Abstract
Purpose
We assessed the efficacy and safety of different modalities using the network meta-analysis for inoperable hepatocellular carcinoma (HCC) with portal vein invasion. The interested modalities included stereotactic body radiotherapy (SBRT) combined with transarterial chemoembolization (TACE), three-dimensional radiotherapy (3D-RT) combined with hepatic arterial infusion chemotherapy (HAIC) or TACE, TACE plus sorafenib, and use of SBRT, HAIC, sorafenib, and TACE alone.
Methods
PubMed and Cochrane Library electronic databases were systematically searched for eligible studies published up to June 2017. We used network meta-analysis to compare the disease control rate (DCR) and severe adverse events for the eight interested regimens included in this analysis. Study quality was assessed following the Grading of Recommendations, Assessment, Development and Evaluations method.
Results
Fifteen studies published between 2010 and 2016 involving a total of 2,359 patients were enrolled in this network meta-analysis. With indirect comparison of DCR and overall safety, the pooled results showed that RT plus HAIC was the most effective regimen in treating advanced HCC with portal vein tumor thrombosis, followed by RT plus TACE. HAIC alone and sorafenib combined with HAIC appeared least effective intervention regimens. The incidence of treatment-related adverse events of grade 3 or 4 occurred less in the patients who received SBRT alone compared with other interested regimens.
Conclusion
3D-RT combined with HAIC or TACE showed more favorable treatment responses compared with other regimens in advanced HCC patients with portal vein tumor thrombosis.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death in Taiwan and worldwide in 2016 and 2015.Citation1 Most HCCs are diagnosed at an advanced stage, and surgical complete resection is not suitable, in particular for patients with portal vein tumor thrombus (PVTT).Citation2–Citation4 Those with PVTT are at an increased risk of liver failure by wide dissemination of tumor throughout the liver.Citation5,Citation6 The prognosis is extremely poor, and the patients have lower tolerance to treatment with limited survival about only several months.Citation7,Citation8
In recent years, various treatment modalities such as transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), three-dimensional radiotherapy (3D-RT), stereotactic body radiotherapy (SBRT), immunotherapy, and sorafenib have been tried for the treatment of advanced HCC with PVTT, but the optimal treatment strategy remains controversial. Recently, some studies reported that TACE or repetitive HAIC in advanced HCC has achieved favorable results.Citation9–Citation13 With the advances in radiation technology, 3D-RT or SBRT has been used for advanced HCC with the benefit of using higher radiation doses directed at the tumor that spare the organ at risk from substantial radiation.Citation14,Citation15 Many studies have reported that 3D-RT plus TACE or HAIC showed good tumor and PVTT response than TACE or HAIC used alone.Citation16–Citation21 However, some studies indicated that adding RT to TACE or HAIC does not improve survival but increases the incidence of adverse events compared with TACE or HAIC alone.Citation19,Citation22 Therefore, the purpose of this network meta-analysis was to evaluate the efficacy and safety of these regimens in terms of disease control rate (DCR) and severe adverse events in advanced HCC patients with PVTT.
Methods
Literature search
We conducted this network meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.Citation23 A systematic literature search of the PubMed and Cochrane Library from inception through November 2017 was performed. The search strategy was based on combinations of the following keywords: (“advanced” [Mesh]) AND (“hepatomas” or “liver cell carcinomas” [MeSH terms]) OR (“hepatocellular carcinoma” [MeSH terms] AND [all fields]) AND (“clinical study” [publication type] OR “cohort as topic” [MeSH terms]) AND (“sorafenib” or “Nexavar”) AND (“hepatic arterial infusion chemotherapy” or “HAIC”) AND (“TACE” or “transarterial chemoembolization”) AND (“ radiotherapy” or “stereotactic body or SBRT”). In addition, we manually examined the titles of all references within the selected articles to identify other potentially appropriate articles. Two authors (AC and HL) evaluated the titles and abstracts independently. Disagreements were discussed until consensus was reached. Letters to the editor, case reports, nonrandomized trials, animal studies, editorials, and posters were excluded. The language was also restricted to English.
