Abstract
Further understanding of psoriasis pathogenesis has led to the development of effective biologic medications. Guselkumab (GUS) is a subcutaneously administered monoclonal antibody that targets the p19 cytokine subunit in IL-23 and IL-39 and is US Food and Drug Administration (FDA) approved for the treatment of moderate-to-severe psoriasis in adult patients. This review evaluates the pharmacology, safety and efficacy of GUS in patients with psoriasis. We performed a literature review by searching online databases including PubMed and Google Scholar. In clinical trials, GUS improved diseases including psoriatic arthritis (PsA) and specific areas of disease (scalp, feet, hands and fingernails). In the Phase III trials VOYAGE 1 and 2, more GUS than adalimumab (ADM) patients experienced a ≥90% reduction in Psoriasis Area and Severity Index (PASI) score (PASI90) (VOYAGE 1: 80.2% vs 53.0%; VOYAGE 2: 75.2% vs 54.8%; P<0.001 for both) and Investigator Global Assessment score of 0 or 1 (VOYAGE 1: 84.2% vs 61.7%; VOAYGE 2: 83.5% vs 64.9%; P<0.001 for both) at Week 24. GUS was also successful in treating patients unresponsive to ADM and ustekinumab in the VOYAGE 2 and NAVIGATE trials, respectively. While long-term data are necessary, GUS appears to have a favorable side effect profile with most common adverse effects including nasopharyngitis and upper respiratory tract infections. GUS is a well-tolerated and effective medication for patients with psoriasis. Continued study of GUS and the p19 subunit will help to determine GUS’s ultimate place in therapy.
Keywords:
Introduction
Psoriasis is an immune disease with an estimated prevalence of ~3% in the United States.Citation1 It is typically diagnosed visually by its characteristic erythematous scaly plaques distributed on the scalp, torso, extensor surfaces and/or throughout the rest of the body.Citation2 The disease greatly reduces patient’s quality of life and is associated with various psychiatric comorbidities including anxiety, depression and suicidality.Citation3,Citation4 Psoriasis can also cause debilitating arthritis and has been linked to numerous systemic pathologies including cardiovascular disease, inflammatory bowel disease and metabolic syndrome.Citation5–Citation7
When deciding on therapeutic options for psoriasis, considerations include disease severity, disease location, joint involvement, cost profile and patient preference.Citation8 Treatments for psoriasis range from topical to systemic medications and include steroids, phototherapy, vitamin A and D derivatives, tars, immunosuppressants and biologics.Citation8 Despite these numerous options, psoriasis can be a difficult disease to treat, and continued investigation is ongoing to discover additional safe and effective interventions.
Biologic medications have transformed the landscape of treatment for moderate-to-severe plaque psoriasis, allowing for better disease control.Citation9 Cytokines and inflammatory mediators involved in the pathogenesis of plaque psoriasis can now be targeted, with some examples of biologics and their targets including adalimumab (ADM; TNF-alpha), ixekizumab (IL-17) and ustekinumab (USM; IL-12/IL-23; ).Citation10,Citation11 The search for better psoriasis treatments is now focusing on the IL-23/IL-17 pathway, including the two subunits of IL-23, p40 and p19.Citation12–Citation14 The p40 subunit is shared with IL-12, while the p19 subunit is present in IL-23 and not in IL-12 ().Citation14,Citation15 USM targets the common p40 subunit, while guselkumab (GUS) targets p19 and as a result IL-23 and not IL-12 ().Citation15,Citation16 IL-23 is a cytokine thought to play a significant role in the pathogenesis of the disease, as it is present at high levels both in the serum and plaques of patients with psoriasis.Citation12,Citation17,Citation18 IL-23 induces the proliferation of proinflammatory Th17 cells, which are key drivers of psoriasis development.Citation19,Citation20 In late 2017, the US Food and Drug Administration (FDA) approved and released GUS to the market. Here, we review the pharmacology, safety and efficacy of GUS in adult patients with moderate-to-severe plaque psoriasis.
Figure 1 IL-12, IL-23 and IL-39 with their receptors and downstream effects.
Abbreviations: USM, ustekinumab; GUS, guselkumab; ADM, adalimumab.
