy. We therefore conducted this study to evaluate the predictors on HCC development and to develop a simple risk score model for HCC prediction in advanced fibrotic chronic hepatitis C patients after pegIFN and RBV therapy.
Patients and methods
Patients
This study was conducted at our institute between October 2003 and November 2016. There were in total 271 patients with biopsy proven chronic HCV infection with advanced fibrosis enrolled into the study (). Chronic hepatitis C infection was defined as seropositive for anti-HCV and HCV RNA for more than 6 months. Clinical and demographic data were recorded within three months before the initiation of therapy. The degree of hepatic inflammation and fibrosis was graded using an Ishak modified scores and read by a single pathologist. Advanced fibrosis and cirrhosis were defined as a fibrosis score ≥4 and ≥5 on the Ishak modification of histologic activity index. The diagnosis of type 2 diabetes was based on a value of fasting glucose ≥126 mg/dL on at least two occasions or ongoing hypoglycemic agent treatment.Citation23 All patients received and completed the pegIFN plus RBV therapy for 24 or 48 weeks according to the reimbursement policy of the National Health Insurance in Taiwan. The prescribed types of pegIFNs were pegIFN-α-2a (180 μg) or weight-based pegIFN-α-2b (1.5 μg/kg). For genotype-1b-HCV–infected patients with body weight <75 kg, the oral RBV dose was 1,000 mg per day and for those with body weight ≥75 kg, 1,200 mg per day. For genotype non-1b-HCV–infected patients, the RBV dose was 800 mg per day. All patients were followed up for 24 weeks after the completion of treatment. RVR was defined as undetectable serum HCV RNA by using PCR at the end of week 4 of therapy. SVR was defined as achievement of undetectable serum HCV RNA by using PCR at the end of treatment that was sustained after the end of six-month follow-up. A non-responder had terminated the treatment course before week 16, and was excluded from our study. We also excluded patients with HCC diagnosed before treatment initiation or within 6 months after the end of therapy, concurrent hepatitis B virus or human immunodeficiency virus infection, toxic hepatitis, autoimmune hepatitis, primary biliary cirrhosis, Wilson’s disease, or hemoglobinopathies. Written informed consent was obtained from all patients enrolled in this study. The study was performed in accordance with the ethical guidelines of the International Conference on Harmonization for Good Clinical Practice and has been approved by the Institutional Review Board of Chang Gung Memorial Hospital (No 201600519B0).
Laboratory test
Anti-HCV tests were conducted using a third-generation enzyme immunoassay kit (AxSYM® HCV Version 3.0; Abbott Laboratories, Abbott Park, IL, USA). Serum HCV RNA was quantified using a real-time polymerase chain reaction (PCR) assay (COBAS® AmpliPrep Instrument and COBAS TaqMan® 48; Hoffman-La Roche Ltd, Basel, Switzerland), with a detection limit of 15 IU/mL. HCV genotyping was determined using a linear probe assay (VERSANTTM HCV Genotype Assay [LiPA]; Bayer AG, Leverkusen, Germany).
