Abstract
Background
To evaluate the clinical significance of degree of pulmonary fissure completeness (PFC) on major in-hospital outcomes following video-assisted thoracoscopic (VATS) lobectomy for non-small-cell lung cancer (NSCLC).
Materials and methods
We carried out a single-center retrospective analysis on the prospectively maintained database at our unit between August 2014 and October 2015. Patients were divided into two groups based on their fissure sum average (FSA). Patients with FSA >1 (1< FSA ≤3) were considered to have incomplete pulmonary fissures (group A), while patients with FSA of 0–1 were considered to have complete pulmonary fissures (group B). Demographic differences in perioperative characteristics and surgical outcomes between these two groups were initially assessed. Then, a multivariate logistic-regression analysis was further conducted to identify the independent predictors for major in-hospital outcomes.
Results
A total of 563 patients undergoing VATS lobectomy for NSCLC were enrolled. There were 190 patients in group A and 373 patients in group B. The overall morbidity and mortality rates of our cohort were 30.6% and 0.5%, respectively. Group A patients had a significantly higher overall morbidity rate than group B patients (42.1% vs 24.7%, P<0.001). Both minor morbidity (40.5% vs 22%, P<0.001) and major morbidity (11.1% vs 5.6%, P=0.021) rates in group A patients were also significantly higher than group B patients. No significant difference was observed in mortality rate between these two groups (1.1% vs 0.3%, P=0.26). The incomplete degree of PFC was significantly correlated with length of stay and chest-tube duration (log-rank P<0.001) after surgery. Finally, the incomplete degree of PFC was found to be predictive of overall morbidity (OR 2.08, P<0.001), minor morbidity (OR 2.39, P<0.001), and major morbidity (OR 2.06, P=0.031) by multivariate logistic-regression analyses.
Conclusion
Degree of PFC is an excellent categorical predictor for both major and minor morbidity after VATS lobectomy for NSCLC.
Introduction
Rationale
Lung cancer is currently the worldwide leading cause of cancer-related deaths and remains the most prevalent cancer in both developed and developing countries. Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, with a poor 5-year survival rate <20%.Citation1 Nowadays, radical surgery is regarded not only as the optimal therapeutic option for early-stage NSCLC but also as a key component of multidisciplinary treatments for advanced-stage NSCLC.Citation2,Citation3 Lobectomy is a standardized surgical procedure in treating operable NSCLC, because of its safety, capacity of en bloc resection, and ability to preserve pulmonary function.Citation4 Approximately 70% of lung cancer patients reported in many previous large-scale trials are treated with lobectomy.Citation5,Citation6
Since the 1990s, video-assisted thoracoscopic surgery (VATS) has emerged as a minimally invasive technique to gain access to the chest cavity, instead of dissecting the intercostal muscles and cutting off the ribs in traditional posterolateral thoracotomy.Citation7 VATS techniques have been dramatically developed and widely utilized in modern surgery for operable NSCLC, offering many more advantages to surgical patients than traditional thoracotomy, especially for pain and stress control, better cosmetic wounds, and preservation of pulmonary function.Citation7,Citation8 However, despite advances in surgical techniques and perioperative care, the morbidity rate remains as high as 24.9%–36.3%.Citation7–Citation9
A number of coexisting invasive clinicopathological variables may increase both morbidity and mortality rates and then cause adverse effects on the lung cancer prognosis. Several major comorbidities that frequently accompany NSCLC, such as COPD, diabetes mellitus (DM), and tuberculosis, and procedural stress responses, such as those related to neoadjuvant (induction) therapy, surgical approaches, and extent of surgery, have significantly unfavorable influences on surgical outcomes.Citation2,Citation5,Citation10–Citation13 All of these factors have been extensively explored in previous studies.
The degree of pulmonary fissure completeness (PFC) is an important issue that thoracic surgeons always deal with when performing VATS lobectomy. Current evidence indicates that incomplete interlobar fissures can increase the surgical difficulty and the probability of prolonged air leak (PAL).Citation14 However, the effects of PFC degree on other major in-hospital outcomes have never been discussed in the literature. A recent cohort study from Lee et alCitation15 confirmed the prognostic value of PFC in patients with stage I adenocarcinoma, but rarely mentioned its impact on in-hospital morbidity or mortality.
Objectives
In the present study, we quantified degrees of PFC with a modified scoring system during the intraoperative period. The primary purpose of this study was to evaluate the predictive roles of PFC for in-hospital morbidity and mortality following VATS lobectomy for operable NSCLC. Our secondary goal was to explore the effects of different degrees of PFC on length of hospital stay in patients undergoing VATS lobectomy.
Materials and methods
Ethics approval and consent to participate
This study was approved by the regional ethics committee of Sichuan University West China Hospital (2016-255), and all relevant procedures were in compliance with the Helsinki Declaration. Written informed consent forms, including the use of participant medical characteristics and files for research in this manuscript, were signed by all enrolled patients.
Study design
This was a single-center retrospective-cohort study conducted on data from a prospectively maintained database of medical records at our unit. It was written in compliance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.Citation16
Patient selection
Settings
We retrospectively analyzed clinical data of consecutive patients undergoing VATS lobectomy for operable NSCLC in our institution between August 2014 and October 2015. Their medical records were carefully reviewed, and all available data for perioperative characteristics and surgical outcomes were extracted for further analyses.
