Abstract
Background
The association of ABO blood group with prognosis of several malignancies has been established. However, its role in hepatocellular carcinoma (HCC) remains unclear.
Patients and methods
In this study, we investigated the prognostic role of ABO blood group in unresectable HCC patients receiving transarterial chemoembolization (TACE) as an initial treatment. Medical records of 2,611 HCC patients were collected, and clinical data of 282 unresectable HCC patients receiving TACE were ultimately analyzed retrospectively. A prognostic nomogram was generated for predicting 1-, 2-, and 3-year overall survival (OS) probability. A total of 114 (40.4%), 69 (24.5%), 64 (22.7%), and 35 (12.4%) HCC patients had blood groups O, A, B, and AB, respectively.
Results
The median OS times for patients with blood groups O, A, B, and AB were 24, 23, 20, and 20 months, respectively. Patients with blood group AB (hazard ratio [HR]=2.050, 95% confidence interval [CI], 1.331–3.157, P=0.001) or group non-O (HR=1.479, 95% CI, 1.110–1.972, P=0.008) had a poorer OS than those with blood group O. The prognostic nomogram, with a c-index of 0.701, was modest in predicting OS of unresectable HCC patients.
Conclusion
Patients with non-O blood group, particularly blood group AB, had a worse OS compared with those having blood type O. ABO blood group can predict the prognosis in patients with unresectable HCC undergoing TACE as an initial therapy. Further external validation in larger cohorts is necessary to confirm their usefulness in clinical practice.
Introduction
Hepatocellular carcinoma (HCC) has been identified as one of the most common and lethal malignancies worldwide.Citation1 Despite advances in diagnosis and treatment of HCC, many patients with HCC still present with intermediate or advanced disease at diagnosis and thus cannot undergo surgical resection. For these patients, transarterial chemoembolization (TACE) is the mainstay of treatment strategy.Citation2–Citation4 However, median survival for these individuals is only ∼1 year, much lower than expected.Citation5 In addition, the prognoses of patients with unresectable stage HCC are variable due to the high heterogeneity in these populations.Citation6 Therefore, efforts to identify new additional predictive markers are of great importance to improve the surveillance of prognosis for unresectable HCC.
Recently, there is increasing evidence showing that the ABO blood group is involved in the pathophysiology of various diseases such as cardiovascular and neoplastic disorders.Citation7,Citation8 The correlations of ABO blood group with survival have been evaluated in various types of malignancies, including nasopharyngeal carcinoma,Citation9 ovarian cancer,Citation10,Citation11 colon cancer,Citation12 and breast cancer.Citation13 A risk of developing cancer of the pancreas, stomach, and pharynx has been shown using 1.6 million blood donors from Swedish and Danish populations.Citation14 Also, another study showed that blood group non-O increased the risk of HCC.Citation15 However, to date, very little is known concerning the association between ABO blood group and the survival for unresectable HCC patients.
To bridge the abovementioned gap, we performed a retrospective study to investigate the prognostic impact of ABO blood group in unresectable HCC patients who underwent TACE as their initial treatment in the Chinese population. The results of this study showed that patients with blood group non-O, particularly blood group AB, had a worse overall survival (OS) than those with blood type O, suggesting that ABO blood group might be used for prognostic stratification and individualized surveillance.
Patients and methods
Patients
This investigation was approved by the institutional review board (IRB) of the First Affiliated Hospital of Xi’an Jiaotong University. Written informed consent was obtained from all enrolled patients before the treatment. The methods were performed in accordance with the approved guidelines. After obtaining IRB approval, we retrospectively reviewed medical records of 2,611 patients with HCC between January 2008 and August 2013 at our institution. The inclusion criteria were as follows: patients whose initial treatment is TACE; patients without extra-hepatic spread; patients in whom liver function is Child–Pugh A and B; and patients without any other malignant tumors. Patients with unresectable HCC met at least one of the following criteria: patient’s refusal resection, multiple lesions of the liver, and the presence of invasion of major blood vessels. Among 2,611 HCC patients in the database, a total of 282 unresectable HCC patients receiving TACE as an initial therapy were ultimately included.