Study selection criteria
The selected studies had to meet the following criteria: 1) nonrandomized or cohort studies; 2) included patients with pathologically proven advanced inoperable HCC with PVTT; 3) detailed data on method, characteristics of patient population, tumor response rates, adverse events, and overall survival; 4) compared at least two arms that consisted of the abovementioned interested regimens; and 5) tumor response rates were defined and evaluated based on the comparison of abdominal computed tomography or magnetic resonance imaging before and after treatment according to the modified Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for HCC.Citation24 A complete response (CR) was defined as disappearance of all target/nontarget lesions after treatment; partial response (PR) was defined as follows: the size of lesion decreased 30% in the sum of the longest diameters of the target lesions; progressive disease (PD) was defined as follows: the size of lesion increased 20% in the sum of the longest diameters of the target lesions; stable disease (SD) was defined as not meeting the criteria of CR, PR, or PD, all other variations.
Data extraction and quality assessment
Two authors (AC and HL) independently reviewed and screened all eligible studies based on the study selection criteria detailed above. The following data were extracted and summarized in a standardized table, including the study’s first author; characteristics of the population; and data to define advanced HCC, interventions, and outcomes such as overall survival and tumor response in terms of DCR and adverse events (). The assessment primary outcome in this study was DCR. The secondary outcomes were median overall survival and grade 3 or 4 adverse events. The DCR was defined as the percentage of a CR, PR, or SD for each study according to the modified RECIST guidelines.
Table 1 Baseline characteristics of studies included in the network meta-analysis
The quality of the included studies was assessed using the Cochrane risk of bias tool (Version 5.1.0). Each study was evaluated independently by two authors explicitly with the following judgment system: low risk of bias, high risk of bias, or unclear (either lack of information or uncertainty for bias).Citation25 The quality of evidence in these studies was evaluated by using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) profiler (GRADEpro) software, Version 3.2 (the Cochrane Information Management System).Citation26
Network meta-analysis
We conducted a network meta-analysis to compare the outcomes among the 15 studies for advanced HCC with PVTT, which included direct (ie, head-to-head) and indirect treatment comparisons.Citation19–Citation22,Citation27–Citation37 We extracted the DCR and grade 3 or 4 adverse events data directly from the studies to calculate the odds ratios (ORs) with 95% confidence intervals (95% CIs). A network meta-analysis was conducted using WinBUGS within Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to synthesize direct and indirect treatment regimens simultaneously and summarize all possible pairwise comparisons between the various regimens as a league table.Citation38,Citation39 The probability of each treatment for a particular outcome would be calculated and ranked first, second, third, etc. by the surface under the cumulative ranking (SUCRA) curve.Citation40 The larger SUCRA number represented the best treatment regimens among the network meta-analysis.
Statistical analysis
The heterogeneity was assessed with the I2 statistic using Review Manager (RevMan) software, Version 5.3.5 (The Cochrane Collaboration, Copenhagen, Denmark).Citation45 I2 statistics were used to measure statistical heterogeneity. I2 is the percentage of total variation attributable to differences observed between the studies rather than sampling error. It is also an intuitive and a simple expression of the inconsistency of studies’ results. I2 values <25% and >75% were regarded as indications of low and high heterogeneity, respectively.Citation41 If significant heterogeneity existed, a fixed-effect statistical model would be used.
The assessment of network inconsistency was conducted by comparing the deviance residuals and deviance information criterion statistics in fitted consistency and inconsistency models to identify inconsistency presented in any loops in the treatment network.Citation42 Inconsistency is caused by imbalances in effect modifiers from study to study, particularly in the direct and indirect evidence. We could select the points on the inconsistency plot to identify which study and treatment contributed to the network inconsistency.
The sensitivity analysis was performed to test the robustness of the network analysis by repeating the main computations using a random-effects model.