Follow-up and assessment of HCC development
The observation duration was started from the date of the end of six-month follow-up after the pegIFN and RBV therapy to the date of HCC diagnosis, last-visit or death of the patient. During the observation period, patients with and without liver cirrhosis returned to the clinic every three and six months for liver biochemistry and liver ultrasonography study. If a liver tumor was suspected by ultrasonography study, further examination was arranged for HCC confirmation. The HCC was diagnosed based on histopathology or two image studies, such as dynamic liver computed tomography or magnetic resonance imaging with typical arterial enhancement, or one image study plus elevated serum AFP level of more than 400 ng/mL.Citation24
Statistical analysis
Continuous variables were expressed as mean±standard deviation. All variables were tested for normal distribution using the Kolmogorov–Smirnov test. The Student’s t-test was used to compare the means of continuous variables and normally distributed data, otherwise the Mann–Whitney U test was used. Categorical data were tested using the chi-square test. Finally, risk factors were assessed by univariate Cox regression analysis, and variables that were statistically significant (P<0.05) were included in multivariate analysis by applying a multivariate Cox regression based on forward elimination of data. Calibration was assessed using the Hosmer–Lemeshow goodness-of-fit test to compare the number of observed and predicted events in risk groups for the entire range of HCC probabilities. Discrimination was assessed by AUROC. The AUROCs were compared using a nonparametric approach. AUROC analyses were also used to calculate cutoff values, sensitivity, specificity, and overall correctness. Finally, cutoff points were calculated by acquiring the best Youden index (sensitivity + specificity - 1). Cumulative survival curves as a function of time were generated using the Kaplan–Meier approach and compared by log-rank test. All statistical tests were two-tailed, with P-values <0.05 being considered as statistically significant. Data were analyzed using SPSS 12.0 software for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Subject characteristics
Baseline demographic and clinical characteristics of non-HCC and HCC patients are shown in . Total 271 patients receiving pegIFN and RBV therapy were enrolled into this study. The median observation duration was 6.0 years (range: 0.9–12.6 years). SVR developed in 57.6% of patients. The overall HCC prevalence of the entire population after pegIFN and RBV therapy was 22.1% (60/271). The mean age of the patients at initiation of therapy was 57.5±10.3 years, with 128 (47.2%) being men. Patients with HCC development after pegIFN and RBV treatment were significantly older at initiation of therapy, had higher pretreatment serum aspartate aminotransferase to alanine aminotransferase ratio, total bilirubin, AFP levels, and higher frequency of liver cirrhosis. Those patients with HCC also had lower pretreatment serum albumin levels, platelet counts, and lower frequency of SVR. Fewer patients with fatty liver and diabetes at initiation of therapy developed HCC after completion of pegIFN and RBV treatment. The mean pretreatment aspartate and alanine aminotransferase level, HCV RNA viral load, and percentage of HCV genotype 1b and male gender, treatment duration, and types of pegIFN (2a vs 2b) prescribed were similar between the non-HCC and HCC patients.
Table 1 Baseline clinical and virologic characteristics of advanced fibrotic chronic hepatitis C patients with and without HCC
Predictors of HCC
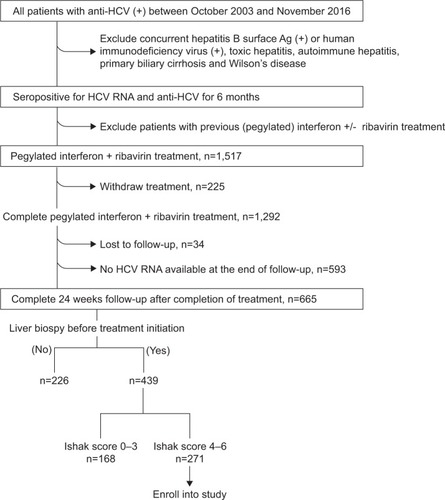
In univariate Cox regression analysis, several clinical factors, including age, serum albumin, aspartate aminotransferase to alanine aminotransferase ratio, total bilirubin, platelet count, AFP, SVR, cirrhosis, fatty liver and diabetes were identified to be significantly associated with HCC development (). ROC curve analysis showed that 59.5 years at initiation of therapy was the best cutoff point to discriminate among HCC and non-HCC occurrence (AUROC: 0.663±0.038, P<0.001). The cutoff points of pretreatment total bilirubin level and platelet count to predict HCC development were 1.1 mg/dL (AUROC: 0.659±0.043, P=0.001) and 146.5 × 103/μL (AUROC: 0.652±0.043, P=0.001). Then we divided the age at initiation of therapy, pretreatment total bilirubin levels and platelet counts into two separate groups according to the cutoff points determined by the ROC curves. In multivariate analysis, the three categorical factors and the rest of the significant factors in univariate analysis were entered in the final stepwise regression analysis. Multivariate Cox regression showed age ≥59.5 years old at initiation of therapy (HR: 2.542, 95% CI: 1.390–4.650, P=0.002), pretreatment total bilirubin ≥1.1 mg/dL (HR: 2.630, 95% CI: 1.420–4.871, P=0.002), pretreatment platelet count <146.5×103/μL (HR: 2.751, 95% CI: 1.373–5.511, P=0.004), no achievement of SVR (HR: 2.331, 95% CI: 1.277–4.253, P=0.006), and no diabetes at baseline (HR: 3.085, 95% CI: 1.283–7.418, P=0.012) were significant predictors of HCC development.
