Abstract
Background
Bisphosphonates (BPs) and denosumab are widely used to treat osteoporosis and complications associated with bone metastases. However, medication-related osteonecrosis of the jaw (MRONJ) is a serious problem.
Objective
The objective of this study was to evaluate the frequency, outcome, and characteristics of patients with drug-induced MRONJ.
Methods
Retrospective pharmacovigilance disproportionality analysis was conducted using the Japanese Adverse Drug Event Report (JADER) database from the Pharmaceuticals and Medical Devices Agency. Adverse event reports submitted to JADER between 2004 and 2017 were analyzed, and the reporting odds ratio (ROR) was calculated.
Results
Among the BPs that cause MRONJ, zoledronate was the most common; therefore, we compared the characteristics of cases of MRONJ induced by zoledronate with those induced by denosumab. Among the 3,875 (68.1% women) cases of MRONJ, zoledronate-related MRONJ accounted for 1,283 (56.0% women) and denosumab-related MRONJ accounted for 322 (55.3% women). MRONJ was more frequent after 70 years of age regardless of the use of either zoledronate or denosumab; onset occurred after 1 year from the denosumab treatment, but it is unknown when onset occurred after zoledronate treatment. The outcomes for MRONJ were poor, with 406 reports on zoledronate (31.6%) and 152 reports on denosumab (47.2%) demonstrating nonrecovery. Zoledronate (ROR: 319.3, 95% CI: 296.0–344.4) had the highest ROR among BP agents. Denosumab had a high ROR (ROR: 155.2, 95% CI: 136.5–176.3). Zoledronate and denosumab were used in similar patient backgrounds, and their use resulted in a similar frequency of MRONJ.
Conclusion
The findings of this comprehensive evaluation of MRONJ using the JADER database will be helpful for prescribing medications to elderly patients.
Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a serious drug reaction. MRONJ is defined as an exposed jawbone or bone that persists for 8 or more weeks, and occurs in the absence of radiation or metastatic disease in the jaws and in the presence of current or previous treatment with a bone resorption inhibitor.Citation1 If the jawbone decomposes, infection may occur because bacteria inhabit the oral cavity, leading to symptoms such as jaw pain and swelling. Although MRONJ is rare (approximately 1%) among patients with cancer exposed to antiresorptive or antiangiogenic medicationsCitation2 and does not affect life prognosis, it remains an important issue, and the refractory symptoms significantly reduce the quality of life for patients.
Bisphosphonates (BPs) are known to cause MRONJ.Citation3–Citation5 BPs have been widely used to treat cancer patients with bone metastases and osteoporosis. BPs, which are analogs of pyrophosphoric acid and have a hydrolysable P-O-P bond, suppress bone resorption by inhibiting osteoclasts. Of note, BPs have a nitrogen side chain (N-BPs) in the P-C-P bond, which is replaced with the P-O-P bond, and exhibit much stronger inhibition of bone resorption than BPs without nitrogen side chains (non-N-BPs).Citation6 BPs also have a greater affinity for bone than non-N-BPs, and are thus more likely to accumulate in bone. Therefore, the inhibition of bone resorption by alendronate with a nitrogen side chain is a 1,000-fold more potent, and that by zoledronate and minodronate, which have a cyclic structure, including two nitrogen side chains, is 10,000-fold more potent than the inhibition by etidronate.Citation7
Denosumab was developed as a therapeutic human monoclonal antibody with a new mechanism of action that inhibits the receptor activator of nuclear factor-kappa B ligand (RANKL) and has been used to treat bone complications. In Japan, use of denosumab was initiated in April 2012 for the treatment of bone complications secondary to multiple myeloma and bone metastases from solid tumors. Subsequently, additional indications for osteoporosis (June 2013) and osteoclastoma (June 2014) were approved.Citation8 Denosumab inhibits bone resorption by osteoclasts similar to BPs, but differs from BPs in that it has a short half-life (approximately 1 month), and neither deposits in the bone nor remains stored in the bone matrix. Additionally, denosumab does not induce apoptosis in osteoclasts.Citation9 Although BPs and denosumab have a different mechanism of action, they are targets for osteoclasts and are involved in the onset of MRONJ. As there is increasing concern that MRONJ is induced by BPs and denosumab, we conducted a comprehensive nationwide overview of BP- and denosumab-related MRONJ, and patient characteristics using the Japanese Adverse Drug Event Report (JADER) database, a spontaneous reporting database from the Pharmaceuticals and Medical Devices Agency (PMDA).