TACE procedures
TACE procedures were performed by two experienced interventional radiologists following the standard practice.Citation16 Briefly, hepatic arteriography was conducted to evaluate the tumor vessels. Subsequently, a catheter was selectively inserted into tumor-feeding artery to close the tumor. A mixture of lipiodol and chemotherapeutic agents (epirubicin and cisplatin) was administered into the tumor-feeding branch. The doses of anticancer agents were adjusted according to liver function and vasculature of the tumor. TACE procedures were repeated thereafter until the tumor was stable.
Clinical variables
Factors that may potentially be related to survival were collected, including age, sex, place of residence, liver cirrhosis, tumor size, differentiation, stage, Child–Pugh grades, hepatitis status, ascites, and pre-operative serum α-fetoprotein (AFP) levels. Information on alcohol consumption and tobacco use was obtained from the patients. Their blood types were obtained from the medical records in the hospital.
Study end points and survival data
Serum AFP level, liver function, and computed tomography (CT) were performed every 3–4 months to assess treatment response. OS was defined as the time from the start of TACE to death from any cause or the last follow-up date. Patients who were still alive at the end of our follow-up were defined as censored data. Outcome data were collected until October 2014 or patient’s death.
Statistical analysis
Data were analyzed using SPSS 23.0 for Windows (IBM Corporation, Armonk, NY, USA). Prognostic nomogram was constructed and analyzed by R 3.3.1 with rms packages.Citation39 Categorical data were grouped and analyzed by using the Pearson χ2 test. The Kaplan–Meier method was applied to estimate the cumulative OS rate, and the differences in survival distributions were tested by the log-rank test. Variables that affected OS (P-value <0.05) on univariate analysis were subsequently included in multivariate analysis by using Cox’s proportional hazards model. All tests were two-sided, and statistical significance was set at P<0.05.
Results
Clinical characteristics
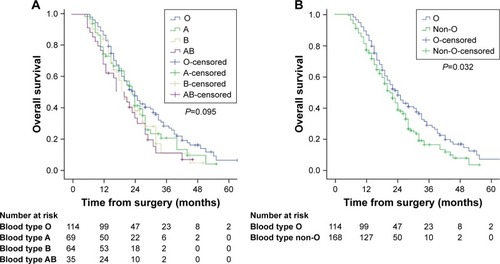
The basic characteristics of enrolled patients are listed in . This study included 282 HCC patients (230 males and 52 females) following TACE as an initial treatment with a median age of 56 years (range: 22–82 years). The distribution of ABO blood group was as follows: O in 114 (40.4%) patients, A in 69 (24.5%), B in 64 (22.7%), and AB in 35 (12.4%). Patient baseline characteristics were well balanced across individual ABO blood group, but significant differences were found between patients with blood type O and non-O group in terms of tumor staging ().
Table 1 Clinicopathological characteristics of patients, stratified by ABO blood type
Correlation of OS with ABO blood group status
Among the 282 patients, there were 212 deaths after a median follow-up of 22 months (range: 4–67 months). The 1-, 2-, and 3-year OS rates for the cohort were 81.2%, 43.2%, and 22.6%, respectively. The median OS times were as follows: blood group O, 24 months (95% confidence interval [CI], 20.05–27.95 months); blood group A, 23 months (95% CI, 19.83–26.16 months); blood group B, 20 months (95% CI, 17.23–22.76 months); blood group AB, 20 months (95% CI, 14.52–25.47 months). As shown in , there were no statistically significant differences between individual ABO blood group and OS (P=0.095). Notably, patients with blood group O had more favorable OS than patients with blood group A, B, or AB. Therefore, we divided the entire cohort into two subgroups (blood group O vs non-O [A, B, and AB]). The median OS was 24 months (95% CI, 20.05–27.95 months) for patients with blood group O and 21 months (95% CI, 18.46–23.53 months) for patients with blood group non-O. As shown in , the OS of patients with blood groups O and non-O was significantly different (P=0.032).
Univariate and multivariate analyses
The univariate analyses suggested that Child–Pugh stage (P<0.001), serum AFP concentration (P=0.001), portal vein tumor thrombus (P=0.050), stage (P<0.001), ascites (P=0.001), tumor size (P=0.006), and blood group (group AB vs group O, P=0.028; group non-O vs group O, P=0.037) were prognostic predictors of OS. Further multivariate analyses identified serum AFP level, tumor stage, tumor size, and ascites as significant prognostic factors of OS. Specifically, compared to blood group O, the hazard ratios (HRs) for patients with blood group AB or non-O were 2.050 (95% CI, 1.331–3.157, P=0.001) and 1.479 (95% CI, 1.110–1.972, P=0.008), respectively, suggesting that these HCC patients with blood group AB or non-O have a worse survival. The details of this analysis are depicted in .