Results
Characteristics of the selected studies
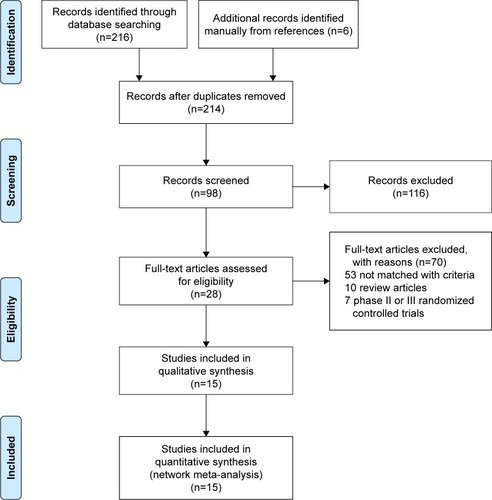
A total of 222 relevant studies were identified through the comprehensive search. After all titles and abstracts were reviewed, 116 studies were excluded because of not matching with the selection criteria. After screening of full-length articles, we found 15 studies that matched the inclusion criteria.Citation19–Citation22,Citation27–Citation37 Twelve studies were retrospective cohort study, and three studies were prospective studies.Citation22,Citation28,Citation32 All the studies were conducted in Asia, except two studies were in Austria and the USA. presents the study characteristics. is the flow diagram of the systematic literature search and selection. A total of 2,359 patients were included in the network meta-analysis, among which 1,061 patients and 319 patients were treated with TACE and HAIC alone.
Quality assessment
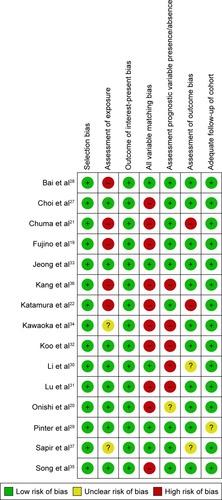
summarizes the risk of bias for the 15 selected studies. All the studies selected exposed cohorts from the same population. Ten studies (66.7%) showed a high risk of bias for the item of comprehensive matching or adjustment for those prognostic variables. Of these 10 studies, five studies (33.3%) showed a high risk of bias for assessment criteria 2 (assessment of exposure) and five studies (33.3%) showed a high risk of bias for the assessment of the presence or absence of prognostic factors. Therefore, the quality of evidence was assessed for the individual studies as moderateCitation22,Citation33,Citation34,Citation36,Citation37 or highCitation12–Citation20,Citation27–Citation32 according to the GRADE method ().
Network meta-analysis
The ranking table in shows the comparison of the efficacy of all treatment regimens against each other in terms of DCR in treating advanced HCC with portal vein invasion. Regimen in the left side was the most effective (OR >1), and regimen in the right side was the least effective (OR <1). From the ranking table generated, it was clear that RT plus HAIC would be the most effective in treating patients with advanced HCC and portal vein invasion (OR range: 4.52–6.40; 95% CI =31.29–9.16 and 2.98–14.03), followed by RT plus TACE (OR range: 3.55–5.03, 95% CI =1.38–9.16 and 2.18–11.82). HAIC alone and sorafenib combined with HAIC appeared the least effective, although it still showed significantly increased DCR when compared with other four regimens. We also compared the efficacy of all interested regimens in terms of the median overall survival retrieved from the included studies. RT plus TACE and RT plus HAIC were still the most effective in treating advanced HCC with portal vein invasion ().
Figure 3 Comparisons of efficacy in terms of disease control rate in advanced HCC scheme.
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; OR, odds ratio; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolization; RT, radiotherapy.
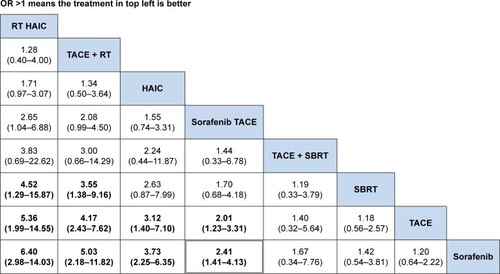
Figure 4 Comparisons of efficacy in terms of median OS in advanced HCC.
Abbreviations: HAIC, hepatic arterial infusion chemotherapy; OR, odds ratio; OS, overall survival; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolization; RT, radiotherapy.
