Abstract
Purpose
SCN1A (3184 A>G) and SCN2A (56G>A) gene encodes α subunit of the neuronal voltage-gated sodium channel, which is a target for carbamazepine (CBZ). Recent studies have demonstrated that polymorphism of SCN1A (3184 A>G) and SCN2A (56G>A) was associated with use of CBZ. However, it has not been determined whether the polymorphism affects CBZ or other antiepileptic drug responsiveness. The aim of the study was to establish whether the SCN1A (3184 A>G) and SCN2A (56G>A) polymorphisms of the SCN1A and SCN2A genes affect responsiveness to CBZ.
Methods
SCN1A (3184 A>G) and SCN2A (56G>A) gene polymorphisms were genotyped in 93 Khyber Pakhtunkhwa epileptic patients treated with CBZ. The association between CBZ responsiveness and the polymorphism was estimated by adjusting for clinical factors affecting the outcome of therapy. The number of seizure episodes was documented at baseline, and the therapy of each of the 93 patients was followed up. The plasma level of CBZ was determined using reverse-phase high-performance liquid chromatography. SCN1A and SCN2A genes were genotyped using RFLP. Data were analyzed using Graph Pad Prism 6.
Results
Mean age of the patients was 18.6±9.3 at the 3rd month and 18.7±9.5 at the 6th month. The baseline dose of CBZ was 468±19.8 mg/d and titrated at the rate of 48±1.4 and 4.0±0.2 mg/d. The difference in plasma level of CBZ was significant (P=0.004) between 3rd and 6th month among different genotypes of SCN1A gene in nonresponder and responder patients. At the 3rd month of the therapy, the poor responders were more likely (P=0.003 and P=0.01) to have variants (3184AG and 3184GG) of SCN1A gene. Similarly, poor responsders were more likely (P=0.0007 and P=0.001) to have variant genotypes (56GA, 56AA) of SCN2A gene at the 3rd month of the therapy.
Conclusion
This study demonstrated a significant association between the SCN1A (3184 AG and GG) and SCN2A (56GA and AA) genotype with CBZ-nonresponsive epilepsy.
Introduction
Epilepsy is a neurological disorder which is characterized by periodic episodes of fits and seizures. Carbamazepine (CBZ) and valproate are considered first-line drugs in the treatment of epilepsy in most parts of the world. Valproate is the drug of choice for generalized tonic–clonic seizure, absence seizure, and myoclonic seizures, while the treatment of choice for simple partial, complex partial, and secondary generalized tonic–clonic seizures is CBZ.Citation1,Citation2 Unfortunately, 30% of epileptic patients are resistant to antiepileptic drugs (AEDs),Citation3 posing a huge financial burden to the patients.Citation4 It has been reported that personal genetic variations affect the response of a drug and the ultimate pharmacoresistance, if any.Citation5 There are polymorphic genes encoding drug targets and drug-metabolizing enzymes that can be used to predict the targeted outcomes of AEDs.Citation6 CBZ is one of the most economical AEDs that is used for the treatment of partial and generalized tonic–clonic seizures.Citation7 Studies have shown that substantial interindividual variations in response still exist to CBZ therapy.Citation8 However, genetic variations are considered one of the main factors that might be responsible for variable response to the drug therapy in individualized patients.Citation9 SCN1A gene expresses the α subunit of voltage-gated sodium channel type 1 that is responsible for firing of neurons in the brain.Citation10,Citation11 These ion channels are molecular targets for many AEDs,Citation12 which block ionic exchange through the respective channels.Citation6,Citation13 Sodium channels have a large pore-forming α subunit associated with two smaller β subunits.Citation10,Citation14 Evidence suggests that AEDs mainly act by blocking the α subunit.Citation13 Previous studies have documented that most of mutations in the sodium channel gene, SCN1A, have been associated with myoclonic epilepsy in infancy, and with familial epilepsy,Citation15–Citation17 with ultimate effectiveness of drug.Citation15,Citation18,Citation19 The polymorphisms in SCN1A and SCN2A alter the sodium channels structure and functions relationship with AEDs, thereby rendering them insensitive to the blocking effect of AEDs. That is why some patients respond well to AEDs while others do not respond.Citation15 It has been found that SCN1A polymorphisms are associated with CBZ-resistant epilepsy.Citation19 SCN2A gene, (c.56G>A), is related to AED resistance in north Indian epileptic patients.Citation16 SCN1A and SCN2A gene polymorphisms have no effect on the pharmacokinetic of CBZ but have an association with the pharmacodynamics of CBZ. SCN1A (3184 A>G) encodes the Thr 1067 Ala and SCN2A (56G>A) encode Arg19Lys. There are certain genes called candidate genes that have a strong association with interindividual drugs response, but some genes are called contributing genes as these may affect the interindividual drug response by interaction with other genes. It has been demonstrated that SCN1A gene polymorphisms are potential risk factors for drug resistance.Citation20 Bao et alCitation21 also suggested that SCN1A gene polymorphisms are significantly associated with drug resistance.