Table 2 Analysis of predictors of HCC using univariate and multivariate Cox regression models
Simple score to predict HCC
Then we used the five significant independent categorical predictors in multivariate Cox regression to form a new simple score for HCC prediction. We assigned patients with age <59.5 years old at initiation of therapy 0 point and age ≥59.5 years old at initiation of therapy 1 point; pretreatment total bilirubin <1.1 mg/dL 0 point and total bilirubin ≥1.1 mg/dL 1 point; pretreatment platelet count ≥146.5×103/μL 0 point and platelet count <146.5× 103/μL 1 point; presence of diabetes at treatment initiation 0 point and no diabetes at treatment initiation 1 point; and post-treatment SVR 0 point and post-treatment non-SVR 1 point. The simple score equaled the sum of points of these five variables.
The simple score had a cutoff point of 2.5 by the ROC curve to predict HCC (). The sensitivity, specificity and overall correctness were 78.5%, 57.5% and 62.2%, respectively. The AUROC of the simple score was 0.774±0.035 (P<0.001) (). Cumulative HCC incidence differed significantly (P<0.001) in advanced fibrotic CHC patients with simple scores ≤2 versus simple scores ≥3 (). The 5-year and 10-year cumulative incidence of HCC development were 4.9% and 10.0% in patients with simple score ≤2; and 25.9% and 44.6% in patients with simple score ≥3.
Figure 2 Area under receiver operating curve (AUROC) analysis of the simple score to predict the occurrence of hepatocellular carcinoma.
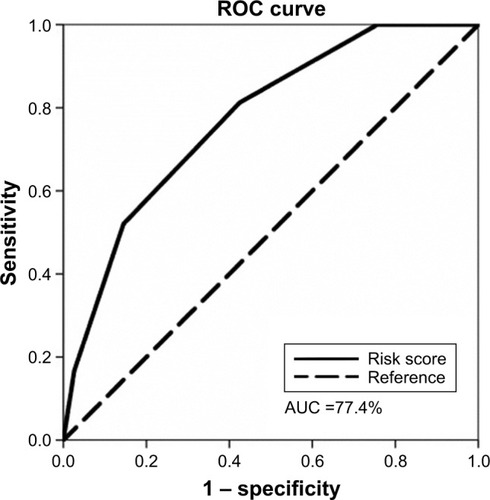
Figure 3 Cumulative incidence rate of hepatocellular carcinoma.
Table 3 Prediction of hepatocellular carcinoma using the new score
Discussion
In this study, we developed a simple scoring system using commonly available clinical and laboratory parameters to predict the risk of HCC development after pegIFN and RBV therapy in previous non-HCC CHC patients with advanced fibrosis. The simple scoring system only included 5 clinical factors, including pretreatment age, serum bilirubin levels, platelet counts, presence of diabetes at treatment initiation and achievement of SVR, which was easier to use in the clinical setting. Patients with a score ≥3 had significant higher risk of HCC development. The 10-year cumulative incidence of HCC was 44.6%. In contrast, only 10.0% of patients with a score ≤2 developed HCC at 10 years. A surveillance program in advanced fibrotic CHC patients after pegIFN and RBV therapy could be scheduled respectively according to high or low risk scoring.