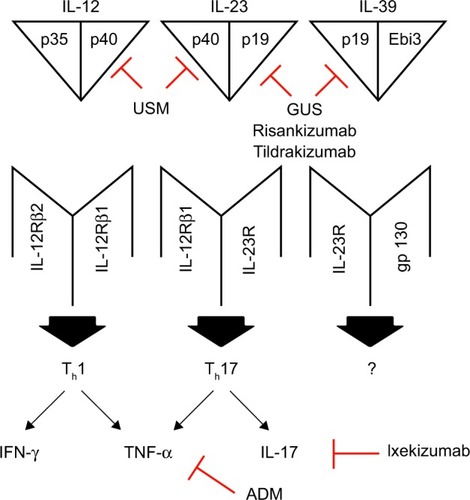
Pharmacodynamics, pharmacokinetics and immunogenicity
GUS is a subcutaneously (SQ) injected human monoclonal antibody that targets IL-23 by binding the IL-23 p19 subunit ().Citation21 IL-23 is a member of a heterodimeric family of cytokines, which also includes IL-12, IL-27, IL-35 and IL-39.Citation22,Citation23 IL-12 and IL-23 are proinflammatory cytokines that drive psoriasis pathogenesis, IL-27 and IL-35 are inhibitory cytokines, and the role of IL-39 in psoriasis is unclear.Citation12,Citation22,Citation23 Within the IL-12 cytokine family, IL-23 and IL-39 contain the p19 subunit.Citation15,Citation22 Variations in the genes encoding p19 and the p19 receptor, IL-23R, are associated with an increased risk of psoriasis, thus highlighting the role of p19 in psoriasis pathogenesis.Citation21,Citation24,Citation25 GUS binds to the IL-23 p19 subunit and prevents IL-23 from binding to IL-23R on the surface of various innate and adaptive immune cells.Citation12,Citation15,Citation21 Through stopping the p19 subunit from binding to IL-23R, the IL-23/Th17 pathway is inhibited, thus reducing its proinflammatory effects.Citation12,Citation21
A Phase I randomized, placebo-controlled clinical trial examined the pharmacokinetics and pharmacodynamics of GUS in 47 healthy participants and 24 participants with moderate-to-severe plaque psoriasis.Citation26 Healthy participants either received a single GUS intravenous (IV) administration (0.03, 0.1, 0.3, 1, 3 or 10 mg/kg), a single GUS SQ injection (3 mg/kg) or placebo treatment.Citation26 Patients with moderate-to-severe plaque psoriasis either received a single GUS SQ injection (10, 30, 100 or 300 mg) or placebo treatment.Citation26
In healthy participants treated with GUS IV, area under the curve for serum drug concentration vs time (AUC0–∞) and peak serum concentration (Cmax) increases were dose dependent, with median AUC0–∞ values ranging from 4.93 to 2,261.8 µg⋅day/mL and median Cmax values ranging from 0.47 to 200.36 µg/mL.Citation26 In addition, in this cohort, median terminal half-life (t1/2) of the drug ranged from 12.5 to 19.5 days, median clearance ranged from 3.58 to 6.1 mL/day/kg and median volume of distribution (Vz) ranged from 97.74 to 117.88 mL/kg.Citation26 For healthy participants receiving GUS SQ, the mean AUC0–∞ was 256.99 µg⋅day/mL and the mean Cmax was 9.46 µg/mL.Citation26
SQ administration in the psoriasis cohort also had dose-dependent AUC0–∞ and Cmax increases, with median AUC0–∞ values ranging from 15.11 to 574.62 µg⋅day/mL and median Cmax values ranging from 0.5 to 22.7 µg/mL.Citation26 Median t1/2 values in this cohort ranged from 15.8 to 17.8 days.Citation26
Throughout the clinical trials examining the immunogenicity of GUS in psoriasis patients, the proportion of patients developing antibodies to GUS ranged from 4% to 9%.Citation15,Citation26–Citation29 These antibodies were generally present in low titers, and their development did not affect the efficacy of the treatment or the incidence of injection site reactions.Citation15,Citation27–Citation29
Treatment efficacy
An initial Phase I clinical trial investigated a single GUS SQ administration in 24 adult patients with moderate-to-severe plaque psoriasis.Citation21 At 12 weeks post injection, 50%, 60%, 60% and 100% of 10, 30, 100 and 300 mg GUS-treated patients, respectively, experienced at least a 75% reduction in Psoriasis Area and Severity Index (PASI) score (PASI75; ).Citation21 Comparatively, no patients in the placebo-treated cohort attained this end point ().Citation21 These results were predominantly stable through 24 weeks post injection, although some loss of efficacy was noted in the 100 mg treated cohort.Citation21 On immunohistochemical evaluation, GUS reduced lesional epidermal thickness and CD3 T-cell and myeloid dendritic cell infiltration (P<0.05 compared to baseline, except for myeloid dendritic cell infiltration with 10 mg GUS; P=0.072).Citation21 Compared to baseline values, GUS reduced serum IL-17A levels in responsive patients at Week 1 (P=0.031) and Week 12 (P=0.0015).Citation21 The placebo-treated cohort had no reduction in epidermal thickness, CD3 T-cell infiltration or serum IL-17A concentration, although there was a decrease in lesional myeloid dendritic cell infiltration compared to baseline (P=0.028).Citation21
Table 1 Summary of clinical trials