Inclusion and exclusion criteria
Target diseases were operable primary NSCLCs. Only standardized single-lobectomy operated on by a completely VATS procedure could be included. Pneumonectomy, bilobectomy, extended lobectomy, sleeve lobectomy, segmentectomy, or wedge resections by either thoracotomy or VATS procedure were considered. In addition, we further excluded data of patients who underwent conversion to thoracotomy during VATS lobectomy, because of the well-defined effects of unexpected conversion on morbidity risks,Citation9 in order to eliminate the confounding influence that might complicate the actual roles of PFC. Patients who received neoadjuvant therapy before surgery were excluded from our study, in order to avoid the confounding-bias risks caused by negative effects of neoadjuvant therapy on major in-hospital outcomes.Citation13 Patients had to have finished the entire clinical pathway according to our institutional policies during their hospitalization. Patients with loss of accurate records on estimated variables or outcomes were not considered.
Follow-up
The end points of our study belong to major in-hospital outcomes. Therefore, a follow-up would be provided on each patient until 30 days after surgery or death in the hospital.
Outcome data, measures, and definitions
We recorded and defined the following outcome data in the present study.
Preoperative variables
Patient baseline characteristics included age, gender, body-mass index, and smoking history (formal/current/never-smoker). Preoperative underlying comorbidities included COPD, tuberculosis, asthma, preoperative respiratory infection (PRI), hypertension, DM, coronary heart disease, previous malignancy, severe liver diseases, renal insufficiency, and steroid use. We defined PRI as the presence of one or more of the following infectious diseases: obstructive pneumonia, aspiration pneumonia, lung abscess, bronchiectasis, and respiratory bacterial/fungal infections. Severe liver diseases involved hepatitis B, hepatitis C, hepatocirrhosis, severe fatty liver and hepatic parasitic infections.Citation4,Citation7,Citation9
Intraoperative variables
Intraoperative variables estimated in the comparative analyses included tumor location, degree of pleural adhesion (none/light/moderate/severe), severity of pleural invasion (none/visceral/parietal), degree of PFC, and duration of surgery.Citation4,Citation7,Citation9
Assessment of pulmonary fissure completeness
Two board-certified surgeons with 8-year clinical experience quantified degrees of PFC independently during the intraoperative period using the fissure-development score system reported by Lee et alCitation15 in their cohort study. In cases of intraoperative mapping where the two surgeons disagreed, a consensus assessment would be determined by a third surgeon with 15-year experience.
For thoracic surgeons, it is widely accepted that the integrity of interlobar fissure in its depth is more clinically meaningful and worthy of evaluation than in its length when dissecting the pulmonary artery at the base of the oblique fissure. As such, in a this study, the fissure-development score was based on a modified assessment model based on the original classification for stratification of PFC:Citation17
grade 0 – fully complete fissure with well-separated lobes;
grade 1 – complete visceral cleft with more than 70% completeness of the interlobar fissure within the lung parenchyma;
grade 2 – partly evident visceral cleft with 30%–70% completeness of the interlobar fissure within the lung parenchyma;
grade 3 – no evident fissural line with absent 30% completeness of the interlobar fissure within the lung parenchyma.
As an important anatomical factor, PFs can be classified as upper major fissures (right/left), lower major fissures (right/left), and minor fissures (right). At least two of these interlobar fissures would be divided when performing VATS lobectomy. Therefore, the fissure sum average (FSA), an average of the fissure-development score of two dissected fissures, was utilized for further grouping and comparison.Citation4
Pathological variables
The following pathological parameters were estimated, including differentiation degree (low/moderate/high), tumor invasion (T status), lymph node-metastasis (N status), and tumour, node, and metastasis (TNM) stage, which were defined according to Union for International Cancer Control TNM Classification (seventh edition).Citation4,Citation7,Citation9
Postoperative outcomes
Primary outcomes of interest
Overall morbidity was defined by the presence of one or more postoperative complications according to the Clavien–Dindo classification system.Citation18 A range of postoperative complications were assigned to five morbidity grades in compliance with this classification system, as reported in our previous studies.Citation19–Citation21 Then, we defined minor morbidity as the presence of any Clavien–Dindo grade I–II complication, including pneumonia, atelectasis, hemoptysis, atrial arrhythmia, sinus irregularity, wound infection, delirium, gastrointestinal discomforts, urinary retention, PAL, subcutaneous emphysema, pneumothorax, and pleural effusion, all of which were cured by conservative treatment.
Clavien–Dindo grade III–IV complications, including severe atelectasis needing bronchoscopic aspiration, acute respiratory distress syndrome, chylothorax, pulmonary embolism, hemothorax, bronchial fistula, ventricular arrhythmia, PAL, subcutaneous emphysema, pneumothorax, and pleural effusion needing chest-tube replacement, thoracentesis, or reoperation, were categorized as major morbidities for further analysis. Furthermore, we defined in-hospital mortality as any death within 30 days after surgery or during the entire perioperative period, which was also regarded as a Clavien–Dindo grade V complication. All of these complications following VATS lobectomy were judged in compliance with US Society of Thoracic Surgeons and European Society of Thoracic Surgeons joint definitions.Citation22
Secondary outcomes of interest
Length of stay was calculated from the operation day to the discharge day. Duration of chest-tube drainage refers to days of pleural drainage.