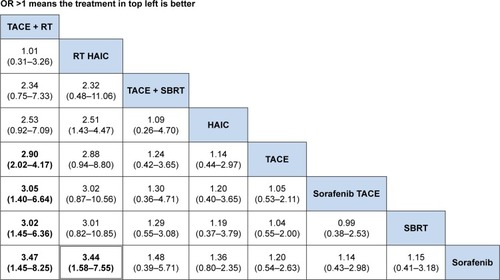
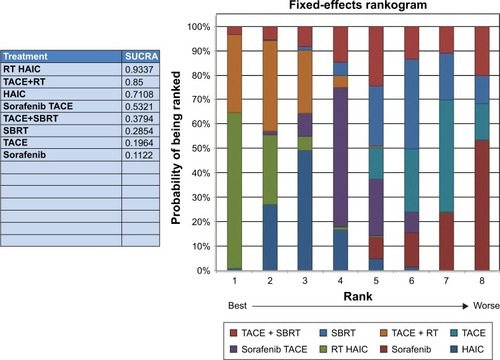
The results of SUCRA curve for each treatment in terms of DCR demonstrated the same results that 3D-RT combined with HAIC was the best regimen (SUCRA 0.9337) followed by 3D-RT combined with TACE (SUCRA 0.85). Sorafenib alone was the worst (SUCRA 0.1122) regimen for mHCC patients with portal vein invasion ().
Figure 5 Rankogram of interested treatment modality.
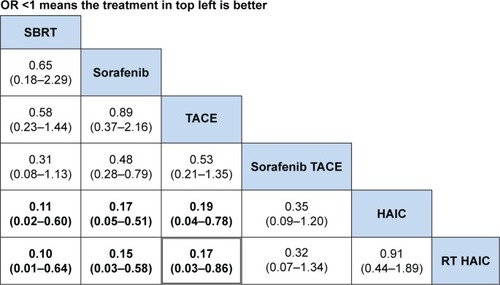
In the safety analysis, retrieving grade 3 or 4 adverse events from all included studies was limited. From the ranking table generated, it was found that regimens with OR <1 would be the safer regimens when compared with every other modalities (). Then, SBRT, sorafenib, or TACE used alone had significant fewer severe adverse events as compared to other regimens. In the subgroup analysis, the severe hematological adverse events (grade 3 or 4) occurred in patients treated with 3D-RT combined with TACE or HAIC and TACE or HAIC used alone were leucopenia and thrombocytopenia. Hand–foot syndrome was the most common severe adverse event in sorafenib regimen (). However, three studies did not have grade 3 or 4 adverse events data available for analysis.Citation30–Citation32
Figure 6 Comparison of adverse events for regimens in advanced HCC OR (95% CI).
Abbreviations: CI, confidence interval; HAIC, hepatic arterial infusion chemotherapy; OR, odds ratio; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolization; RT, radiotherapy.
Assessment of inconsistency and heterogeneity
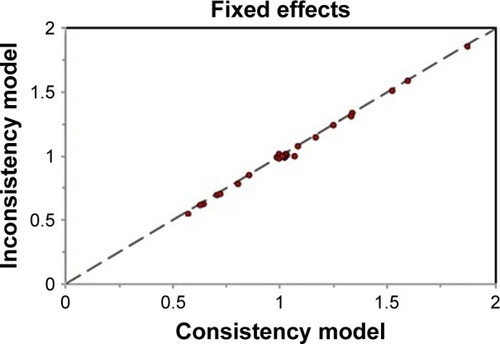
The assessment of inconsistency in the network meta-analysis was presented by the inconsistency plot in fixed-effects model. From the inconsistency plot in , it was found that all studies were along with the line of equality, which may indicate that the effect modifiers are more evenly balanced across the network; it is more likely to be no potential inconsistency between different treatment modalities.
From the results of pooled data for heterogeneity (I2), it was found that between-studies variation within compared treatment modalities was 19%. In the subgroup analysis of included treatment regimens, the heterogeneity (I2) values for TACE plus sorafenib versus TACE or sorafenib alone and HAIC + RT versus HAIC alone were 20% and 19%, respectively, which indicated low heterogeneity (I2<25%). The heterogeneity (I2) for other regimens was 0% ().