A Phase II randomized, double-blinded, placebo-controlled trial compared GUS to ADM in 293 patients with moderate-to-severe plaque psoriasis over 52 weeks.Citation15 The ADM cohort received standard dosing, while the GUS cohort received either 5, 50, or 200 mg at 0 and 4 weeks and then every 12 weeks or 15 or 100 mg every 8 weeks.Citation15 At Week 16, the placebo-treated cohort crossed over to 100 mg GUS with 8-week dosing intervals.Citation15 By Week 16, 34%, 61%, 79%, 86% and 83% of 5, 15, 50, 100 and 200 mg GUS-treated cohorts reached a Physician Global Assessment (PGA) score of 0 or 1 ().Citation15 Comparatively, 7% of placebo and 58% of the ADM cohort achieved this end point ().Citation15 In addition, at Week 16, ≥90% PASI score improvement (PASI90) was seen in 34%, 34%, 45%, 62% and 57% of 5, 15, 50, 100 and 200 mg GUS-treated cohorts, respectively, compared to 2% of placebo and 44% of the ADM group ().Citation15 Disease improvement with GUS persisted through Week 40, as most dosage subgroups experienced relative preservation of PGA 0 or 1 and PASI75.Citation15 Some loss of efficacy was observed as the time of the next scheduled GUS injection approached and was seen more often when dosed every 12 weeks as opposed to every 8 weeks.Citation15 Crossover from placebo to GUS also had
A Phase IIa, randomized trial investigated GUS for the treatment of psoriatic arthritis (PsA) in 149 patients.Citation30 Patients received either GUS 100 mg at 0 and 4 weeks and then every 8 weeks or placebo.Citation30 At Week 24, more GUS-treated patients experienced 20% (58.0% vs 18.4%; P<0.001), 50% (34.0% vs 10.2%; P=0.002) and 70% (14.0% vs 2.0%; P=0.023 [post hoc]) American College of Rheumatology score improvements compared to the placebo group.Citation30 Furthermore, greater improvements in multiple psoriatic arthropathy measures – such as Leeds Enthesitis Index (median percentage change from baseline: −100.0% vs −33.3%; P=0.009), Dactylitis Score (median percentage change from baseline: −100.0% vs −33.3%; P<0.001) and Psoriatic Arthritis Disease Activity Score (mean change from baseline: −2.50 to −0.49; P<0.001) – were observed in the GUS-treated cohort compared to placebo.Citation30
In VOYAGE 1, the first of three Phase III randomized, double-blinded, placebo-controlled GUS clinical trials, GUS was generally more effective than placebo and ADM.Citation27 A total of 837 patients were randomized to one of three treatment groups, including GUS (100 mg at 0 and 4 weeks and then every 8 weeks), ADM (standard dosing) or placebo, followed over 48 weeks.Citation27 The placebo group was crossed over to GUS 16 weeks into the study.Citation27
At Week 16, 85.1% of GUS patients had an Investigator Global Assessment (IGA) score of 0 or 1, compared to 65.9% and 6.9% of ADM- and placebo-treated groups, respectively (P<0.001 compared to the placebo group; ).Citation27 Furthermore, 73% of GUS patients experienced PASI90 at Week 16 compared to 3% of the placebo group and 50% of the ADM group (P<0.001 compared to the placebo group; ).Citation27 At Week 24, more GUS patients than ADM patients had an IGA score of 0 or 1 (84.2% vs 61.7, respectively; P<0.001) and attained PASI90 (80.2% vs 53.0%, respectively; P<0.001; ).Citation27 GUS treatment response was persistent, with improved PASI and IGA scores through Week 48.Citation27 Crossover from placebo to GUS improved disease measures comparable to measures seen in the original GUS cohort.Citation27
VOYAGE 2 differed from the VOYAGE 1 study as it examined noncontinuous GUS treatment and GUS treatment for patients unresponsive to ADM injection.Citation28 The multi-center, randomized, double-blinded, placebo-controlled study included 992 subjects.Citation28 At Week 28, the “randomized withdrawal and retreatment” segment began.Citation28 Responsive patients were those who attained PASI90.Citation28 Unresponsive patients in the original GUS cohort continued their current regimen, while responders were re-randomized into one of two groups – one group that continued GUS 100 mg every 8 weeks or another group that received placebo until a defined loss of efficacy, at which point they were reinitiated on GUS.Citation28 In the placebo-to-GUS crossover cohort, unresponsive patients continued their current regimen, while responders received placebo until a defined loss of efficacy was observed and were then reinitiated on GUS.Citation28 Patients unresponsive to ADM were converted to GUS, while patients responsive to ADM received placebo until a defined loss of efficacy was observed and were then initiated on GUS.Citation28 At Week 16, 84% of patients in the initial GUS cohort had IGA scores of 0 or 1 compared to 68% and 9% of the ADM- and placebo-treated cohorts, respectively (P<0.001 for both; ).Citation28 Similarly, at Week 16, 70.0% of patients in the GUS cohort reached PASI90 compared to 46.8% of ADM-treated group or 2.4% of placebo-treated group (P<0.001 for both; ).Citation28 Of patients who started on and continued GUS to Week 28, 19.9% were classified as nonresponders compared to 47.3% of the ADM cohort.Citation28 Overall, continuous GUS attained better clinical responses than noncontinuous treatment.Citation28 At Week 48, 89% of patients receiving continuous GUS experienced PASI90 compared to 37% of patients in the placebo re-randomized cohort (P<0.001).Citation28 For patients responsive to GUS and re-randomized to placebo, loss of PASI90 was observed in a median time of 15.2 weeks.Citation28 GUS also effectively treated patients unresponsive to ADM.Citation28 At the end of the study, 66.1% of patients initially unresponsive to ADM who were converted to GUS attained PASI90.Citation28
