Abstract
Purpose
Unplanned reoperation (URO) after radical gastrectomy for gastric cancer (GC) mostly results from serious postoperative complications. At present, there is still controversy over the predictive factors for URO. Our goal was to identify the risk factors for URO and to investigate its potential impact on long-term survival.
Patients and methods
We included 2,852 GC patients who underwent a gastrectomy. Multivariate logistic regression analyses were performed to determine the risk factors for URO. Patients were randomly selected from the non-URO group by 1:4 propensity score matching with multiple parameters with patients from the URO group. The survival disparity of 34 URO patients and 136 non-URO patients was examined using the Kaplan–Meier method and the multivariate Cox proportional hazard model.
Results
The incidence of URO was 1.4% (39/2, 852). The primary cause of URO was intra-abdominal bleeding (53.9%, 21/39). Multivariate logistic regression analyses revealed that male gender (OR = 4.630, 95% CI = 1.412–15.152, P = 0.011), diabetes (OR = 4.189, 95% CI = 1.705–10.290, P = 0.002), and preoperative hypoproteinemia (OR = 2.305, 95% CI = 1.079–4.923, P = 0.031) were independent risk factors for URO. With regard to early surgical outcomes, patients undergoing URO had a longer hospital stay (P < 0.001), higher incidence of postoperative complications (P < 0.001), and greater mortality (P < 0.001) compared with the non-URO group. No significant correlation was found between URO and cancer-specific survival in univariate (P = 0.275) and multivariate (P = 0.090) survival analyses.
Conclusion
Male gender, diabetes, and preoperative hypoproteinemia were suggested as independent risk factors for URO. URO was associated with longer hospital stay and increased perioperative mortality, but might not be correlated with long-term mortality.
Introduction
Gastric cancer (GC) ranks fifth for cancer incidence and third for cancer deaths worldwide,Citation1 and surgery is the most important therapeutic strategy in patients with resectable GC. China is classified as a high-incidence area for GC, with the third highest incidence and the second leading cause of death among all cancers.Citation2 Despite the improvements of preoperative management and surgical technique, postoperative complications are still a common problem. Due to the intricacy of operative procedures, the incidence of complications after radical gastrectomy can be as high as 20%.Citation3 Reportedly, 2%–10% patients with these complications need reoperation.Citation4,Citation5 Unplanned reoperation (URO) may result in psychological and physical problems, increase financial burden, prolong hospital stay, and even lead to death.Citation6–Citation9 Therefore, decreasing the incidence of postoperative complications and reoperation rate is of great importance in clinical practice.
Several studies reported the risk factors for GC postoperative complications.Citation3,Citation10–Citation13 Deguchi et al showed that pulmonary insufficiency and the duration of the operation are independent risk factors for anastomotic leakage.Citation10 According to Kobayashi et al’s study, dissection of the pancreas head area is an unfavorable predictor for postoperative pancreatic fistula after laparoscopic gastrectomy.Citation13 However, substantial heterogeneity existed in previous studies and their findings were inconsistent. Furthermore, our knowledge of the impact of URO on long-term survival after radical gastrectomy is still limited. Thus, investigation into the relationship between URO and tumor prognosis is duly warranted.
The aim of this study is to systematically evaluate the risk factors for URO after radical gastrectomy and to explore the potential correlation between URO and long-term survival outcomes for patients. Our results may increase the awareness of URO among surgeons and prevent the occurrence of these undesirable postoperative events after surgery.