Table 2 Cox regression analysis of potential prognosticators
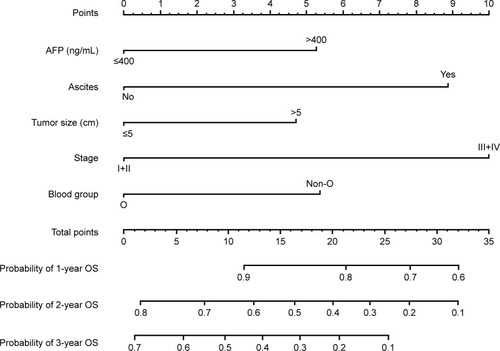
Prognostic nomogram for OS
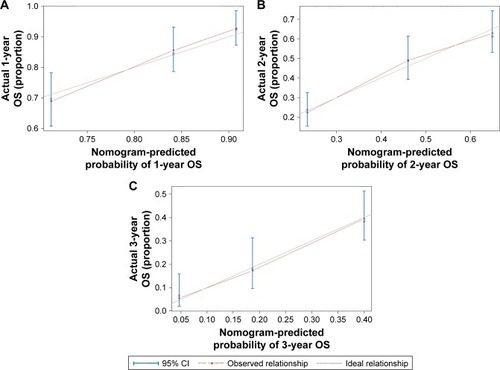
As shown in , we further conducted a nomogram to predict the probability of death for individual HCC patients within 1, 2, and 3 years after TACE. The significant prognostic variables (AFP level, tumor stage, tumor size, ascites, and blood group) were used to build the nomogram. Calibration curves for nomogram are shown in , which performed well with the ideal model. Harrell’s c-index was 0.701 for OS, indicating that the nomogram had an excellent predictive accuracy.
Discussion
Prognostic biomarkers are expected to guide therapeutic options and surveillance strategies. Although prognostic factors have been identified for the whole HCC population, including AFP, tumor size, vascular invasion, and cirrhosis, useful clinical factors are not validated for predicting the outcomes of unresectable HCC receiving TACE.Citation17 Recently, ABO blood group has been reported to correlate with increasing the risk of HCC.Citation18,Citation19 However, few investigations have examined its prognostic role in unresectable HCC patients following TACE. Therefore, we assess the association of ABO blood group and the prognosis of unresectable HCC patients who underwent TACE as an initial treatment in the Chinese population for the first time.
In this study, the results suggested that patients with AB blood group or non-O have a poorer OS than those with blood group O. Also, we developed a novel nomogram incorporating tumor stage, tumor size, ascites, serum AFP level, and blood type, which predicts OS for HCC patients receiving TACE more precisely. Our results are consistent with the previous findings in nasopharyngeal carcinoma,Citation20 pancreatic, and renal cancer patients.Citation21,Citation22 These studies indicated that the clinical outcome of patients with blood O is better than that of non-O patients. Particularly, individuals with blood group non-O may have a genetic background that predisposes to the risk of cancer. A recent large-scale epidemiologic study reported that blood group A correlated with the elevated risk of stomach cancer and blood group non-O correlated with the development of pancreatic cancer.Citation23 Similarly, Kakava et alCitation24 showed that head and neck cancer was more common in a population with blood group A compared to other blood groups. Another study indicated that blood group A was related to the increased risk of developing glioblastoma, while blood group O reduced the risk.Citation25 However, some research suggested that ABO blood group had no significant prognostic impact on non-small-cell lung cancer,Citation26 urothelial carcinoma of the bladder,Citation27 and triple-negative breast cancer.Citation28 Racial variability, heterogeneity of blood group distribution, and differences in demographics may lead to these conflicting results.