In both VOYAGE 1 and VOYAGE 2, GUS improved specific areas of disease, including the scalp, feet, hands and fingernails.Citation27,Citation28 At Week 16 of both studies, more GUS-treated patients than placebo-treated patients attained a fingernail Physician Global Assessment (f-PGA) score of 0 or 1 (39.1%/52.0% vs 15.9%/14.6%), Physician Global Assessment of hands and/or feet (hf-PGA) score of 0 or 1 (73.3%/77.2% vs 14.0%/14.3%) and scalp-specific Investigator Global Assessment (ss-IGA) score of 0 or 1 (83.4%/80.6% vs 14.5%/10.9%; P<0.001 for both; ).Citation27,Citation28 At Week 24 of both studies, more GUS patients than ADM patients had an hf-PGA score of 0 or 1 (78.9%/81.6% vs 56.8%/66.1%) and ss-IGA score of 0 or 1 (84.5%/85.3% vs 69.2%/67.5%; ).Citation27,Citation28 At Week 24 of VOYAGE 1, less GUS patients than ADM patients attained f-PGA scores of 0 or 1 (56.3% vs 62.4%), although at Week 48, more GUS patients than ADM patients achieved this measure (74.7% vs 61.8%; P=0.038; ).Citation27 Similarly, at Week 24 of VOYAGE 2, less GUS patients than ADM patients had an f-PGA score of 0 or 1 (62.6% vs 66.9%; ).Citation28
A subsequent study combined the data from VOYAGE 1 and VOYAGE 2 to determine if specific patient populations had distinctive responses to GUS.Citation31 Patients were stratified by age, gender, weight, body mass index, ethnicity, pretreatment disease features and previous treatments.Citation31 Overall, in almost all the examined subgroups, GUS had greater efficacy than placebo and ADM.Citation31
The final Phase III trial, NAVIGATE, examined GUS safety and efficacy for patients unresponsive to USM.Citation29 In the initial 16-week open-label segment of the study, patients received USM at Weeks 0 and 4.Citation29 At Week 16, patients with IGA scores of 0 or 1 were considered responsive and remained on USM, while patients with IGA scores of ≥2 were considered unresponsive and randomized to either receive GUS 100 mg at Weeks 16 and 20 and then every 8 weeks or continue USM.Citation29 Overall, patients on GUS had better clinical outcomes than patients in the randomized USM group.Citation29 The primary end point of the study was the number of patient visits with recorded IGA scores of 0 or 1 and with a minimum 2-point IGA score improvement from Week 16 scores.Citation29 From Week 28 to Week 40, the GUS-treated cohort achieved a greater average number of these visits than the randomized USM cohort (1.5 vs 0.7 visits; P≤0.001).Citation29 In addition, from Week 28 to Week 40, GUS-treated patients had a greater average number of visits with PASI90 compared to randomized USM patients (2.2 vs 1.1 visits; P≤0.001).Citation29 GUS remained more effective than randomized USM through Week 52, with 51.1% of GUS patients compared to 24.1% of randomized USM patients reaching PASI90 (P<0.001; ).Citation29
Safety and adverse events (AEs)
Side effects of GUS are typically mild, and the medication is well tolerated.Citation15,Citation21,Citation26–Citation29 A total of 48%–74% of patients treated with GUS experience at least one AE, similar to the frequency of AE seen in ADM treatment.Citation15,Citation21,Citation26–Citation29 In any clinical trial, <3% of patients receiving GUS discontinued their treatment due to AEs and <7% experienced serious AEs.Citation15,Citation21,Citation26–Citation29 The most common AE of GUS is infection, most frequently nasopharyngitis and upper respiratory tract infections.Citation15,Citation21,Citation26–Citation29 The majority of infections observed did not require treatment, and serious infections were generally rare.Citation15,Citation21,Citation26–Citation29 No opportunistic infections, active tuberculosis (TB) infections or hypersensitivity reactions were noted for GUS patients in any reported studies, and of 105 patients with latent TB who were administered both TB prophylaxis and GUS, none experienced disease reactivation.Citation15,Citation21,Citation26–Citation29,Citation32 Other less common AEs included injection site reaction, headache, arthralgia, pruritus, cardiovascular events and cancer.Citation15,Citation21,Citation26–Citation29 In the NAVIGATE trial, a higher rate of musculoskeletal AE, such as PsA, was noted with GUS treatment compared to USM treatment.Citation29 Throughout all the described clinical trials, <2% of GUS patients were noted to have cardiovascular events compared to none in the placebo groups.Citation15,Citation21,Citation26–Citation29 Rates of cardiovascular events were generally comparable between GUS and ADM.Citation15,Citation21,Citation27,Citation28 Malignancies observed in GUS-treated groups included nonmelanoma skin cancer, prostate cancer, breast cancer, bladder cancer, grade 3 cervical intraepithelial neoplasia and squamous cell carcinoma of the head and neck.Citation15,Citation27–Citation29