Patients and methods
Patient population
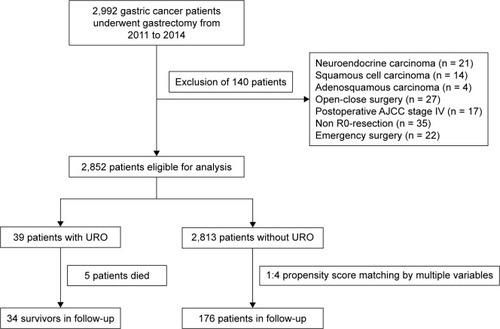
This study was designed as a retrospective analysis of GC patients treated with gastrectomy in the Department of Gastrointestinal Surgery, the First Affiliated Hospital of Wannan Medical College. A total of 2,992 patients undergoing gastrectomy for GC between January 2011 and December 2014 were enrolled. Exclusion criteria were as follows: 1) patients diagnosed with non-adenocarcinoma: squamous cell carcinoma (n = 14), adenosquamous carcinoma (n = 4), and neuroendocrine carcinoma (n = 21); 2) patients who did not receive D2 radical resection: open-close surgery (n = 27), non-R0 resection (n = 35), and postoperative American Joint Committee on Cancer (AJCC) stage IV (n = 17); and 3) patients undergoing emergency surgery (n = 22). Together, 2,852 patients were ultimately included for further analyses. Among them, 39 patients were termed as the URO group and 2,813 as the non-URO group (). This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. The current research was reviewed and approved by the ethical review board of the First Affiliated Hospital of Wannan Medical College. Written informed consent was obtained from all patients.
Data collection and definitions
Patient information, medical history, concomitant diseases, primary surgical treatment, postoperative complications, clinical outcomes, and follow-up data were retrieved from the electronic database of the First Affiliated Hospital of Wannan Medical College. The postoperative pathological stages were determined according to the AJCC 8th Edition of Gastric Cancer TNM Staging.Citation14
In this study, URO was defined as the status that the patient had to receive reoperation under general anesthesia and tracheal intubation due to severe postoperative complications caused by the initial gastrectomy for GC. Neo-adjuvant chemotherapy was administered to patients with a preoperative staging cT3/4 or cN+, based on the decision after multidisciplinary discussion.Citation15 Adjuvant combination chemotherapy was routinely implemented to all patients with postoperative staging II and III with oxaliplatin plus 5-FU/leucovorin (FOLFOX) or capecitabine (CapeOX) for 4–6 cycles. Cancer-specific survival refers to the time from the first gastrectomy to death of recurrence and metastasis.
Operative techniques
All operations were performed by experienced surgeons specializing in gastrointestinal surgery at our center. The surgeons had performed at least 100 D2 radical gastrectomies. Anticoagulants were discontinued 1 week before surgery. Depending on the location of the foci, total or partial gastrectomy was performed with D2 lymph node dissection, according to the new Japanese classifications and treatment guidelines for GC.Citation16 The following methods were adopted for reconstruction after gastrectomy: Roux-en-Y reconstruction following total gastrectomy, Billroth-I or Billroth-II reconstruction following distal gastrectomy, and esophagogastrostomy following proximal gastrectomy.
Follow-up
As shown in , five patients who died within 30 days after URO were excluded from survival analysis. We followed-up all patients surviving the procedure of URO (n = 34) and 136 patients in the non-URO group who were randomly selected by propensity score matching based on the clinicopathological features, including postoperative AJCC stage, age, gender, body mass index, concomitant diseases, diabetes, preoperative hypoproteinemia, and preoperative anemia, with a ratio of 1:4. Follow-up data were obtained by telephone or email and the outpatient/inpatient clinical records. The last follow-up time was December 2017.
Statistical analysis
The risk factors included in the univariate analysis were age, gender, concomitant diseases, diabetes, preoperative hypoproteinemia, preoperative anemia, tumor size, type of surgical resection, type of reconstruction, surgical approach, combined organ resection, neoadjuvant chemotherapy, and postoperative AJCC stage. Categorical data were shown as percentages and were analyzed with Fisher’s exact test. Continuous data were presented as mean ± standard errors of the mean, and differences between groups were analyzed by the Mann–Whitney U test. All clinical variables were included in a multivariate logistic regression model. Moreover, propensity score analysis was performed as a superior and more refined statistical method of adjusting for potential baseline confounding variables.Citation17 The baseline risk profiles of the matched patients were compared to assure that no major differences in baseline patient characteristics persisted. The prognostic value of URO for cancer-specific survival was finally assessed using the Kaplan–Meier method and multivariate Cox proportional hazard model analysis after the propensity score matching. R statistical software was used to conduct propensity score matching, and all analyses were performed using the SPSS 24.0 software (IBM Corporation, Armonk, NY, USA). A two-sided P-value < 0.05 was considered statistically significant.