Grouping criteria
With reference to the cutoff proposed by Lee et al,Citation15 patients were divided into two groups based on their FSA. Patients with an FSA >1 (1< FSA ≤3) were classified as group A, indicating that they had incomplete PFs. On the contrary, patients with an FSA of 0–1 were classified as group B, suggesting that they had good completeness of PFs. This cutoff of FSA >1 would be included in the multivariate logistic-regression model to stratify patients at high surgical risk.
Surgical procedure and perioperative care
VATS lobectomy with systematic mediastinal lymph-node dissection was performed via three-portal access using a single-direction thoracoscopic technique previously described by Liu et al:Citation23,Citation24 a modified “hilum-first-fissure last” technique that avoids early dissections through the fissural parenchyma. Staple-line buttressing was then implemented on the bronchial stumps of all enrolled patients.
Surgical patients were managed in compliance with our standardized clinical pathway, including comprehensive routine assessments, antibiotic prophylaxis, and pulmonary rehabilitation physiotherapy.Citation25 All these patients received intravenous patient-controlled analgesia for postoperative pain control. A chest tube was placed on the suction device (−20 cm H2O) at the end of the operation and then either alternated or removed according to our institutional policies. A chest radiograph was done to stratify levels of lung recruitment on postlobectomy day 1. Chest-tube removal was allowed with pleural drainage <200 mL in 24 hours and air leak (AL) cessation detected in the chest-drainage system. Analgesic management was stopped after removing the chest tube.Citation4,Citation7,Citation9
Statistical analysis
Continuous data are presented as the means with SDs and medians with IQRs, while categorical data are presented as patient numbers and percentages. In the univariate analysis, we used Pearson’s χ2 or Fisher’s exact test to compare categorical variables and Student’s t-test to compare mean values of continuous variables.
Then, a multivariate logistic-regression analysis was performed to identify independent risk factors for major in-hospital morbidities. Dichotomous data with univariate P<0.05 were put into the logistic-regression model, utilizing the Hosmer–Lemeshow test for precision and the C-statistic for calibration. Finally, ORs with 95% CIs were derived from multivariate logistic-regression analysis. Statistical significance was set at P<0.05. All statistical analyses were accomplished with SPSS 22.0 software (IBM Corporation, Armonk, NY, USA).
Results
Basic characteristics and outcomes
Patient characteristics
There were 563 patients meeting the eligibility criteria who underwent VATS lobectomy for primary NSCLC during the study period included in the present study. Patient baseline characteristics and surgical outcomes are outlined in . Our cohort consisted of 359 male (63.8%) and 204 female patients (36.2%), with a mean age of 62.2±8.3 years (median 62 years, IQR 57–69 years). Mean body-mass index was 23.4±2.9 kg/m2 (median 23.3 kg/m2, IQR 21.3–25.4 kg/m2). A total of 316 were active smokers (56.1%), and 403 patients (71.6%) suffered from one or more underlying comorbidities, as shown in . There were 237 patients that received adjuvant chemotherapy followed by VATS lobectomy (42.1%).
Table 1 Patient characteristics and outcomes
Tumors located in the right upper lobe (n=185) accounted for the largest proportion of the entire cohort – 32.9%. The mean operation time of the cohort was 129.3±42.4 (median 120, IQR 110–140) minutes. The majority of patients were diagnosed with stage I–II NSCLC (n=493, 87.5%). Lung adenocarcinoma accounted for 70.2% (n=395) of all enrolled cases, followed by squamous-cell carcinoma in 150 patients (26.6%), large-cell carcinoma in eleven (2%), and other subtypes of NSCLC in seven (1.2%). In addition, lymph-node metastasis was confirmed by pathological criteria after surgery in 150 patients (26.6%) ().
Degree of pulmonary fissure completeness
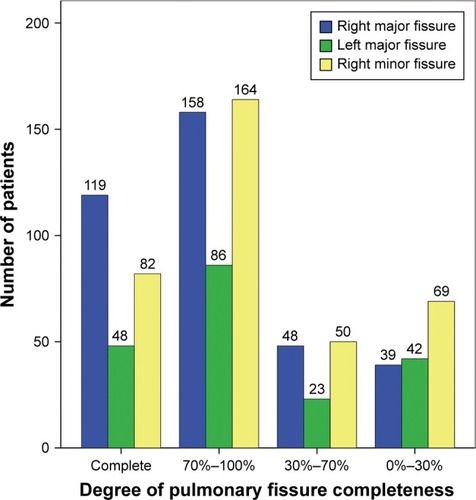
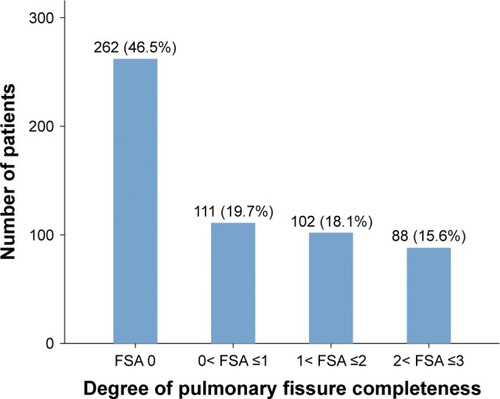
The degree of PFC estimated by fissure-development score in all enrolled patients is shown in . There were 262 patients with an FSA of 0 (46.5%), 111 patients with FSA 0–1 (19.7%), 102 patients with 1< FSA ≤2 (18.1%), and 88 patients with 2< FSA ≤3 (15.6%), respectively (). Therefore, a total of 190 patients were included in group A (33.7%), and the remaining 373 patients with FSA 0–1 were included in group B (66.3%).