Patient-focused perspectives
GUS improved quality of life end points.Citation15,Citation27–Citation30,Citation33 A Phase II clinical trial measured GUS treatment on patients’ quality of life with the Dermatology Quality of Life Index (DLQI).Citation15 At Week 16, GUS at any of the five administered dosages (5, 15, 50, 100 and 200 mg) improved DLQI scores compared to placebo (P≤0.01).Citation15 DLQI scores of 0 or 1 through the first 16 weeks of the study were observed in 26%, 34%, 42%, 63% and 70% of the 5, 15, 50, 100 and 200 mg GUS cohorts, respectively, compared to 7% of placebo and 49% of the ADM cohort (P<0.001 for comparison to placebo; ).Citation15 In the Phase IIa study examining GUS for the treatment of PsA, GUS improved more quality of life measures than placebo, including mean change from baseline in Medical Outcomes Study 36-Item Short Form (SF-36) Physical (6.59 vs 0.46, respectively; P<0.001) and Mental (4.95 vs 0.42, respectively; P=0.002) Component Scores and Health Assessment Questionnaire without Disability Index (HAQ-DI) scores (−0.42 to −0.06; P<0.001).Citation30
VOYAGE 1 added the Psoriasis Symptoms and Signs Diary (PSSD) as a quality of life measure.Citation27 Through Week 16, 56.3% of the GUS group attained a DLQI score of 0 or 1 compared to 4.2% of the placebo group (P<0.001; ).Citation27 GUS improved PSSD symptom scores compared to placebo at Week 16 (41.9±24.6 vs 3.0±19.6, respectively; P<0.001; ).Citation27 Furthermore, GUS had a greater impact on these measures compared to ADM through Week 48 (62.5% vs 38.9%, respectively, achieving DLQI scores of 0 or 1 and mean PSSD symptom score reductions of 45.3±25.5 vs 32.5±31.1, respectively; P<0.001 for both; ).Citation27
In VOYAGE 2, GUS improved quality of life measures more than placebo and ADM treatments.Citation28 At Week 16, 51.7% of GUS patients achieved a DLQI score of 0 or 1, with a mean PSSD symptom score reduction of 40.4±26.5 ().Citation28 Comparatively, also at Week 16, 3.3% of the placebo group and 39.0% of the ADM group attained a DLQI score of 0 or 1, with mean PSSD symptom score reductions of 8.3±23.7 and 32.8±24.9, respectively ().Citation28 Through Week 24, more GUS-treated patients than ADM-treated patients attained DLQI scores of 0 or 1 (57.6% vs 41.1%, respectively; P<0.001; ).Citation28 Mean PSSD symptom score reductions at Week 24 were also greater for GUS than ADM (42.1±26.8 vs 31.9±27.0, respectively; P<0.001; ).Citation28 Through Week 48, continuous GUS treatment improved DLQI and PSSD scores more than noncontinuous treatment (P<0.001 for both).Citation28
Another VOYAGE 2 analysis investigated the impact of GUS on treatment (P<0.001 for both).Citation28 Another VOYAGE 2 analysis investigated the impact of GUS on anxiety and depression by utilizing the Hospital Anxiety and Depression Scale (HADS).Citation33 Through 16 weeks of the study, GUS improved both mean anxiety (HADS-A) and depression (HADS-D) HADS subcomponent scores than placebo (HADS-A: −1.7±3.4 to −0.2±2.9; HADS-D: −1.6±3.6 to −0.1±2.9; P<0.001 for both).Citation33 Through Week 24, GUS reduced mean HADS-A and HADS-D scores more than ADM (HADS-A: −2.0±3.6 to −1.0±3.6; P<0.001; HADS-D: −1.7±3.8 to −1.1±3.5; P=0.6), indicating greater improvements in anxiety and depression, although the difference in mean HADS-D scores between the two groups was not statistically significant (P=0.06).Citation33 Furthermore, at Week 16, a higher percentage of GUS-treated patients with HADS scores consistent with anxiety and depression attained HADS scores below the defined threshold for mild anxiety and depression compared to placebo-treated patients (HADS-A: 51.4% vs 25.9%, respectively; HADS-D: 59.2% vs 27.0%, respectively; P<0.001 for both).Citation33 Similarly, at Week 24, a higher percentage of GUS-treated patients compared to ADM-treated patients reached HADS scores below this threshold (HADS-A: 58.4% vs 42.9%, respectively; P=0.028; HADS-D: 59.8% vs 46.4%, respectively; P=0.079), although the difference between the two groups was not statistically significant for the depression specific HADS-D measure (P=0.079).Citation33
In NAVIGATE, GUS had better DLQI and PSSD score improvements compared to randomized USM.Citation29 At Week 52, within the group of patients with DLQI scores >1 at Week 16, 38.8% of GUS patients achieved DLQI scores of 0 or 1 compared to 19% of patients in the randomized USM group (P=0.002; ).Citation29 Similarly, at Week 52, among patients who had PSSD symptom scores >0 at Week 16, 20.3% of patients in the GUS cohort achieved a PSSD symptom score of 0 compared to 9.5% of patients in the randomized USM cohort (P<0.05).Citation29