Methods
In this study, we used data from the public release of PMDA’s JADER database, which is freely available and contains information on the adverse drug events and patients in Japan since April 1 2004. We used data from JADER to which adverse event reports were submitted between April 2004 and January 2017. The data structure of JADER consists of four data sets: patient demographic information (DEMO), drug information (DRUG), adverse events (REAC), and medical history. In REAC table, the Medical Dictionary for Regulatory Activities (MedDRA) is used to codify adverse effects that are indicated as “Preferred Term (PT).”
After we removed duplicate data from each table, the DEMO table was then merged with the REAC and DRUG tables using the ID numbers. In each case, the contribution of the medication to adverse events was classified into three categories: “suspected medicine,” “concomitant medicine,” and “interaction.” A “suspected medicine” is defined as a pharmaceutical product with which an adverse event is suspected to be associated. When the reporter suspects an interaction, he/she reports it as an “interaction.” A “concomitant medicine” is defined as another pharmaceutical product used at the time of the adverse event.
We only extracted cases that were classified as “suspected medicine” and analyzed the reports of suspected drugs and adverse drug events, which we selected as “MRONJ” in the Preferred Term coded in MedDRA. We compiled a cross-tabulation table based on two classifications: the presence or absence of MRONJ and the presence or absence of the suspected medicine. Then, we calculated the reporting odds ratio (ROR). The ROR is rate of reporting a specific adverse event caused by a particular drug divided by the rate of the same adverse event caused by all other drugs present in the database. In addition, the ROR was frequently used with the spontaneous reporting database as an index of the relative risk for drug-related adverse events. A signal was considered to be present when the lower limit of the 95% CI of the ROR is >1.
In this database, age, height, and weight information are indicated in the form of age in decades, height in centimeter-denominated ranges, and weight in kilogram-denominated ranges. Because these data are not continuous variables, we could not conduct multiple analyses using them. All analyses were performed with JMP Pro 12 (SAS Institute Inc. Cary, NC, USA).
Results
As shown in , a total of 5,195,890 reports were obtained after the combination of three tables using the ID numbers: DRUG (2,850,470 reports), REAC (709,826 reports), and DEMO (449,558 patients). Of those, we extracted all suspected drugs causing adverse events (1,984,122 reports), and 3,875 reports of MRONJ were analyzed. The annual number of reports during 2004–2016 is shown in . The number of reports of drug-induced MRONJ has been increasing. The number of deaths from MRONJ was extremely low, but nonrecovery with drug-induced MRONJ was common.
Figure 1 Flowchart of this study.
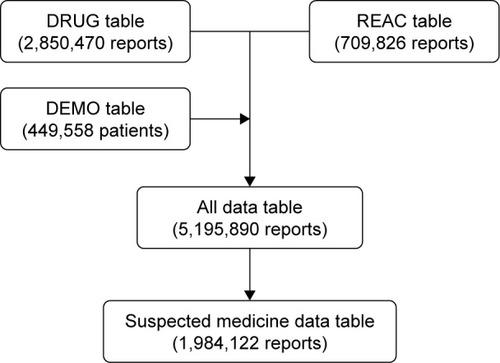
Table 1 Number of reports of MRONJ and its outcome between 2004 and 2016
The drugs reported for MRONJ were predominantly anti-resorptive agents such as BP agents and RANKL inhibitors. Among the BP agents (alendronate, ibandronate, etidronate, zoledronate, pamidronate, minodronate, and risedronate), the most commonly reported agent was zoledronate and the least common was etidronate (). Then, we focused on zoledronate and compared it with the new drug, denosumab. Characteristics of the patients with MRONJ induced by zoledronate and denosumab are shown in . Among the reports of zoledronate-related MRONJ (1,283 reports), 718 had a higher percentage of women (56.0%). The reasons for the use of zoledronate included bone metastases (65.8%), plasma cell myeloma (10.4%), and breast cancer (5.9%). More than 30% of the reports were on patients in their seventies. The timing of zoledronate-related MRONJ onset was mostly unknown (51.8%), but secondary after 1 year from the start of medication, and approximately 30% of the reports indicated no recovery. Similarly, among the reports of denosumab-related MRONJ, 178 had a higher percentage of women (55.3%). The reasons for the use of denosumab were bone metastases (50.9%), osteoporosis (12.4%), and off-label use (10.6%). More than 30% of the reports were on patients in their seventies. As with BPs, MRONJ onset occurred after 1 year from the start of denosumab, and approximately half (152 reports, 47.2%) of the reports on denosumab-related MRONJ described nonrecovery.