Figure 2 Proportions of four different degrees of pulmonary fissure completeness.
When compared with group B patients, group A patients had significantly higher mean age (P<0.001) and duration of surgery (P<0.001), and higher rates of smoking history (P=0.041), tuberculosis (P=0.011), DM (P=0.003), malignancy history (P=0.016), right upper lobe-located lesion (P=0.002), dense pleural adhesion (P<0.001), and lowly differentiated tumors (P=0.006). No difference was found in the other clinicopathological variables between these two groups ().
Postoperative outcomes
A total of 298 postoperative complications developed in 172 patients, with an overall morbidity rate of 30.6%. Minor and major morbidity rates were 28.2% (n=159) and 7.5% (n=42), respectively. There were three deaths during the in-hospital period, with a mortality rate of 0.5%, as shown in . Incidence of individual complications is presented in . The five most frequent complications were PAL (n=72, 12.8%), pneumonia (n=69, 12.3%), atelectasis (n=37, 6.6%), subcutaneous emphysema (n=32, 5.7%), and pneumothorax (n=23, 4.1%). With regard to secondary outcomes, the mean duration of chest-tube drainage and length of stay of the entire cohort were 4.4±3 (median 3, IQR 2–6) and 6.9±3.5 (median 6, IQR 4–8) days, respectively.
Table 2 Individual postoperative complications
Effect of degree of PFC on postoperative complications
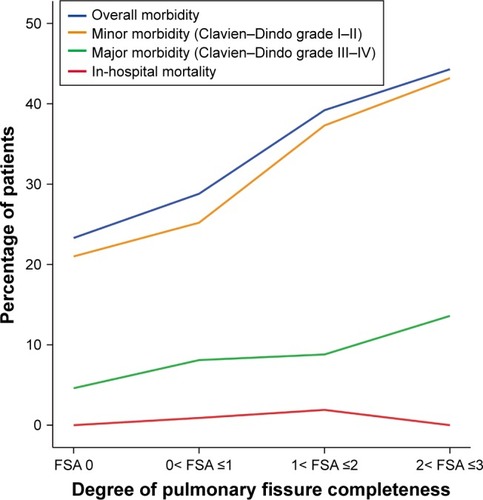
Overall morbidity and mortality
Percentages of both overall morbidity and in-hospital mortality distributed over four different degrees of PFC are shown in . The overall morbidity rate showed a steady tendency to increase with increasing FSA score, as shown in . There were 80 patients in the group A that developed postoperative complications, while 92 patients in group B had postoperative complications. The overall morbidity rate in group A patients was significantly higher than in group B patients (42.1% vs 24.7%, P<0.001; ). With regard to in-hospital mortality, two of three deaths belonged to group A, and the remaining one was from group B. No significant difference was found in mortality rate between these two groups (1.1% vs 0.3%, P=0.26; ).
Minor and major morbidity
Both minor and major morbidity rates were found to have a steady tendency to increase with increasing FSA score, as shown in . Compared to group B patients, group A patients had both significantly higher rates of minor morbidity (40.5% vs 22%, P<0.001; ) and major morbidity (11.1 vs 5.6%, P=0.021; ).
Individual complications
shows the five most frequent individual complications distributed over four different degrees of PFC. We found that only the incidence of pneumonia showed a steady tendency to increase with increasing FSA score. The incidence of PAL, atelectasis, subcutaneous emphysema, and pneumothorax all showed little fluctuation until reaching their peaks in the patients with 2< FSA ≤3.
Figure 4 Tendency of the five most frequent individual complications with increasing FSA.
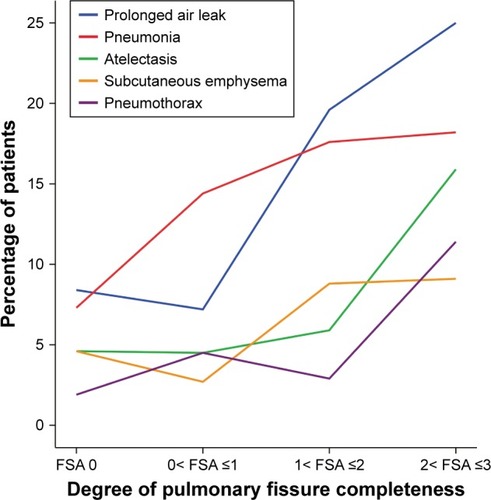
Incidence of individual complications between group A and group B is shown in . Incidence of five most frequent complications in group A patients, including PAL (22.1% vs 8%, P<0.001), pneumonia (17.4% vs 9.7%, P=0.008), atelectasis (10.5% vs 4.6%, P=0.007), subcutaneous emphysema (8.9% vs 4%, P=0.017), and pneumothorax (6.8% vs 2.7%, P=0.018) was significantly higher than group B patients. In addition, group A patients also had significantly higher incidence of pleural effusion (2.6% vs 0.5%, P=0.047) and atrial arrhythmia (2.6 vs 0.5%, P=0.047) than group B patients. No significant difference was found in other complications between these two groups.