Discussion
Overall, GUS is an excellent treatment option for adult patients with moderate-to-severe plaque psoriasis. It targets the p19 cytokine subunit, which is present in the IL-23 and IL-39 members of the IL-12 heterodimeric cytokine family, but not in IL-12 ().Citation14,Citation15,Citation22,Citation23 For comparison, USM targets the p40 cytokine subunit, which is present in both IL-23 and IL-12 ().Citation16 IL-23 plays a crucial role in psoriasis pathogenesis, and targeting IL-23 through the p19 subunit is an effective treatment strategy.Citation15,Citation21,Citation26–Citation29
GUS provides effective disease control and is more ffective than the established TNF-alpha inhibitor ADM.Citation15,Citation27,Citation28 Furthermore, GUS’s success in treating patients with incomplete responses to ADM and USM highlights its role as an alternative treatment option for patients failing initial biologic medications.Citation28,Citation29 The effectiveness of GUS in treating scalp, hand, feet and fingernail diseases also suggests that it is a good option for patients with refractory disease in these difficult treat areas.Citation27,Citation28 In the Phase IIa clinical trial, GUS was effective in treating PsA.Citation30 For patients with PsA, TNF-alpha inhibitors are generally first-line options, with IL-17 inhibitors and USM as alternatives.Citation34 GUS is a good option for moderate-to-severe psoriasis whether patients have PsA or not and may be a reasonable treatment option for PsA patients failing TNF-alpha inhibitors.
While there are no clinical trials that directly compare GUS to IL-17 inhibitors, they appear to have similar efficacies. At Week 12 of Phase III clinical trials, the IL-17A antagonists ixekizumab and secukinumab had 70.7% and 59.2% PASI90 response rates, respectively, while the IL-17A receptor antagonist brodalumab had a 70.3% PASI90 response rate.Citation35–Citation37 These efficacies are comparable to GUS, which had a 73.3% PASI90 response rate at Week 16 of the Phase III VOYAGE 1 trial.Citation27
Even though both GUS and USM block IL-23, they have different efficacies.Citation29 One potential explanation is that IL-12 may have protective effects in psoriasis.Citation38 In one study, imiquimod-treated mice lacking IL-12 signaling components developed worse psoriasis compared to wild-type mice.Citation38 Therefore, inhibition of IL-12 may impair therapeutic efficacy in psoriasis.Citation38 Another possibility is that other p19 cytokines, such as IL-39, play a role in psoriasis pathogenesis. GUS’s effects might extend beyond inhibition of IL-23 through the p19 subunit and include effects from blocking other p19 cytokines. While more complete blockage of p19 may have increased efficacy, it may also produce additional side effects.
USM is a very safe medication even when used long term, which suggests that inhibiting the IL-12/IL-23 pathway produces a favorable side effect profile.Citation39 GUS appears to also have a favorable side effect profile, although long- term data are necessary to fully evaluate its safety.Citation15,Citation21,Citation26–Citation29 Considering that GUS inhibits IL-23 without blocking IL-12, many people believe that it should be at least as safe as USM; such assumptions are not warranted, as the immune system is inscrutable. Moreover, because GUS inhibits IL-39, a cytokine not inhibited by USM, in addition to IL-23, it seems inappropriate to assume that USM’s excellent safety profile necessarily extends to GUS. The natural experiment of genetic defects in p19 expression is reassuring. In one study, mice without IL-23 p19 had impaired T-cell immune responses with otherwise normal development.Citation40
While IL-17 antagonists exacerbate inflammatory bowel disease,Citation41 biologics antagonizing IL-23 (including an IL12/23 blocker and an IL23/39 blocker) improve inflammatory bowel disease.Citation42,Citation43 As a result, GUS may be a good choice for patients with both moderate-to-severe plaque psoriasis and concurrent inflammatory bowel disease. Finally, GUS did not cause disease reactivation in patients with latent TB who also received TB prophylaxis.Citation32 In clinical trials evaluating USM, there were similarly no reported reactivations of latent TB in patients receiving concurrent isoniazid prophylaxis.Citation34,Citation44 USM is currently the preferred biologic agent for patients with concomitant latent TB, with IL-17 inhibitors as second-line options.Citation34 Considering their similar mechanisms, GUS is a reasonable alternative to USM for patients with both psoriasis and latent TB.