Table 2 Number of reports of MRONJ induced by antiresorptive agents between 2004 and 2016
Table 3 Characteristics of the patients with MRONJ induced by zoledronate or denosumab
The ROR of antiresorptive agents is shown in . As the lower limit of the 95% CI of the ROR was >1, all of the antiresorptive medicines were considered to have a signal. Zoledronate had the strongest signal (ROR: 319.3; 95% CI: 296.0–344.4) among the BPs. Denosumab also had a strong signal (ROR: 155.2; 95% CI: 136.5–176.3). In general, the use of anticancer drugs, angiogenesis inhibitors, or adrenocortical steroid hormones is known to be a risk factor for MRONJ. Therefore, we analyzed the association between BPs or denosumab and the use of anticancer drugs, angiogenesis inhibitors, or adrenocortical steroid hormones. However, no relationship between the onset of MRONJ and concomitant administration of these drugs was found.
Table 4 The RORs of MRONJ by antiresorptive agents
Discussion
In this study, we comprehensively overviewed the occurrence and characteristics of patients with MRONJ induced by BPs and denosumab using the JADER database. The number of reports of MRONJ was 3,875 in 2016. For BP agents, zoledronate was found to be the most common medicine. Focusing on zoledronate among the BP agents, MRONJ occurred more often in women ≥70 years old and after use for more than 1 year; no recovery was observed in more than 30% of the reports. Similar results were noted for denosumab. This is the first survey to provide important insights regarding postapproval knowledge for rare and adverse drug events such as MRONJ.
Zoledronate and denosumab have different mechanisms of action; therefore, we expected MRONJ not to occur in patients taking denosumab. However, denosumab-related MRONJ occurred at almost the same frequency as BP-agent-related MRONJ. These results were consistent with Phase III trialsCitation10 and a report by the Food and Drug Administration’s Adverse Event Reporting System (FAERS).Citation11 In the FAERS, most MRONJ cases in the setting for prevention of skeletal-related events occurred due to pamidronate and zoledronate. These results were consistent with ours, but the ROR was different. In the FAERS database, the ROR for denosumab-related MRONJ was 13.8, whereas it was 155.2 in our study. This difference is partly because denosumab has an antibody preparation that may cause differences in the effects and frequency of adverse events according to individual or pathological conditions. Another reason for the difference is that the use of denosumab is limited in the US, whereas there is no such restriction in Japan.
Several studies have reported that the use of anticancer drugs, such as an angiogenesis inhibitors and adrenocortical steroid hormones, is also a risk factor for MRONJ.Citation12–Citation14 However, the angiogenesis inhibitor bevacizumab was found to be minimally associated with the onset of MRONJ.Citation15 In our study, no concomitant drugs were used with denosumab or zoledronate in cases of MRONJ. Of note, MRONJ was not induced by zoledronate when it was used for benign bone disease, ie, osteoporosis, whereas 40/322 (12.4%) of the MRONJ cases induced by denosumab occurred when it was used for osteoporosis (). This is partly because the dose of zoledronate for malignant bone disease is usually 4 mg intravenous every 4 weeks compared with 5 mg every 12 months for osteoporosis; the dose of denosumab for malignant bone disease is 120 mg every 4 weeks compared with 60 mg every 6 months for osteoporosis. These risk factors should be confirmed in further epidemiological studies such as case–control or cohort studies.
In our study, the total number of cases of MRONJ induced by denosumab was less than that induced by zoledronate. However, it is possible that the number of cases of MRONJ due to denosumab will increase with the expanded use. In 2017, denosumab was approved for the treatment of bone erosion from rheumatoid arthritis.Citation16,Citation17 Furthermore, denosumab has greater preventive effects against skeletal-related events, but leads to almost the same frequency of MRONJ as zoledronate (2.0%, denosumab; 1.4%, zoledronate).Citation18 Therefore, the number of reports of denosumab-related MRONJ may increase.
The occurrence of MRONJ is associated with specific medical and dental conditions and procedures, including dental procedures and conditions that increase the risk of bone trauma. Most commonly, MRONJ is associated with invasive bone procedures such as tooth extractions.Citation19 Therefore, one of the most important prophylactic methods for MRONJ is keeping the oral cavity clean. As regular dental check-ups reduce the incidence of MRONJ,Citation20,Citation21 clinical staff need to explain this to patients in detail and closely coordinate with the dentist or oral surgeon. Further studies are needed to prevent MRONJ. Similar to reports on denosumab-related MRONJ,Citation11,Citation22 we noted a decline in MRONJ reports in 2015 and 2016. This finding is partly because several publications of case reports increased the awareness of health care providers regarding the risk for MRONJ. Saad et alCitation10 stated that educating physicians about oral health before and during bone-targeted therapy may help reduce the MRONJ incidence and improve outcomes. Therefore, serious outcomes of MRONJ induced by BPs or denosumab should be improved in the near future.