Effect of degree of PFC on length of hospital stay
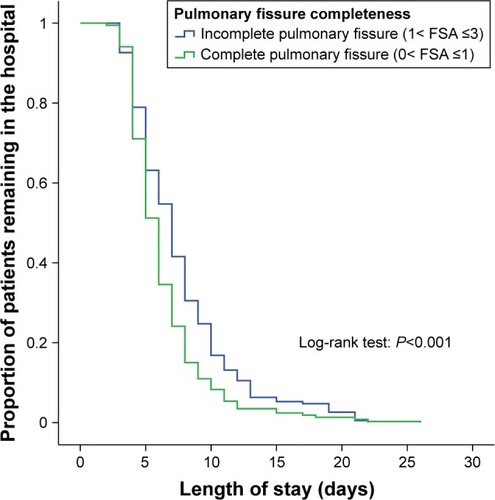
Length of stay
A Kaplan–Meier curve revealing the length of stay between group A and group B patients is shown in . The mean length of stay in group A (mean 7.7 days; 95% CI 7.1–8.2 days) was significantly longer than that group B (mean 6.4 days, 95% CI 6.1–6.7 days; log-rank P<0.001), indicating that incomplete degree of PFC was significantly associated with prolonged length of stay.
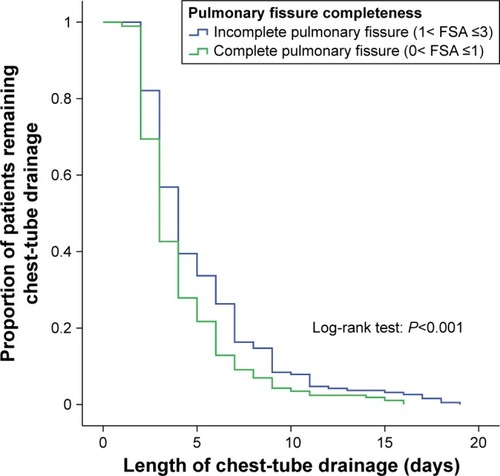
Duration of chest-tube drainage
shows the days of pleural drainage in groups A and B. Chest-tube duration in group A (mean 5.1 days, 95% CI 4.6–5.6 days) was significantly longer than group B (mean 4.1 days, 95% CI 3.8–4.4 days; log-rank P<0.001), revealing a significant relationship between incomplete PF and days of pleural drainage after surgery.
Univariate analyses on risk factors for postoperative morbidity
Relationships among perioperative patient characteristics and development of postoperative complications are shown in .
Table 3 Association between patient baseline characteristics and development of postoperative complications
Overall morbidity
Compared to patients without any complications, patients with any complications had significantly higher mean age (P<0.001), longer duration of surgery (P<0.001), and higher rates of male gender (P=0.006), COPD (P<0.001), PRI (P<0.001), hypertension (P=0.025), coronary heart disease (P=0.009), and incomplete PF (FSA >1, P<0.001).
Minor morbidity
Patients who developed any minor morbidity were found to have significantly higher mean age (P<0.001), longer duration of surgery (P<0.001), and higher rates of male gender (P=0.004), smoking history (P=0.009), COPD (P<0.001), PRI (P<0.001), hypertension (P=0.010), coronary heart disease (P=0.011), and incomplete PF (FSA >1, P<0.001) than those with no minor morbidity.
Major morbidity
Finally, when compared to patients with no major morbidity, patients who developed any major morbidity had significantly higher mean age (P=0.022), longer duration of surgery (P=0.006), and higher rates of male gender (P=0.038), PRI (P<0.001), steroid use (P=0.031), and incomplete PF (FSA >1, P=0.021), but significantly lower rates of DM (P=0.025) and lymph-node metastasis (P=0.025).
Multivariate analysis on risk factors for postoperative morbidity
Overall morbidity
A multivariate logistic-regression model including all eight significant clinical parameters was established, as shown in . The C-statistic for our logistic-regression model was 0.72, with a 95% CI of 0.67–0.77 (P<0.001), as shown in . Finally, we identified the PRI (OR 2.36, 95% CI 1.3–4.31; P=0.005), COPD (OR 1.83, 95% CI 1.17–2.87; P=0.009), incomplete PF (FSA >1, OR 2.08, 95% CI 1.38–3.14; P<0.001), and operation time >120 minutes (OR 2.37, 95% CI 1.55–3.65; P<0.001) as independent risk factors for overall morbidity after adjusting the confounding influence by the multivariate logistic-regression analysis.
Figure 7 Receiver-operating-characteristic analysis on discriminative power of multivariate logistic-regression model for predicting overall morbidity.