The underlying mechanisms by which ABO blood group influences the survival of HCC patients remain largely unknown. Several hypotheses may explain the underlying biological basis. ABO antigens have been recognized for their role as cancer-associated antigens.Citation29,Citation30 Terada and NakanumaCitation31 revealed that ABO antigens are usually present on HCC tissue but not expressed on normal liver. Thus, the aberrant antigen changes might be involved in HCC progression. Second, non-O blood group individuals have higher circulating plasma levels of vWF and factor VIII, and consequently contribute to developing venous thromboembolism.Citation32 These coagulation factors are modulators of angiogenesis and are involved in carcinogenesis.Citation33 In addition, hypercoagulability plays significant roles in tumor metastasis and poor prognosis.Citation34 Third, there is a close link between blood group antigens and systemic inflammation response, which facilitates the development and progression of HCC. Studies revealed that single-nucleotide polymorphisms (SNPs) at the ABO gene locus correlated with circulating levels of inflammatory markers, including tumor necrosis factor-alpha (TNF-α) and soluble intercellular adhesion molecule-1 (sICAM-1).Citation35 These inflammatory cytokines are involved in the regulation of hepatocarcinogenesis. Particularly, sICAM-1 has been associated with occurrence and prognosis of HCC.Citation36 The low sICAM-1 level may promote tumor progression.Citation37 Individuals with non-O blood group express low level of sICAM-1 in comparison with those with blood group O.Citation38 These biological bases may partially explain the correlation of blood group with OS of HCC.
To the best of our knowledge, there are few reports on the association of blood group and survival for HCC. Moreover, it remains unclear whether blood group plays a role in the biological behavior of HCC cells or the TACE surgery process. Further investigations are needed to figure out these questions. The present study can provide an important reference for future research and can inspire more works to explore the correlation and mechanism between blood group and prognosis of HCC.
There are several limitations in this study. First, the patient cohort in this retrospective study is not representative of the general population. Selection bias may exist. Second, although ABO blood group is linked to race, the ethnic makeup in this study is Chinese, perhaps limiting the universality of our findings. Third, because sample size of the study was relatively small, we did not explore the impact of ABO blood group on survival based on different clinicopathological features. Despite the abovementioned limitations, the results of this study expand the current understanding of the relationship between ABO blood group and prognosis of HCC. Obtaining serum blood type is convenient; the effect of blood group on tumor biology or response to therapy should be further explored.
Conclusion
ABO blood group correlates with the prognosis of patients receiving TACE as an initial treatment for unresectable HCC. Patients with blood group non-O, particularly blood group AB, had a worse OS than those with blood group O. Further clinical and experimental investigations are necessary to confirm this association and to elucidate the related mechanisms.
Acknowledgments
This study was supported by the National Natural Science Foundation of China Program (No 81372581).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2018CA Cancer J Clin201868173029313949
- TsurusakiMMurakamiTSurgical and locoregional therapy of HCC: TACELiver Cancer20154316517526675172
- YangBLiCLGuoWHIntra-arterial ethanol embolization augments response to TACE for treatment of HCC with portal venous tumor thrombusBMC Cancer201818110129378532
- JiangBGWangNHuangJTumor SOCS3 methylation status predicts the treatment response to TACE and prognosis in HCC patientsOncotarget2017817286212862728404963
- HuoYREslickGDTranscatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysisJAMA Oncology20151675676526182200
- MokdadAAMurphyCCPruittSLEffect of hospital safety net designation on treatment use and survival in hepatocellular carcinomaCancer2018124474375129072773
- LiumbrunoGMFranchiniMBeyond immunohaematology: the role of the ABO blood group in human diseasesBlood Transfus201311449149924120598
- FranchiniMMengoliCLippiGRelationship between ABO blood group and pregnancy complications: a systematic literature analysisBlood Transfus201614544144827177402
- PengHChenLLiWFPrognostic correlations between ABO blood group and pre-treatment plasma Epstein-Barr virus DNA in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapyPLoS One20161111e016619427835689