The sensitivity analysis showed that the network results of the random-effects model were consistent with that of the fixed-effects model. The pooled OR remained unchanged in heterogeneity (I2=19) and slightly changed in OR (1.96 [1.51, 2.56] in random-effects model; 1.99 [1.58, 2.50] in fixed-effects model).
Discussion
To the best of our knowledge, this is the first network meta-analysis to compare the efficacy and safety of the treatment regimens in patients with advanced HCC and portal vein invasion. PVTT is a well-known poor prognostic factor for HCC patients, whose median survival time was only 2.7 months.Citation43 In this network meta-analysis, we collected the direct and indirect comparative data and assessed the tumor control rate, median survival rate, and severe adverse events in advanced HCC patients with PVTT undergoing different treatment modalities. The pooled results demonstrated that, in patients with advanced HCC with PVTT, those who were treated with local control combination regimens, such as 3D-RT plus TACE (ORRT + TACE =3.55–5.03; 95% CI =1.38–9.16 and 2.98–11.82) and 3D-RT plus HAIC (ORRT + HAIC =4.52–6.40; 95% CI =31.29–9.16 and 2.98–14.03), could reach significantly higher PVTT and tumor response as well as better median overall survival (ORRT + TACE =2.90–3.47; 95% CI =2.02–4.17 and 1.45–8.25; ORRT + HAIC =3.44; 95% CI =1.58–7.55) compared with those other six regimens. The combination regimens were well tolerated with statistically nonsignificant and significant severe grade 3 or 4 adverse events (ORTACE + Sorafenib =0.32; 95% CI =0.07-1.34; ORRT + HAIC =0.91; 95% CI =0.44-1.89; ORRT + TACE =0.07; 95% CI =0.01–0.3). Based on these results, it was suggested that either TACE or HAIC in combination with 3D-RT is a better choice for advanced HCC patients with PVTT. No network meta-analysis has previously been published focusing on comparing different treatment modalities for patients with advanced HCC and portal vein invasion. Therefore, we compared the results of HAIC or TACE or sorafenib combined with 3D-RT with those from conventional meta-analyses published recently. We found that the results in our analysis may be considered as a further evidence to support the previous three conventional meta-analyses because the conventional meta-analysis conducted by Zhang et alCitation45 and Huo and EslickCitation44 did not include all patients with portal vein invasion and the outcomes were only presented in terms of objective response rate. Thus, their results may be overestimation.Citation44,Citation45 Only the meta-analysis conducted by Zhao et al was similar to our study to enroll patients with advanced HCC with portal vein invasion.Citation46
Various treatment modalities have been tried to treat advanced HCC patients with PVTT, but the optimal treatment remains controversial. Surgical resection has been considered as the first preferred modality for highly selected patients with reserved liver function and localized PVTT in the distal branch. TACE is considered as a first-line treatment for patients with HCC, Barcelona Cancer Clinic Liver Cancer (BCLC) stage B with an excellent liver function, and asymptomatic multinodular tumors without macroscopic vascular invasion or extrahepatic spread. HAIC has been used for advanced HCC without distant metastasis with improved survival in Asian countries, such as Japan, Korea, and China. SBRT is a newer treatment modality for patients with local-ized HCC and not eligible to TACE.Citation47 3D-RT and SBRT are emerging as practical treatment modalities for localized HCC. Although the BCLC guideline recommended sorafenib as the standard systematic therapy for advanced HCC patients, and sorafenib alone has limited efficacy for HCC patients with limiting extrahepatic spread. With the advances in the technology of radiation therapy, SBRT is a treatment option with local control rates of 75% to 100% at 1–2 years of survival.Citation48,Citation49 Recently, many studies demonstrated that the combination of 3D-RT or SBRT with HAIC or TACE and TACE combined with sorafenib to treat PVTT were superior to any single treatment option.Citation44 The mechanisms of the combination of radiotherapy (SBRT or 3D-RT) and TACE may involve 1) the reduction of the radiation target volume and the increase of radiation dose precisely delivered to the target lesion and thus sparing uninvolved normal liver tissue; 2) the administration of RT after TACE may contribute to killing or inhibiting any residual tumor cells after TACE;Citation31,Citation36 and 3) PVTT is sensitive to RT, which can boost the treatment responses of tumors and PVTT.Citation50,Citation51 As for the mechanisms of combination of TACE and sorafenib, this multimodality may involve the embolization of the hepatic artery and reduce the portal vein blood supply to HCC.Citation52,Citation53 Sorafenib is an orally active Raf kinase inhibitor with effects on tumor cell proliferation and tumor angiogenesis that acts by inhibiting the serine–threonine kinases Raf-1 and B-Raf. It also inhibits vascular endothelial growth factor receptors 1, 2, and 3; platelet-derived growth factor receptor β; and receptor tyrosine kinase receptor tyrosine kinases.Citation54
The network meta-analysis is a useful method for incorporating information from both direct and indirect treatment comparisons in a network of studies using novel statistical methods. It can help us to make a quantitative comparison of the efficacy and safety of various competing treatments in a single analysis.