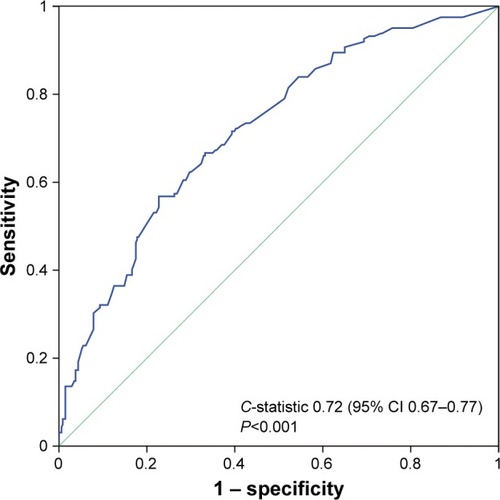
Table 4 Multivariate analysis of risk factors for postoperative complications
Minor morbidity
There were nine significant contributing factors derived from the univariate analysis, as shown in . The multivariate logistic-regression model involving these nine clinicopathological variables, with a C-statistic of 0.74 (95% CI 0.69–0.79; P<0.001), demonstrated that COPD (OR 1.95, 95% CI 1.22–3.11; P=0.005), PRI (OR 2.86, 95% CI 1.56–5.23; P=0.001), incomplete PF (FSA >1, OR 2.39, 95% CI 1.57–3.65; P<0.001), and operation time >120 minutes (OR 2.11, 95% CI 1.36–3.28; P=0.001) were independently predictive of the occurrence of minor morbidity.
Major morbidity
shows six high-risk contributing factors derived from the univariate analysis. According to the multivariate logistic-regression model involving these six clinicopathological parameters, which got a C-statistic of 0.69 (95% CI 0.6–0.77, P<0.001), history of PRI (OR 3.37, 95% CI 1.51–7.54; P=0.003), incomplete PF (FSA >1, OR 2.06, 95% CI 1.07–3.95; P=0.031), and operation time >120 minutes (OR 2.95, 95% CI 1.3–6.72; P=0.01) were finally found to be independent risk factors for major morbidity.
Discussion
Key results
The main findings of the present study were that patients with incomplete PFs had significantly higher overall morbidity rate, minor morbidity rate, and major morbidity rate than patients with well-developed PFs. Incomplete degree of PFC was significantly associated with higher incidence of PAL, pneumonia, atelectasis, subcutaneous emphysema, pneumothorax, pleural effusion, and atrial arrhythmia. Patients with incomplete PFs were also found to have significantly prolonged length of stay and pleural drainage than patients with well-developed PFs. The multivariate logistic-regression analysis demonstrated that incomplete degree of PFC was a strongly independent risk factor for overall morbidity, minor morbidity, and major morbidity.
Interpretations
Nowadays, the identification and measurement of PFC can be achieved by several perioperative methods. Imaging techniques, especially high-resolution computerized tomography (CT) and multidetector CT, with their 3-D reconstructions, have mostly been used to assess the integrity of interlobar fissures preoperatively, although these computerized methods still need to be validated, especially in patients with heterogeneously diseased lungs.Citation26,Citation27 By visual inspection of thin-section CT images, an interlobar fissure can be seen as a thin line that separates neighboring lobes, while the line is generally disconnected by vessels or lung parenchyma in cases of incompleteness. Perioperative mapping is performed only on patients with serious pulmonary diseases, although this method depends on the surgeon’s area of interest and expertise.Citation26 An autopsy can also help to distinguish adjacent lobes, but with the obvious drawback that it can only be performed on eviscerated lungs postoperatively.
Prior studies have demonstrated that incomplete degree of PFC was a major risk factor for PAL, but showed no evidence of other major in-hospital outcomes.Citation14,Citation28,Citation29 The earliest studyCitation30 indicated that incomplete PFs had no impact on 5-year survival of resected NSCLC. However, no detail about in-hospital outcome was presented in that study. In an another cohort of 297 stage I adenocarcinoma patients, Lee et alCitation15 evaluated the prognostic roles of degree of PFC, and concluded that incomplete PFs had adverse effects on 5-year survival, but showed no significant impact on either in-hospital morbidity or mortality. Effects of PFC on any individual complication were not further reported.
In our series, we quantified degree of PFC by using the fissure-development score that was reported by Lee et al,Citation15 and recorded details in the intraoperative period. The FSA of two dissected fissures during VATS lobectomy was calculated and then utilized to establish our grouping criterion. As Lee et alCitation15 previously described, FSA >1 was chosen as the cutoff to indicate the incomplete degree of PFC. After that, we found a significantly higher incidence of PAL in patients with incomplete PFs, which was similar to results in the literature.Citation25 In addition, we further identified that patients with incomplete interlobar fissures had significantly higher incidence of pneumonia, atelectasis, subcutaneous emphysema, pneumothorax, pleural effusion, and atrial arrhythmia than patients with complete interlobar fissures. However, no significant difference was found in in-hospital mortality between patients with incomplete PFs and incomplete PFs. We speculated that the possible reason may have been the limited sample availability for in-hospital deaths (n=3) in our cohort, resulting in attenuated analytical power when evaluating the effects of degree of PFC on in-hospital mortality.
We further utilized the Clavien–Dindo classification system to categorize all postoperative complications into minor and major morbidity. Incomplete degree of PFC was found to be significantly associated with risks of minor and major morbidity. Kaplan–Meier analysis further showed that both length of stay and chest-tube drainage in patients with incomplete PFs were significantly longer than in patients with complete PFs. That may be because of their higher probability of postoperative morbidity, which needs much more time to be treated in hospital. Finally, after minimizing bias risks from other confounding factors by multivariate logistic-regression analysis, incomplete degree of PFC was considered a strongly independent risk factor for overall morbidity, minor morbidity, and major morbidity after VATS lobectomy. We speculate the following three reasons might be considered when trying to explain this phenomenon.