Advanced fibrosis has been considered a strong negative predictor for SVR in pegIFN and RBV combination treatment. There has been a lot of debate on the use of this combination therapy to treat CHC patients with advanced fibrosis, because of a high frequency of adverse effects, a high rate of treatment discontinuation, and decreased SVR. Previous reports have shown that an overall SVR rate for patients with advanced fibrosis is 24%–52%.Citation21,Citation22,Citation25 There were also several clinical adverse effects that developed during therapy, including pruritus (40%), malaise (36%), anemia (31%), insomnia (29%), anorexia (28%), dizziness (27%), thrombocytopenia (26%), fever (23%), dyspnea (23%), and myalgia (23%) in our study. However, several studies have demonstrated that CHC patients with advanced fibrosis achieving SVR after IFN-based therapy have reduced risks of developing liver decompensation, HCC, and liver-related mortality.Citation8–Citation11 Moreover, a large proportion of patients in those studies received conventional IFN rather than pegIFN. Our study enrolled only pegIFN and RBV treated CHC patients with advanced fibrosis and showed that the combination therapy decreased the risk of HCC occurrence significantly. In our present study, SVR developed in 57.6% of patients. However, in those sustained responders, the risk of HCC could not be fully eliminated after pegIFN and RBV therapy. There were 23 (14.7%) patients with SVR who developed HCC during follow-up. The sensitivity and specificity of non-SVR to predict HCC were 61.7% and 63.0% with an AUROC: 0.623 (P=0.004), which were lower than the simple score with cutoff point: 2.5 (AUROC: 0.774; P<0.001, sensitivity: 81.3%, specificity: 57.5%). Therefore, the simple score ≥3 was a much better predictor to predict HCC development after pegIFN and RBV therapy and those patients with simple score ≥3 should be closely followed for HCC surveillance.
Liver cirrhosis at treatment initiation has been shown to be a significant predictor for HCC occurrence in CHC patients treated with IFN-based therapy or DAAs.Citation26,Citation27 Our study also showed that more HCC patients had cirrhosis at treatment initiation. However, the discrimination between severe bridging fibrosis (Ishak modified fibrosis score 4) and cirrhosis (Ishak modified fibrosis score 5–6) disappeared after adjustment for other risk factors in the multivariate Cox regression analysis.
Serum AFP measurement was frequently used for HCC surveys because it is inexpensive, simple to perform, and commonly available. Our report as well as previous studies have also shown that an elevated serum AFP level was a significant predictor of HCC development in CHC patients.Citation2,Citation16,Citation28 Although the patients with HCC had higher pretreatment serum AFP levels than those without HCC in the present study, we found the serum AFP level was not a predictor for HCC development in advanced fibrotic CHC patients. In addition, AFP alone was not recommended for HCC survey because of its low sensitivity and specificity for detecting HCC. At a serum cutoff level of 20 ng/mL, AFP has low sensitivity ranging from 25% to 65% for detecting HCC.Citation29 Moreover, only one third of patients with HCC had elevated serum AFP levels higher than 100 ng/mL.Citation30 Hu et al showed an association between serum AFP levels and liver disease activity, suggesting that AFP production was increased during liver necroinflammation.Citation31 AFP elevation was also noted in patients with advanced chronic hepatitis C without evidence of HCC.Citation31–Citation34 Our advanced fibrotic patients with HCC had higher AST to ALT ratio, representing a more prominent liver necroinflammation, and may thus have resulted in more elevated serum AFP levels.
Diabetes has been shown to impair the treatment response to IFN-based therapy and increase the HCC risk in non-cirrhotic CHC patients treated with IFN-based therapy.Citation35–Citation38 In contrast to those reports, our study found that presence of diabetes at treatment initiation was a predictor of reduced HCC risk in advanced fibrotic CHC patients. The possible explanation for this conflicting result was that in the study of Hung et al,Citation38 the diabetic patients were significantly older in age, and had higher fibrosis scores, and lower platelet counts and SVR rates than those of the non-diabetic patients. These factors may mask the protective influence of diabetes. Conversely, the baseline clinical factors and achievements of SVR rate between the diabetic and non-diabetic patients were similar in our present study (62.9% vs 56.0%; P=0.333). An increase of SVR rate in diabetic patients may provide an increased benefit in HCC prevention. Interestingly, 82% (51/62) of the diabetes patients had been treated with metformin. Previous studies have shown the use of metformin was associated with a significant reduction of HCC risk compared with the use of other hypoglycemia agents.Citation39,Citation40 The use of metformin may be associated with reduced risk of HCC in our present study. The conflicting data might also be associated with different patient data collection and more careful diet control, resulting in less hepatotoxin exposure in diabetic patients.