Limitations
Analysis using JADER as a self-reporting database has several advantages as it covers numerous medications and a wide range of patients, reflects clinical circumstances, and can detect unknown or severe side effects. However, the present study using the JADER database needs to be interpreted in the context of its potential limitations. First, the ROR does not provide a robust indication of the signal strength. In spontaneous reporting systems, such as JADER, control populations are not included; therefore, the ROR is different from the “odds ratio” that is commonly used in epidemiological studies. In clinical terms, the ROR indicates an increased risk of adverse event reporting, and not the risk of adverse events. Second, as a consequence of it being a self-reporting database, there are underreported cases, typing errors, reported biases, and a lack of detailed clinical information. Finally, the association between patients and medications is unclear in this database. If several medications are administered together, it is difficult to identify the drug causing the adverse events. Therefore, careful attention must be paid to the interpretation of the results from JADER.Citation23,Citation24
Conclusion
In conclusion, analysis of data from JADER suggests that the frequency of MRONJ due to BPs was higher than that due to denosumab, but the characteristics of patient with MRONJ induced by denosumab were similar with those of patients with MRONJ induced by zoledronate. These findings can be used to update information used for prescriptions, especially for elderly patients.
Disclosure
K Hosohata received research support from the Science Research Promotion Fund. The authors report no other conflicts of interest in this work.
References
- YonedaTHaginoHJapanese Allied Committee on Osteonecrosis of the Jaw, et al. Erratum to: Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the JawJ Bone Miner Metab20173512028120102
- DodsonTBThe frequency of medication-related osteonecrosis of the jaw and its associated risk factorsOral Maxillofac Surg Clin North Am201527450951626362367
- KhoslaSBurrDCauleyJBisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral ResearchJ Bone Miner Res200722101479149117663640
- MarxREPamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemicJ Oral Maxillofac Surg20036191115111712966493
- MaxillofacialSAdvisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial SurgeonsAmerican Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jawsJ Oral Maxillofac Surg200765336937617307580
- RogersMJCrockettJCCoxonFPMönkkönenJBiochemical and molecular mechanisms of action of bisphosphonatesBone2011491344121111853
- EndoYKumamotoHNakamuraMUnderlying mechanisms and therapeutic strategies for bisphosphonate-related osteonecrosis of the jaw (BRONJ)Biol Pharm Bull201740673975028566618
- ChawlaSHenshawRSeegerLSafety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 studyLancet Oncol201314990190823867211
- BaronRFerrariSRussellRGDenosumab and bisphosphonates: different mechanisms of action and effectsBone201148467769221145999
- SaadFBrownJEvan PoznakCIncidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastasesAnn Oncol20122351341134721986094
- ZhangXHamadehISSongSOsteonecrosis of the jaw in the united states food and drug administration’s adverse event reporting system (faers)J Bone Miner Res201631233634026288087
- Aragon-ChingJBNingYMChenCCHigher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agentsCancer Invest200927222122619235596
- VahtsevanosKKyrgidisAVerrouELongitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jawJ Clin Oncol200927325356536219805682
- SivolellaSLumachiFStelliniEFaveroLDenosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe diseaseAnticancer Res20133351793179723645723
- GuarneriVMilesDRobertNBevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancerBreast Cancer Res Treat2010122118118820361252
- MochizukiTYanoKIkariKEffects of denosumab treatment on bone mineral density and joint destruction in patients with rheumatoid arthritisJ Bone Miner Metab201836443143828681148
- YueJGriffithJFXiaoFRepair of bone erosion in rheumatoid arthritis by denosumab: A high-resolution peripheral quantitative computed tomography studyArthritis Care Res201769811561163
- StopeckATLiptonABodyJJDenosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind studyJ Clin Oncol201028355132513921060033
- JeongHGHwangJJLeeJHKimYHNaJYHanSSRisk factors of osteonecrosis of the jaw after tooth extraction in osteoporotic patients on oral bisphosphonatesImaging Sci Dent2017471455028361029
- DimopoulosMAKastritisEBamiaCReduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acidAnn Oncol200920111712018689864
- RipamontiCIManiezzoMCampaTDecreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of MilanAnn Oncol2009201137145
- FuscoVGalassiCBerrutiADecreasing frequency of osteonecrosis of the jaw in cancer and myeloma patients treated with bisphosphonates: the experience of the oncology network of piedmont and aosta valley (north-Western Italy)ISRN Oncol2013201367202723533811
- van PuijenbroekEPBateALeufkensHGLindquistMOrreREgbertsACA comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactionsPharmacoepidemiol Drug Saf200211131011998548
- HatahiraHAbeJHaneYDrug-induced gingival hyperplasia: a retrospective study using spontaneous reporting system databasesJ Pharm Health Care Sci201731928729910