Study limitation
The limitations of our network meta-analysis should be considered in the interpretation of the results. First, the baseline characteristics of the enrolled studies may influence the heterogeneity and the results, such as the dose of HAIC used which depends on patients’ body surface area, the location of portal vein thrombus, type of tumor, and the primary tumor size, and there were slightly differences between the studies and may be confounding factor. Second, selection bias resulting from cohort data because patients or clinical investigators may select treatment modalities depending on patients’ physical condition or investigator’s decision. Third, the small sample size of some studies may cause overestimation of the outcomes. However, our study has advantages that 1) the selected patients had mHCC with portal vein invasion, which may reduce heterogeneity or inconsistency and 2) the primary outcome is more comprehensive than the published conventional meta-analysis.
Conclusion
The network meta-analysis provided evidence that the combination of HAIC or TACE or sorafenib with 3D-RT improved survival and better outcome. Future randomized controlled trials are needed to confirm the advantages of combined therapy of interested modalities over those modalities used alone for mHCC with portal vein invasion.
Disclosure
The authors report no conflicts of interest for this work.
References
- FerlayJSoerjomataramIErvikM [webpage on the Internet]Cancer incidence and mortality worldwide: IARC Cancer Base No. 11Lyon, FranceInternational Agency for Research on Cancer2014 Available from: http://globocan.iarc.frAccessed February 24, 2015
- AriiSYamaokaYFutagawaSResults of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of JapanHepatology20003261224122911093728
- LlovetJMBruixJSystematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survivalHepatology200337242944212540794
- IkaiIAriiSKojiroMReevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide surveyCancer2004101479680215305412
- XueTCXieXYZhangLYinXZhangBHRenZGTransarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysisBMC Gastroenterol2013136023566041
- ChengSHLinYMChuangVPA pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinomaJ Gastroenterol Hepatol199914101025103310530500
- WooHYHeoJNew perspectives on the management of hepatocellular carcinoma with portal vein thrombosisClin Mol Hepatol201521211512126157747
- YinJBoWTSunJNew evidence and perspectives on the management of hepatocellular carcinoma with portal vein tumor thrombusJ Clin Transl Hepatol20175216917628660155
- SilvaJPBergerNGTsaiSTransarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysisHPB (Oxford)201719865966628552299
- ParkJYAhnSHYoonYJRepetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinomaCancer2007110112913717508408
- AndoETanakaMYamashitaFHepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 casesCancer200295358859512209752
- KimHYKimJDBaeSHA comparative study of high-dose hepatic arterial infusion chemotherapy and transarterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinomaKorean J Hepatol201016435536121415578
- EunJRLeeHJMoonHJKimTNKimJWChangJCHepatic arterial infusion chemotherapy using high-dose 5-fluorouracil and cisplatin with or without interferon-alpha for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosisScand J Gastroenterol200944121477148619958061
- FussMSalterBJHermanTSThomasCRJrExternal beam radiation therapy for hepatocellular carcinoma: potential of intensity-modulated and image-guided radiation therapyGastroenterology20041275 Suppl 1S206S21715508086
- AndolinoDLJohnsonCSMaluccioMStereotactic body radiotherapy for primary hepatocellular carcinomaInt J Radiat Oncol Biol Phys2011814e447e45321645977
- MengMBCuiYLLuYTranscatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysisRadiother Oncol200992218419419042048
- TazawaJMaedaMSakaiYRadiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvementJ Gastroenterol Hepatol200116666066511422619
- SeongJParkHCHanKHClinical results of 3-dimensional conformal radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma in the cirrhotic patientsHepatol Res2003271303512957204
- FujinoHKimuraTAikataHRole of 3-D conformal radiotherapy for major portal vein tumor thrombosis combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinomaHepatol Res201445660761725052365