Previous reports have shown the HCV genotype 1b was associated with higher risk of HCC.Citation17,Citation41 In the present study we did not find a significant difference of HCC risk between advanced fibrotic CHC patients with HCV genotype 1b and non-1b. IFN has also been considered to have anticarcinogenic activity in addition to its antiviral effect to decrease the risk of HCC.Citation42 However, the treatment duration (48 weeks vs 24 weeks) or types of pegIFN prescribed in our study did not influence the rate of HCC development. From previous studies, the contribution of gender to the risk of HCC development is controversial.Citation17,Citation38,Citation41,Citation43 Our study found that gender was not a risk factor for HCC occurrence in CHC patients with advanced fibrosis.
Conclusion
The simple score has a better discriminatory power for HCC prediction in CHC patients with advanced fibrosis. SVR after pegIFN and RBV therapy could reduce the risk of HCC in those patients. However, the risks of HCC still remained after achieving SVR, especially in those with a score ≥3, who should be intensively followed for HCC surveillance. For high-risk patients who failed the pegIFN and RBV therapy, more aggressive treatment with DAAs to eradicate the virus should be considered in order to reduce the risk of HCC.
Author contributions
Ching-Chih Hu, Cheng-Hao Weng, and Rong-Nan Chien conceived the study. Ching-Chih Hu, Cheng-Hao Weng and Ching-Fang Hu analyzed the data. Yen-Ting Chen, Man-Chin Hua and Li-Wei Chen contributed to data collection. Ching-Chih Hu and Cheng-Hao Weng contribute to manuscript writing, and Rong-Nan Chien contributed to reviewing and revising the paper. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Abbreviations
| AFP | = | α-fetoprotein |
| AUROC | = | area under receiver operating characteristic |
| CHC | = | chronic hepatitis C |
| DAA | = | direct-acting antiviral agents |
| HCV | = | hepatitis C virus |
| HCC | = | hepatocellular carcinoma |
| IFN | = | interferon |
| pegIFN | = | pegylated interferon |
| RBV | = | ribavirin |
| ROC | = | receiver operating characteristic |
| RVR | = | rapid virologic response |
| SVR | = | sustained virologic response |
Acknowledgments
This study was supported by the Chang Gung Memory Hospital Research Grant CMRPG2F0071 and CMRPG2D0313.
Disclosure
The authors report no conflicts of interest in this work.
References
- AlterMJEpidemiology of hepatitis C virus infectionWorld J Gastroenterol200713172436244117552026
- TsukumaHHiyamaTTanakaSRisk factors for hepatocellular carcinoma among patients with chronic liver diseaseN Engl J Med199332825179718017684822
- HuCCLinCLKuoYLEfficacy and safety of ribavirin plus pegylated interferon alfa in geriatric patients with chronic hepatitis CAliment Pharmacol Ther2013371819023121150
- FassioEHepatitis C and hepatocellular carcinomaAnn Hepatol20109Suppl11912220714008
- PerzJFArmstrongGLFarringtonLAHutinYJBellBPThe contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwideJ Hepatol200645452953816879891
- ColomboMde FranchisRDel NinnoEHepatocellular carcinoma in Italian patients with cirrhosisN Engl J Med1991325106756801651452
- FattovichGGiustinaGDegosFMorbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patientsGastroenterology199711224634729024300
- BrunoSStroffoliniTColomboMSustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective studyHepatology200745357958717326216
- ShiratoriYItoYYokosukaOAntiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survivalAnn Intern Med2005142210511415657158
- HungCHLeeCMLuSNLong-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosisJ Viral Hepat200613640941416842444
- CardosoACMoucariRFigueiredo-MendesCImpact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosisJ Hepatol201052565265720346533
- RutterKStattermayerAFBeinhardtSSuccessful anti-viral treatment improves survival of patients with advanced liver disease due to chronic hepatitis CAliment Pharmacol Ther201541652153125616017
- HsuCSHuangCJKaoJHInterferon-based therapy decreases risks of hepatocellular carcinoma and complications of cirrhosis in chronic hepatitis C patientsPLoS One201387e7045823894660
- StraderDBWrightTThomasDLSeeffLBAmerican Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis CHepatology20043941147117115057920