In addition to GUS, other p19 inhibitors are increasingly becoming the focus of clinical trials.Citation45–Citation47 Risankizumab and tildrakizumab are both humanized monoclonal antibodies that similarly target p19.Citation45,Citation46 In a Phase II clinical trial, more risankizumab-treated patients than USM-treated patients attained PASI90 at Week 12 (77% vs 40%, respectively; P<0.001).Citation46 These results were corroborated in two recent Phase III trials, UltlMMa-1 and UltlMMa-2, in which more risankizumab-treated patients attained PASI90 at Week 16 compared to USM-treated patients (UltlMMa-1: 75.3% vs 42.0%, respectively; UltlMMa-2: 74.8% vs 47.5%, respectively; P<0.001 for both).Citation47 At Week 12 of another Phase III clinical trial, more patients treated with tildrakizumab than with etanercept attained PASI75 (66% for 200 mg tildrakizumab vs 61% for 100 mg tildrakizumab vs 48% for etanercept; P<0.0001 and P<0.001 for 200 and 100 mg tildrakizumab vs etanercept, respectively).Citation45 Based on these studies, p19 inhibition may be a more effective treatment strategy than TNF-alpha and IL-12/IL-23 inhibition, although not all p19 inhibitors appear to have the same degree of efficacy. Further understanding of psoriasis pathogenesis will clarify the exact roles and mechanisms of IL-23 and the p19 subunit.
The FDA has instructed that additional studies be reported on long-term malignancy risk, the safety of GUS for pregnant patients and long-term risk of other AEs.Citation32 Prolonged follow-up and large long-term clinical trials will ultimately determine the safety and efficacy of GUS.
Conclusion
GUS is an effective novel monoclonal antibody that is FDA approved for the treatment of moderate-to-severe plaque psoriasis in adult patients. It targets the p19 subunit of IL-23 and IL-39, which appears to play a critical role in psoriasis pathogenesis.Citation21 The medication is well tolerated, and side effects are generally mild and most commonly include infections.Citation15,Citation21,Citation26–Citation29 In the reported clinical trials, GUS markedly improved disease with corresponding improvements in quality of life measures.Citation15,Citation21,Citation27–Citation29 The medication also successfully treated difficult to treat areas of the body, including the scalp, hands, feet and fingernails.Citation27,Citation28 In addition to its impact on cutaneous lesions, GUS may be effective in treating PsA.Citation30 Furthermore, GUS was more effective than ADM and was successful in treating patients with incomplete responses to ADM and USM.Citation15,Citation27–Citation29 Long-term investigation of both side effects and efficacy is required.
Disclosure
Dr Feldman has received research, speaking and/or consulting support from Galderma, GSK/Stiefel, Almirall, LEO Pharma, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, AbbVie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. The authors report no other conflicts of interest in this work.
References
- HelmickCGLee-HanHHirschSCBairdTLBartlettCLPrevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination SurveysAm J Prev Med2014471374524746373
- KimWBJeromeDYeungJDiagnosis and management of psoriasisCan Fam Physician201763427828528404701
- KurdSKTroxelABCrits-ChristophPGelfandJMThe risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort studyArch Dermatol2010146889189520713823
- WeissSCKimballABLiewehrDJBlauveltATurnerMLEmanuelEJQuantifying the harmful effect of psoriasis on health-related quality of lifeJ Am Acad Dermatol200247451251812271293
- KimballABGladmanDGelfandJMNational Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screeningJ Am Acad Dermatol20085861031104218313171
- OliveiraMFRochaBODuarteGVPsoriasis: classical and emerging comorbiditiesAn Bras Dermatol201590192025672294
- LanganSMSeminaraNMShinDBPrevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United KingdomJ Invest Dermatol20121323 Pt 155656222113483
- Canadian Psoriasis Guidelines AddendumC2016 Addendum to the Canadian Guidelines for the Management of Plaque Psoriasis 2009J Cutan Med Surg201620537543127421294
- SmithCHAnsteyAVBarkerJNBritish Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009Br J Dermatol20091615987101919857207
- BonifatiCAmeglioFCytokines in psoriasisInt J Dermatol199938424125110321938
- CarrascosaJMJacobsIPeterselDStrohalRBiosimilar drugs for psoriasis: principles, present, and near futureDermatol Ther201882173194
- di CesareAdi MeglioPNestleFOThe IL-23/Th17 axis in the immunopathogenesis of psoriasisJ Invest Dermatol200912961339135019322214
- OppmannBLesleyRBlomBNovel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12Immunity200013571572511114383