Results
Severe complications, primary causes, and management of URO
We included 2,104 males and 748 females in this study. The mean age was 65.0 ± 9.3 years. Among the enrolled 2,852 patients, 39 cases (mean age: 62.1 ± 10.1 years) underwent URO, including 36 males and three females. The primary causes of URO were intra-abdominal bleeding (15/39, 38.5%), anastomotic leakage and intra-abdominal bleeding (6/39, 15.4%), incision dehiscence (6/39, 15.4%), intestinal obstruction (4/39, 10.2%), anastomotic leakage and intra-abdominal infection (3/39, 7.7%), jejunal perforation (3/39, 7.7%), and anastomotic bleeding (2/39, 5.1%).
The total incidence of URO resulting from intra-abdominal bleeding was 53.9% (21/39), and all bleeding cases were confirmed by arterial angiography or open surgery. Splenic area was the most common site of bleeding found during the reoperation (5/21, 23.8%). The primary causes of bleeding were associated with operative technique failure (13/21, 61.9%) and anastomotic leakage (6/21, 28.6%). Regarding the treatment of bleeding caused by anastomotic leakage, suturing the bleeding vessels and adequate abdominal drainage were necessary. Among the 21 patients with postoperative bleeding, 18 patients recovered after URO, while three patients died. Eight patients required a third operation due to re-bleeding. The detailed causes, treatments, and outcomes of URO patients with intra-abdominal bleeding are presented in .
Table 1 Intra-abdominal bleeding position, cause, and treatment
Risk factors for URO
As shown in , three factors including male gender (P = 0.005), diabetes (P = 0.010), and preoperative hypoproteinemia (P = 0.035) were closely correlated with URO. Furthermore, the multivariate analysis using logistic regression demonstrated that male gender (OR = 4.630, 95% CI = 1.412–15.152, P = 0.011), diabetes (OR = 4.189, 95% CI = 1.705–10.290, P = 0.002), and preoperative hypoproteinemia (OR = 2.305, 95% CI = 1.079–4.923, P = 0.031) were independent risk factors for URO ().
Table 2 Univariate analysis of potential influencing factors for URO
Table 3 Multivariate logistic regression analysis of risk factors for URO
Early surgical outcomes of the URO patients
After reoperation, 19 patients developed postoperative complications, including re-bleeding, pulmonary infection, ileus, and incision infection. The incidence of postoperative complications after URO was 48.7% (19/39), whereas the incidence was 14.0% (396/2,813) in the non-URO group. Furthermore, patients with URO had a significantly increased time of hospital stay (35.03 ± 14.85 vs 15.52 ± 6.16 days, P < 0.001). Compared with the non-URO group (six out of the 2,813 patients), five out of 39 patients with URO died of disseminated intravascular coagulation (DIC) or multiple organ dysfunction syndrome during hospitalization. The mortality was 12.8% (5/39) in the URO group and 0.2% (6/2,813) in the non-URO group (P < 0.001). Together, URO was correlated with prolonged hospital stay, increased in-hospital complications, and higher perioperative mortality ().