- WrightTLTreatment of patients with hepatitis C and cirrhosisHepatology2002365 Suppl 1S185S19412407593
- IkedaKSaitohSKoidaIA multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosisHepatology199318147537686879
- BrunoSCrosignaniAMaisonneuvePRossiSSiliniEMondelliMUHepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort studyHepatology20074651350135617680653
- AlemanSRahbinNWeilandOA risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosisClin Infect Dis201357223023623616492
- Fernandez-RodriguezCMAlonsoSMartinezSMPeginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: outcomes and factors predicting responseAm J Gastroenterol20101051021642172 quiz 217320700116
- McHutchisonJGGordonSCSchiffERInterferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy GroupN Engl J Med199833921148514929819446
- FriedMWShiffmanMLReddyKRPeginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infectionN Engl J Med20023471397598212324553
- MannsMPMcHutchisonJGGordonSCPeginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trialLancet2001358928695896511583749
- Expert Committee on the Diagnosis and Classification of Diabetes MellitusReport of the Expert Committee on the Diagnosis and Classification of Diabetes MellitusDiabetes Care1997207118311979203460
- BruixJShermanMPractice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinomaHepatology20054251208123616250051
- HuCCLinCLChangLCInterleukin-28B gene non-TT allele strongly predicts treatment failure for genotype 1 infected chronic hepatitis C patients with advanced fibrosis: a case control studyBMC Infect Dis20151515625888020
- NagaokiYAikataHNakanoNDevelopment of hepatocellular carcinoma in patients with hepatitis C virus infection who achieved sustained virological response following interferon therapy: A large-scale, long-term cohort studyJ Gastroenterol Hepatol20163151009101526584407
- ContiFBuonfiglioliFScuteriAEarly occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antiviralsJ Hepatol201665472773327349488
- HuCCWengCHLinCLNew score to predict risk of hepatocellular carcinoma in chronic hepatitis C patients after pegylated interferon and ribavirin therapyOncotargetIn press2017
- PaulSBGulatiMSSreenivasVEvaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoproteinOncology200772Suppl 111712318087192
- TorzilliGMinagawaMTakayamaTInoueKHuiAMKubotaKOhtomoKMakuuchiMAccurate preoperative evaluation of liver mass lesions without fine-needle biopsyHepatology19993088989310498639
- HuKQKyuloNLLimNElhazinBHillebrandDJBockTClinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinomaAm J Gastroenterol200499586086515128351
- BayatiNSilvermanALGordonSCSerum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis CAm J Gastroenterol19989312245224569860408
- ChuCWHwangSJLuoJCClinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis CJ Clin Gastroenterol200132324024411246354
- Di BisceglieAMSterlingRKChungRTSerum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C TrialJ Hepatol200543343444116136646
- Romero-GomezMDel Mar ViloriaMAndradeRJInsulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patientsGastroenterology2005128363664115765399
- KonishiIHoriikeNHiasaYDiabetes mellitus reduces the therapeutic effectiveness of interferon-alpha2b plus ribavirin therapy in patients with chronic hepatitis CHepatol Res200737533133617441805
- KawamuraYAraseYIkedaKDiabetes enhances hepatocarcinogenesis in noncirrhotic, interferon-treated hepatitis C patientsAm J Med201012310951956e95120920698
- HungCHLeeCMWangJHImpact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapyInt J Cancer2011128102344235220669224
- DonadonVBalbiMMasMDCasarinPZanetteGMetformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver diseaseLiver Int20103075075820331505
- LaiSWChenPCLiaoKFMuoCHLinCCSungFCRisk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort studyAm J Gastroenterol2012107465222085817
- ChangKCWuYYHungCHClinical-guide risk prediction of hepatocellular carcinoma development in chronic hepatitis C patients after interferon-based therapyBr J Cancer201310992481248824084770
- TengWHsiehYCLuiKWEradication of hepatitis C virus profoundly prolongs survival in hepatocellular carcinoma patients receiving transarterial chemoembolizationJ Viral Hepat201724121160116728643457
- ChangKCHungCHLuSNA novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapyJ Antimicrob Chemother201267112766277222899800