- ZhouJYangLCHeZYLiFYWuSGSunJYPrognostic impact of ABO blood group on the survival in patients with ovarian cancerJ Cancer201561097097526316893
- CozziGDLevinsonRTTooleHBlood type, ABO genetic variants, and ovarian cancer survivalPLoS One2017124e017511928448592
- CaoXWenZSSunYJLiYZhangLHanYJPrognostic value of ABO blood group in patients with surgically resected colon cancerBr J Cancer2014111117418024901236
- ParkSKimKSKimJSKorean Breast Cancer SocietyPrognostic value of ABO blood types in young patients with breast cancer; a nationwide study in Korean Breast Cancer SocietyMed Oncol201734611828500618
- VasanSKHwangJRostgaardKABO blood group and risk of cancer: a register-based cohort study of 1.6 million blood donorsCancer Epidemiol201644404327459465
- IavaroneMDella CorteCPelucchiCRisk of hepatocellular carcinoma in relation to ABO blood typeDig Liver Dis2016481949626611335
- TsurusakiMMurakamiTSurgical and locoregional therapy of HCC: TACELiver Cancer20154316517526675172
- FarinatiFVitaleASpolveratoGDevelopment and validation of a new prognostic system for patients with hepatocellular carcinomaPLoS Med2016134e100200627116206
- LiXXuHDingZJinQGaoPAssociation between ABO blood group and HCV-related hepatocellular carcinoma risk in ChinaMedicine20169549e558727930575
- WuTMaXAWangGQABO blood type correlates with survival in hepatocellular carcinoma following hepatectomySci Rep201771441228667286
- OuyangPYSuZMaoYPLiuQXieFYPrognostic value of ABO blood group in southern Chinese patients with established nasopharyngeal carcinomaBr J Cancer201310992462246624022193
- RahbariNNBorkUHinzUAB0 blood group and prognosis in patients with pancreatic cancerBMC Cancer20121231922838843
- KoKParkYHJeongCWKuJHKimHHKwakCPrognostic significance of blood type A in patients with renal cell carcinomaUrol J20161342765277227576883
- SunWWenCPLinJABO blood types and cancer risk – a cohort study of 339,432 subjects in TaiwanCancer Epidemiol201539215015625600007
- KakavaKKarelasIKoutrafourisIRelationship between ABO blood groups and head and neck cancer among Greek patientsJ BUON201621359459627569078
- AllouhMZAl BarbarawiMMHiasatMYAl-QarallehMAAbabnehEIGlioblastoma and ABO blood groups: further evidence of an association between the distribution of blood group antigens and brain tumoursBlood Transfus201615654354727416574
- UnalDErogluCKurtulNOguzATasdemirAKaplanBABO blood groups are not associated with treatment response and prognosis in patients with local advanced non-small cell lung cancerAsian Pac J Cancer Prev20131463945394823886212
- KlatteTXylinasERiekenMEffect of ABO blood type on mortality in patients with urothelial carcinoma of the bladder treated with radical cystectomyUrol Oncol201432562563024495451
- YuJGaoFKlimbergVSMargenthalerJAABO blood type/Rh factor and the incidence and outcomes for patients with triple-negative breast cancerAnn Surg Oncol201219103159316422878611
- DuanYFZhuFLiXDAssociation between ABO gene polymorphism (rs505922) and cancer risk: a meta-analysisTumour Biol20153675081508725656610
- WangFMZhangYZhangGMLiuYNSunLJLiuYAssociation of ABO blood types and clinicopathological features of prostate cancerDis Markers20172017923748129129952
- TeradaTNakanumaYExpression of ABH blood group antigens, receptors of Ulex europaeus agglutinin I, and factor VIII-related antigen on sinusoidal endothelial cells in adenomatous hyperplasia in human cirrhotic liversHum Pathol19912254864931851720
- ShahsavarzadehTJavanmardSHSaadatniaMImpact of factor VIII and von Willebrand factor plasma levels on cerebral venous and sinus thrombosis: are they independent risk factors?Eur Neurol201166424324621967990
- ZhouSWelsbyIIs ABO blood group truly a risk factor for thrombosis and adverse outcomes?World J Cardiol20146998599225276299
- TsimafeyeuIVDemidovLVMadzhugaAVSomonovaOVYelizarovaALHypercoagulability as a prognostic factor for survival in patients with metastatic renal cell carcinomaJ Exp Clin Cancer Res2009283019254383
- ZhangWXuQZhuangYChenYNovel association of soluble intercellular adhesion molecule 1 and soluble P-selectin with the ABO blood group in a Chinese populationExp Ther Med201612290991427446295
- ShimizuYMinemuraMTsukishiroTSerum concentration of intercellular adhesion molecule-1 in patients with hepatocellular carcinoma is a marker of the disease progression and prognosisHepatology19952225255317543436
- KobayashiHBoelteKCLinPCEndothelial cell adhesion molecules and cancer progressionCurr Med Chem200714437738617305540
- LarsonNBBellEJDeckerPAABO blood group associations with markers of endothelial dysfunction in the Multi-Ethnic Study of AtherosclerosisAtherosclerosis201625142242927298014
- The R Project for Statistical Computing Available from: https://www.r-project.org/Accessed May 15, 2018