Table 4 Early surgical outcomes for patients with or without URO
Assessment of URO as a prognostic factor for long-term survival
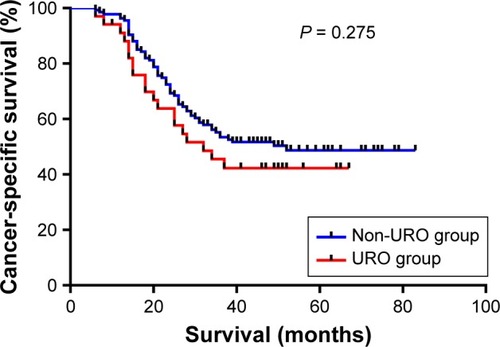
A total of 34 URO patients and 136 propensity matched patients without URO were included in the long-term follow-up analysis. The propensity score in patients who underwent URO was 0.028 ± 0.005, compared with 0.030 ± 0.004 in those who did not undergo URO (P = 0.782), indicating no significant bias in the patient characteristics. The median follow-up time was 56 months. The 1- and 3-year cancer-specific survival rates of patients with URO were 88.23% (30/34) and 47.1% (16/34), and 97.8% (133/136) and 56.6% (77/136) in patients without URO. According to the univariate and multivariate survival analyses, URO was not associated with cancer-specific survival (P = 0.275) ( and ). In addition, AJCC stage (HR = 7.004, 95% CI = 2.130–23.034, P = 0.001) and preoperative hypoproteinemia (HR = 1.831, 95% CI = 1.126–2.978, P = 0.015) significantly affected the cancer-specific survival in this study. Taken together, URO might not be correlated with long-term cancer-specific survival of GC patients.
Table 5 Prognostic factors for cancer-specific survival after curative gastric cancer resection in univariate and multivariable analyses
Discussion
Reoperation following surgery is correlated with higher morbidity, mortality, and cost to the health care system.Citation18 The URO rate, recognized as a compelling evaluation index of surgical quality, has drawn increasing attention from surgeons.Citation19 URO is typically associated with a more complicated postoperative course, particularly for complex surgical procedures such as gastric resection. Knowledge regarding the risk factors and indications for URO could provide valuable insight. In this study, we elucidated the causes, risk factors, early outcomes, and long-term cancer-specific survival of URO after radical gastrectomy for GC. Notably, URO was found not to be correlated with the long-term cancer-specific survival according to our data.
In recent years, the incidence of URO has declined over the past years with the rapid development of surgical techniques. According to Sah et al’s study, URO incidence after open gastrectomy for GC is 2.2%, and the primary causes of URO are intra-abdominal bleeding and anastomotic leakage.Citation20 Li et al reported that the morbidity of patients undergoing URO after laparoscopic gastrectomy is 1.1%, and the main reasons for URO are intra-abdominal hemorrhage and anastomotic bleeding.Citation21 This study enrolled a much larger population and suggested that the URO incidence was 1.4%, with intra-abdominal bleeding and anastomotic leakage being the major causes. In addition, our data showed that intra-abdominal bleeding was the most common complication after gastrectomy.
Previous studies indicated that early postoperative bleeding is associated with surgeons’ technical failure,Citation12,Citation22 such as lack of experience in the procedure of hemostasis and vessel ligatures, and anastomotic bleeding. Abdominal arterial bleeding usually occurs in splenic artery, hepatic artery, and gastroduodenal artery, with the incidence ranging from 0.6% to 3.3%, and the main reason for hemorrhage was anastomotic leakage.Citation23,Citation24 Esophagojejunal anastomotic leakage after total gastrectomy and Roux-en-Y reconstruction was the primary reason for delayed massive abdominal bleeding. Severe postoperative hemorrhage was an extremely intractable complication which lacks standardized treatment, leading to high postoperative mortality.Citation4,Citation8 In this study, three out of eight re-bleeding patients died of DIC, while the remaining patients eventually recovered after one or more transcatheter arterial embolizations (TAEs) and/or surgical operations. Early postoperative arterial bleeding can be managed effectively by immediate re-laparotomy, with a satisfactory clinical outcome.Citation25 However, re-laparotomy is often difficult to perform in the latter phase as the bleeding site is hard to find due to intraperitoneal adhesion and inflammatory reaction. Alternatively, TAE is a less invasive approach, irrespective of tissue adhesion and edema caused by the initial surgery. Nevertheless, TAE had a higher re-bleeding rate compared to re-laparotomy.Citation26,Citation27 In case radiological intervention fails, immediate shift to surgical treatment is compulsory.Citation28,Citation29
As URO can cause great harm to patients, it is crucial for surgeons to evaluate the risk factors for URO before surgery, which might assist surgeons in preventing URO. Several risk factors for URO after radical gastrectomy proposed by previous studies were not completely coincident. According to Yi et al’s report,Citation5 tumor size and type of operation are risk factors for URO. One study performed by Oh et al suggested that male gender and increased mean age are closely correlated with the incidence of URO.Citation4 Consistently, Li et al indicated that there are more elderly, male, and overweight patients in the URO group.Citation21 In this study, we found that male gender, diabetes, and preoperative hypoproteinemia were independent risk factors for URO.