- LupardusPJGarciaKCThe structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12J Mol Biol2008382493194118680750
- GordonKBDuffinKCBissonnetteRA Phase 2 trial of guselkumab versus adalimumab for plaque psoriasisN Engl J Med2015373213614426154787
- LuoJWuSJLacyERStructural basis for the dual recognition of IL-12 and IL-23 by ustekinumabJ Mol Biol2010402579781220691190
- LeeETrepicchioWLOestreicherJLIncreased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgarisJ Exp Med2004199112513014707118
- Michalak-StomaABartosińskaJKowalMJuszkiewicz-BorowiecMGerkowiczAChodorowskaGSerum levels of selected Th17 and Th22 cytokines in psoriatic patientsDis Markers201335662563124288431
- BettelliEOukkaMKuchrooVKT(H)-17 cells in the circle of immunity and autoimmunityNat Immunol20078434535017375096
- RaychaudhuriSPRole of IL-17 in psoriasis and psoriatic arthritisClin Rev Allergy Immunol201344218319322362575
- SofenHSmithSMathesonRTGuselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasisJ Allergy Clin Immunol201413341032104024679469
- WangXWeiYXiaoHA novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like miceEur J Immunol20164661343135027019190
- VignaliDAKuchrooVKIL-12 family cytokines: immunological playmakersNat Immunol201213872272822814351
- CargillMSchrodiSJChangMA large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genesAm J Hum Genet200780227329017236132
- NairRPDuffinKCHelmsCGenome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathwaysNat Genet200941219920419169254
- ZhuangYCalderonCMarciniakSJFirst-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasisEur J Clin Pharmacol201672111303131027515978
- BlauveltAPappKAGriffithsCEEfficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trialJ Am Acad Dermatol201776340541728057360
- ReichKArmstrongAWFoleyPEfficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trialJ Am Acad Dermatol201776341843128057361
- LangleyRGTsaiTFFlavinSEfficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trialBr J Dermatol2018178111412328635018
- DeodharAGottliebABoehnckeWHEfficacy and safety results of guselkumab, an anti-Il23 monoclonal antibody, in patients with active psoriatic arthritis over 24 weeks: a phase 2a, randomized, double-blind, placebo-controlled studyAnn Rheum Dis201776142143
- GordonKBBlauveltAFoleyPEfficacy of guselkumab in subpopulations of patients with moderate-to-severe plaque psoriasis: a pooled analysis of the phase III VOYAGE 1 and VOYAGE 2 studiesBr J Dermatol2018178113213928940259
- Center for Drug Evaluation and ResearchMulti-discipline review/summary application numberResearch CfDEa 016761061Orig1s000
- GordonKBArmstrongAWHanCAnxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 studyJ Eur Acad Dermatol VenereolSuppl 1 Epub2018428
- AminMNoDJEgebergAWuJJDjNJjWChoosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say?Am J Clin Dermatol201819111329080066
- GriffithsCEReichKLebwohlMComparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trialsLancet2015386999354155126072109
- LangleyRGElewskiBELebwohlMSecukinumab in plaque psoriasis – results of two phase 3 trialsN Engl J Med2014371432633825007392
- PappKAReichKPaulCA prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasisBr J Dermatol2016175227328626914406
- KuligPMusiolSFreibergerSNIL-12 protects from psoriasiform skin inflammationNat Commun201671346627892456
- PappKAGriffithsCEGordonKLong-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-upBr J Dermatol2013168484485423301632
- GhilardiNKljavinNChenQLucasSGurneyALde SauvageFJCompromised humoral and delayed-type hypersensitivity responses in IL-23-deficient miceJ Immunol200417252827283314978083
- HohenbergerMCardwellLAOussedikEFeldmanSRInterleukin-17 inhibition: role in psoriasis and inflammatory bowel diseaseJ Dermatolog Treat2018291131828521565
- SandbornWJGasinkCGaoLLUstekinumab induction and maintenance therapy in refractory Crohn’s diseaseN Engl J Med2012367161519152823075178
- FeaganBGSandbornWJD’HaensGInduction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 studyLancet2017389100801699170928411872
- TsaiTFHoVSongMThe safety of ustekinumab treatment in patients with moderate-to-severe psoriasis and latent tuberculosis infectionBr J Dermatol201216751145115222803615
- ReichKPappKABlauveltATildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSUR-FACE 2): results from two randomised controlled, phase 3 trialsLancet20173901009127628828596043
- PappKABlauveltABukhaloMRisankizumab versus ustekinumab for moderate-to-severe plaque psoriasisN Engl J Med2017376161551156028423301
- GordonKBEfficacy and safety of risankizumab: results from two double-blind, placebo- and ustekinumab-controlled, phase 3 trials in moderate-to-severe plaque psoriasisPaper presented at: American Academy of Dermatology2018San Diego, CA