First, when performing VATS lobectomy, division of lung parenchyma overlying the artery within the fused interlobar fissures could easily produce an AL, because this procedure is usually finished by using electrocautery or blunt/sharp dissection.Citation31,Citation32 If alveolar ALs persist without timely and effective control, a devastating PAL, sometimes with induced pneumothorax, can seriously impair lung recruitment, resulting in the development of pulmonary edema, pneumonia, and pleural effusion. Therefore, patients with poorly developed PFs are more likely to have a much higher overall morbidity rate.
Second, another important disease that may predispose to the occurrence of postoperative complications is respiratory inflammation. On the one hand, both history of COPD and PRI were found to be predictive of overall morbidity in our multivariate logistic-regression models. The proportion of fused PFs was generally higher in both patients with COPD and those with PRI, which may be attributable to the excessive exudation induced by chronic inflammatory responses. The reduced area of gas exchange and inadequate distance from the alveolar surface to the capillary endothelium in such patients with less discrete interlobar fissures can lead to a large decline in cardiopulmonary functional reserve and increase morbidity risks.Citation4
On the other hand, the presence of pleural adhesions, which can increase the risks of PAL and pleural morbidity following elective pulmonary resections,Citation33 was found in 29.7% of all our enrolled cases. History of COPD and PRI was more frequent in the patients with pleural adhesions, revealing a close relationship between severity of pleural adhesions and inflammatory responses of the chest. More importantly, patients with less discrete PFs were found to have a significantly higher probability of dense pleural adhesions. That might because the inflammatory process rarely spared the visceral pleura over the internal chest wall or within the interlobar fissures. Therefore, such patients with incomplete PFs were more likely to suffer from pleural and procedural morbidities.
Finally, since PF acts as an interface between neighboring lobes, we hypothesize that the poor integrity of PFs may hinder potential interlobar collateral ventilation, which helps to maintain a certain degree of pulmonary function within obstructive areas.Citation34 Although there is no conclusive evidence supporting this assumption, a large decline in pulmonary functional reserve in patients with poorly developed PFs has the ability to increase the risk of morbidity.
Generalizability
In clinical practice, comprehensive risk assessment can affect surgeons’ decisions on surgical procedures. According to our study results, it may be more efficient to integrate the degree of PFC into an assessment model to stratify the surgical risk of lung cancer patients, in order to decide the best therapeutic strategy. In addition, for young surgeons, it may help to select participants in their early learning curve or a teaching program of VATS techniques, in order to avoid a high morbidity rate and train themselves more effectively.
Limitations
Several limitations must be taken into account regarding the interpretations. First, the present study was subject to the inherent limitations of any single-center retrospective-cohort study. Potential selection bias might still complicate our findings, although a multivariate logistic-regression analysis involved as many clinicopathological variables as possible to attempt to minimize the bias risks from potential confounding factors. Second, the sample in our single-center study was relatively small, which may limit the analytical power. Third, there has been no standardized assessment tool on the degree of PFC until now. The fissure-development score and corresponding cutoff proposed by Lee et alCitation15 were consulted and modified in our cohort study, but essentially this subjective intraoperative mapping may be limited by the surgeon’s area of interest and expertise. Fourth, morbidity rate can also depend on surgeon experience. However, it may be difficult to conduct a quantitative analysis on this artificial factor appropriately. This is another important limitation that cannot be ignored. Fifth, according to our primary objectives, only VATS lobectomy was analyzed, so our findings may not be generalized to sublobar resections. Finally, complete details of laboratory indices were not available in all enrolled patients, so their relevant clinical significance was not analyzed.
Conclusion
The present study demonstrates that degree of PFC is an excellent categorical predictor of overall morbidity, minor morbidity, and major morbidity in patients undergoing VATS lobectomy for NSCLC. Incomplete degree of PFC is also significantly associated with prolonged duration of chest-tube drainage and length of stay following VATS lobectomy. Our results suggest that degree of PFC should be considered when informing surgical patients about the morbidity risks and selecting suitable cases in a surgeon’s early learning curve of VATS techniques. Large-scale cohort studies are needed further to confirm and modify our findings in the future.
Acknowledgments
Special thanks to the English-language-polishing contributions from Mr Stanley Crawford, Institution of Medical English, West China Medical School of Sichuan University, Chengdu, China. This study was supported by the Foundation of Science and Technology Support Plan Department of Sichuan Province (2015SZ0158).
Disclosure
The authors report no conflicts of interest in this work.