Additionally, we suggested that URO might have no impact on the long-term cancer-specific survival of the patients after radical gastrectomy, though URO impaired the early surgical outcomes. Several studies concluded that intraoperative blood loss, TNM stage, operation duration, and neoadjuvant chemotherapy are prognostic factors for patient survival.Citation30 Nakauchi et al found that tumor size, pathological N factor ≥2, and postoperative pancreatic fistula combined with intra-abdominal abscesses are associated with the 3-year recurrence free survival.Citation31 This study suggested that there was no significant relationship between URO and long-term cancer-specific survival. Given that the occurrence of URO is multifactorial, we conducted a matched follow-up study to minimize the potential effect of these confounding variables. The patients from the URO group and the non-URO group were 1:4 propensity score matched according to multiple clinicopathological characteristics. Multivariate Cox analysis revealed that TNM stage III and preoperative hypoproteinemia were significant unfavorable prognostic factors for cancer-specific survival, further confirming the robustness and reliability of our results.
In spite of our efforts to conduct a comprehensive and accurate analysis, there were still several limitations in this study. First, the clinical characteristics of the Asian population may result in potential selection bias which limits its universal value. Our findings should be considered valid in the geographical-ethnical-social context and need to be verified in different populations with different phenotypes. Second, this was a retrospective study performed in a single center. Large multicenter prospective studies are duly warranted to further verify our results. Third, despite the large total sample size in this study, the sample size of URO patients was relatively small because of the low incidence of URO. Fourth, due to disparities among the treatment strategies of patients and intricate complications, potential bias might affect our results. Therefore, multi-institutional prospective cohort studies are needed for further confirmation. However, this study included a larger population than previous pertinent research, and the follow-up data were analyzed to evaluate the long-term survival of URO patients for the first time.
Conclusion
This study suggests that male gender, diabetes, and preoperative hypoproteinemia might be risk factors for URO after gastrectomy for GC. URO was associated with increased time of hospital stay and higher perioperative mortality but might not be correlated with long-term survival. These findings may assist in the identification of patients at high risk for URO that may benefit from preoperative counseling, optimization, and tailoring of postoperative management to reduce the rate of reoperation.
Acknowledgments
This study was funded by the Natural Science Research of Anhui Education Department Key Project (No. KJ2018A0246) and Anhui Natural Science Foundation (No.1508085MH147).
Disclosure
The authors report no conflicts of interest in this work.
References
- Global Burden of Disease Cancer CollaborationFitzmauriceCAllenCGlobal, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease studyJAMA Oncol20173452454827918777
- YangLIncidence and mortality of gastric cancer in ChinaWorld J Gastroenterol2006121172016440411
- PedrazzaniCMarrelliDRamponeBPostoperative complications and functional results after subtotal gastrectomy with Billroth II reconstruction for primary gastric cancerDig Dis Sci20075281757176317404848
- OhSJChoiWBSongJComplications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single instituteJ Gastrointest Surg200913223924518850251
- YiHWKimSMKimSHComplications leading reoperation after gastrectomy in patients with gastric cancer: frequency, type, and potential causesJ Gastric Cancer201313424224624511420
- FerlayJShinHRBrayFEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- SelbyLVGennarelliRLSchnorrGCAssociation of hospital costs with complications following total gastrectomy for gastric adenocarcinomaJAMA Surg20171521095395828658485