- OnishiHNousoKNakamuraSEfficacy of hepatic arterial infusion chemotherapy in combination with irradiation for advanced hepatocellular carcinoma with portal vein invasionHepatol Int20159110511225788384
- ChumaMTaguchiHYamamotoYEfficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiationJ Gastroenterol Hepatol20112671123113221501224
- KatamuraYAikataHTakakiSIntra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosisJ Gastroenterol200944549250219330281
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementInt J Surg20108533634120171303
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis2010301526020175033
- HigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011)The Cochrane Collaboration2014 Available from: www.cochrane-handbook.org
- GRADEproThe Cochrane IMS Available from: http://ims.cochrane.org/revman/gradeproAccessed November 12, 2017
- ChoiGHShimJHKimMJSorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analysesRadiology2013269260361123864102
- BaiWWangYJZhaoYSorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching studyJ Dig Dis201314418119023324079
- PinterMHuckeFGraziadeiIAdvanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenibRadiology2012263259059922438359
- LiXLGuoWXHongXDEfficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score analysisHepatol Res201646111088109826783741
- LuDHFeiZLZhouJPHuZTHaoWSA comparison between three-dimensional conformal radiotherapy combined with interventional treatment and interventional treatment alone for hepatocellular carcinoma with portal vein tumour thrombosisJ Med Imaging Radiat Oncol201559110911425088249
- KooJEKimJHLimYSCombination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombusInt J Radiat Oncol Biol Phys201078118018719926229
- JeongSWJangJYLeeJEThe efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinomaAsia Pac J Clin Oncol20128216417122524575
- KawaokaTAikataHHyogoHComparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinomaJ Dig Dis201516950551226121102
- SongDSSongMJBaeSHA comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosisJ Gastroenterol201550444545425027973
- KangJNieQDURStereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosisMol Clin Oncol201421435024649306
- SapirETaoYSchipperMJStereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinomaInt J Radiat Oncol Biol Phys2018100112213029066120
- MRC Biostatistics UnitUniversity of Cambridge [webpage on the Internet] Available from: https://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs/
- MillsEJThorlundKIoannidisJPDemystifying trial networks and network meta-analysisBMJ2013346f291423674332
- SalantiGAdesAEIoannidisJPGraphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorialJ Clin Epidemiol201164216317120688472
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- DiasSWeltonNJSuttonAJCaldwellDMLuGAdesAEEvidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trialsMed Decis Making201333564165623804508
- LlovetJMBustamanteJCastellsANatural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trialsHepatology199929162679862851
- HuoYREslickGDTranscatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysisJAMA Oncol20151675676526182200
- ZhangLHuPChenXBiePTransarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysisPLoS One201496e10030524945380
- ZhaoQZhuKYueJComparison of intra-arterial chemoembolization with and without radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a meta-analysisTher Clin Risk Manag201613213128053537
- TseRVHawkinsMLockwoodGPhase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinomaJ Clin Oncol200826465766418172187
- BujoldAMasseyCAKimJJSequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinomaJ Clin Oncol201331131631163923547075
- CammàCCabibboGPettaSCost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinomaHepatology20135731046105423299720
- YoonSMLimYSWonHJRadiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomesInt J Radiat Oncol Biol Phys20128252004201121621346
- KimTHKimDYParkJWThree-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitableAm J Clin Oncol200629656857517148993
- LiXFengGSZhengCSZhuoCKLiuXExpression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor levelWorld J Gastroenterol200410192878288215334691
- ZhangXWangKWangMTransarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysisOncotarget2017817294162942728177886
- KeatingGMSantoroASorafenib: a review of its use in advanced hepatocellular carcinomaDrugs200969222324019228077