References
- XuYDingVWZhangHZhangXJablonsDHeBSpotlight on afatinib and its potential in the treatment of squamous cell lung cancer: the evidence so farTher Clin Risk Manag20161280781627307741
- LiSWangZHuangJSystematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist?Eur J Cardiothorac Surg201751581782828040677
- LeADAlzghariSKJeanGWLa-BeckNMUpdate on targeted therapies for advanced non-small cell lung cancer: nivolumab in contextTher Clin Risk Manag20171322323628260909
- LiSZhouKWangMLinRFanJCheGDegree of pulmonary fissure completeness can predict postoperative cardiopulmonary complications and length of hospital stay in patients undergoing video-assisted thoracoscopic lobectomy for early-stage lung cancerInteract Cardiovasc Thorac Surg2018261253329049746
- FernandezFGKosinskiASBurfeindWThe Society of Thoracic Surgeons lung cancer resection risk model: higher quality data and superior outcomesAnn Thorac Surg2016102237037727209606
- ThomasPABerbisJFalcozPENational perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional statusEur J Cardiothorac Surg201445465265924062351
- LiSZhouKDuHShenCLiYCheGBody surface area is a novel predictor for surgical complications following video-assisted thoracoscopic surgery for lung adenocarcinoma: a retrospective cohort studyBMC Surg2017176928606134
- LaursenLØPetersenRHHansenHJJensenTKRavnJKongeLVideo-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomyEur J Cardiothorac Surg201649387087526088592
- LiSJZhouKShenCBody surface area: a novel predictor for conversion to thoracotomy in patients undergoing video-assisted thoracoscopic lung cancer lobectomyJ Thorac Dis2017982383239628932543
- IachinaMJakobsenEMøllerHThe effect of different comorbidities on survival of non-small cells lung cancer patientsLung2015193229129725516286
- LiSJFanJZhouJRenYTShenCCheGWDiabetes mellitus and risk of bronchopleural fistula after pulmonary resections: a meta-analysisAnn Thorac Surg2016102132833927063612
- LiSJZhouXDHuangJLiuJTianLCheGWA systematic review and meta-analysis: does chronic obstructive pulmonary disease predispose to bronchopleural fistula formation in patients undergoing lung cancer surgery?J Thorac Dis2016871625163827499951
- LiSFanJLiuJNeoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14 912 patientsJpn J Clin Oncol201646653454627052116
- LiSJZhouKLiYJEfficacy of the fissureless technique on decreasing the incidence of prolonged air leak after pulmonary lobectomy: a systematic review and meta-analysisInt J Surg20174211028414119
- LeeSLeeJGLeeCYKimDJChungKYPulmonary fissure development is a prognostic factor for patients with resected stage I lung adenocarcinomaJ Surg Oncol2016114784885227633283
- von ElmEAltmanDGEggerMStrengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studiesBMJ2007335762480680817947786
- CraigSRWalkerWSA proposed anatomical classification of the pulmonary fissuresJ R Coll Surg Edinb19974242332349276555
- DindoDDemartinesNClavienPAClassification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a surveyAnn Surg2004240220521315273542
- LaiYHuangJYangMSuJLiuJCheGSeven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trialJ Surg Res2017209303628032568
- HuangJLaiYGaoKSurfactant protein D: a sensitive predictor for efficiency of preoperative pulmonary rehabilitationInt J Surg20174113614228385654
- LiPLaiYZhouKCheGAnalysis of postoperative complications and risk factors of patients with lung cancer through Clavien-Dindo classificationChin J Lung Cancer2017204264271
- FernandezFGFalcozPEKozowerBDSalatiMWrightCDBrunelliAThe Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminologyAnn Thorac Surg201599136837625555970
- LiuLCheGPuQA new concept of endoscopic lung cancer resection: Single-direction thoracoscopic lobectomySurg Oncol2010192e71e7719500971
- LiuCPuQGuoCNon-grasping en bloc mediastinal lymph node dissection for video-assisted thoracoscopic lung cancer surgeryBMC Surg2015153825884998
- LaiYSuJQiuPSystematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trialInteract Cardiovasc Thorac Surg201725347648328520962
- SedlackovaZCtvrtlikFMiroslavHPrevalence of incomplete inter-lobar fissures of the lungBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub2016160449149427829688
- Koenigkam-SantosMde PaulaWDOwsijewitschMIncomplete pulmonary fissures evaluated by volumetric thin-section CT: semi-quantitative evaluation for small fissure gaps identification, description of prevalence and severity of fissural defectsEur J Radiol201382122365237024016827
- BrunelliACassiviSDHalgrenLRisk factors for prolonged air leak after pulmonary resectionThorac Surg Clin201020335936420619226
- StamenovicDBostanciKMesserschmidtAJahnTSchneiderTFissureless fissure-last video-assisted thoracoscopic lobectomy for all lung lobes: a better alternative to decrease the incidence of prolonged air leak?Eur J Cardiothorac Surg201650111812326792925
- KamiyoshiharaMKawashimaOSakataSHiraiTIshikawaSMorishitaYDoes an incomplete interlobar fissure influence survival or recurrence in resected non-small-cell lung cancer?Lung Cancer1999251333810466860
- DrahushNMillerADSmithJSRoyerAMSpivaMHeadrickJRJrStandardized approach to prolonged air leak reduction after pulmonary resectionAnn Thorac Surg201610162097210127083245
- LiSLvWZhouKCheGDoes the fissureless technique decrease the incidence of prolonged air leak after pulmonary lobectomy?Interact Cardiovasc Thorac Surg201725112212428379438
- KouritasVKKefaloyannisETcherveniakovPDo pleural adhesions influence the outcome of patients undergoing major lung resection?Interact Cardiovasc Thorac Surg201725461361928962506
- MarshallHDeppeMHParra-RoblesJDirect visualisation of collateral ventilation in COPD with hyperpolarized gas MRIThorax201267761361722286930