- TuRHLinJXZhengCHComplications and failure to rescue following laparoscopic or open gastrectomy for gastric cancer: a propensity-matched analysisSurg Endosc20173152325233727620911
- TrakulthongCPhunmaneeAMortality risk factors during readmission at the Department of MedicineTher Clin Risk Manag2017131551155429255362
- DeguchiYFukagawaTMoritaSIdentification of risk factors for esophagojejunal anastomotic leakage after gastric surgeryWorld J Surg20123671617162222415758
- ChenJHCaiSRZhaiETSurvival prognosis and clinicopathological features of the lymph nodes along the left gastric artery in gastric cancer: implications for D2 lymphadenectomyInt J Clin Exp Pathol2015811143651437326823752
- De AngelisFAbdelgawadMRizzelloMMattiaCSilecchiaGPerioperative hemorrhagic complications after laparoscopic sleeve gastrectomy: four-year experience of a bariatric center of excellenceSurg Endosc20173193547355128008466
- KobayashiNShinoharaHHarutaSProcess of pancreas head as a risk factor for postoperative pancreatic fistula in laparoscopic gastric cancer surgeryWorld J Surg20164092194220127142626
- IkomaNBlumMEstrellaJSEvaluation of the American Joint Committee on Cancer 8th edition staging system for gastric cancer patients after preoperative therapyGastric Cancer2018211748328643144
- Van LaethemJLCarneiroFDucreuxMThe multidisciplinary management of gastro-oesophageal junction tumours: European Society of Digestive Oncology (ESDO): Expert discussion and report from the 16th ESMO World Congress on Gastrointestinal Cancer, BarcelonaDig Liver Dis201648111283128927590840
- Japanese Gastric Cancer AssociationJapanese gastric cancer treatment guidelines 2010 (ver. 3)Gastric Cancer201114211312321573742
- EbingerSMWarschkowRTarantinoISchmiedBMMartiLAnastomotic leakage after curative rectal cancer resection has no impact on long-term survival: a propensity score analysisInt J Colorectal Dis201530121667167526245949
- KroonHMBreslauPJLardenoyeJWCan the incidence of unplanned reoperations be used as an indicator of quality of care in surgery?Am J Med Qual200722319820217485561
- MichaelsADMullenMGGuidryCAUnplanned reoperation following colorectal surgery: indications and operationsJ Gastrointest Surg20172191480148528523487
- SahBKChenMMYanMZhuZGReoperation for early postoperative complications after gastric cancer surgery in a Chinese hospitalWorld J Gastroenterol20101619810320039455
- LiPHuangCMTuRHRisk factors affecting unplanned reoperation after laparoscopic gastrectomy for gastric cancer: experience from a high-volume centerSurg Endosc201731103922393128205027
- KhoursheedMAl-BaderIMouzannarAPostoperative bleeding and leakage after sleeve gastrectomy: a single-center experienceObes Surg20162612300727730464
- KoderaYSasakoMYamamotoSIdentification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancerBr J Surg20059291103110916106493
- MitaKItoHMurabayashiRPostoperative bleeding complications after gastric cancer surgery in patients receiving anticoagulation and/or antiplatelet agentsAnn Surg Oncol201219123745375222805868
- ParkJYKimYWEomBWUnique patterns and proper management of postgastrectomy bleeding in patients with gastric cancerSurgery201415561023102924856122
- SpivakHAzranCSpectreGLidermannGBlumenfeldOSleeve gastrectomy postoperative hemorrhage is linked to type-2 diabetes and not to surgical techniqueObes Surg201727112927293228523403
- YapFYOmeneBOPatelMNTranscatheter embolotherapy for gastrointestinal bleeding: a single center review of safety, efficacy, and clinical outcomesDig Dis Sci20135871976198423361570
- JilesenAPTolJABuschOREmergency management in patients with late hemorrhage after pancreatoduodenectomy for a periampullary tumorWorld J Surg20143892438244724791669
- HanKAhmedBMKimMDClinical outcome of transarterial embolization for postgastrectomy arterial bleedingGastric Cancer201720588789428194589
- WangLWangXAHaoJQLong-term outcomes after radical gastrectomy in gastric cancer patients with overt bleedingWorld J Gastroenterol20152147133161332426715815
- NakauchiMSudaKKadoyaSTechnical aspects and short-and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective studySurg Endosc201630104632463926703126