Aims of the study
The current study was focused on finding out the association of SCN1A and SCN2A genes polymorphisms with poor response to CBZ therapy in epileptic patients of Khyber Pakhtunkhwa, Peshawar, Pakistan. It is important to mention here that such types of studies are carried out in most populations of the world with variable conclusions. This study was carried out for the first time in the Khyber Pakhtunkhwa, Pakistan, population, and so this will add new knowledge to the existing literature.
Ethics approval and consent to participate
The study was approved by the Ethics Board of the Khyber Medical University, Peshawar via approval no DIR/KMU-EB/AC/000165 and the study complied with Helsinki’s declaration.
Methods
Selection of patients
Ninety three patients suffering from different types of epilepsies, without any comorbidity, were purposively enrolled in the study at Outpatient Department of Neurology, Government Lady Reading Hospital, Peshawar, Pakistan, from August 2015 to June 2017. They were prescribed CBZ for the first time. Medical history, careful physical examinations, and complete investigations were recorded on a standardized proforma by respective ward physicians. All patients signed written consents form after explanation of the steps and the aims of the study were made in their local language(s).
Sample size calculation, study design, and protocols
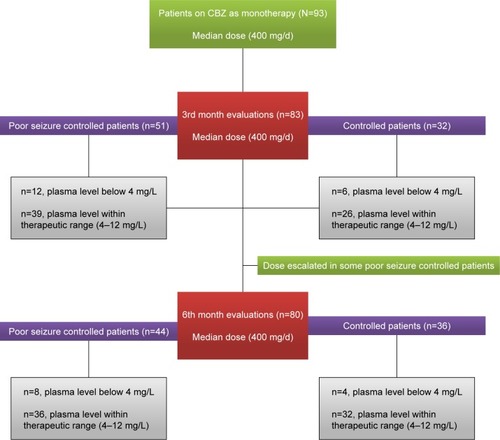
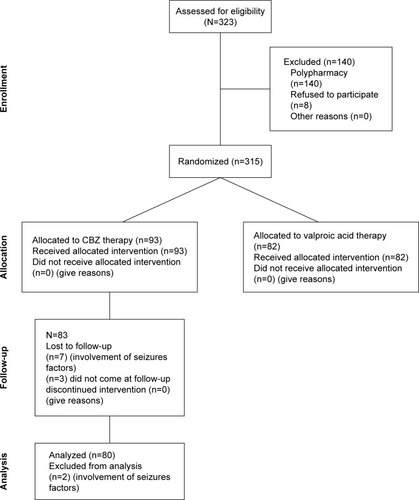
Sample size (n=80) were calculated at 95% CI, SD 4, and margin of error 1. The α score was 0.025, and Z score was 1.95. The value of β in sample size calculation is 0.8. Keeping in view 20% dropout rate, the total patients included in the study was 93. Also, it was a hospital based longitudinal study done with a 6-month follow-up period. Baseline interviews were carried out for determining types, evaluation of seizures, and subsequent initiation of the CBZ therapy. All the enrolled patients were treated with CBZ (Tegrol 200 and 400 mg) monotherapy on the basis of their seizure control keeping in view their types of epilepsies. Patients were asked to visit the Outpatient Department of Neurology Department of Lady Reading Hospital, on a monthly basis, for monitoring of their clinical prognosis and following for adherence to CBZ therapy. The enrolled patients were evaluated at the 3rd and 6th month of the CBZ therapy for clinical scoring to assess epilepsy management and determine their mean plasma levels for CBZ. The doses of CBZ in patients with poor seizure control were escalated, based on patients’ prognosis, by their respective ward/neurophysicians. The protocol of study is comprehensively shown in and . According to guideline 6-month follow-up are short but most biochemical factors can be affected by CBZ within 6th month and more follow-up period affect the quality of life of patients. The patients whose seizure were not controlled were prescribed with other AEDs or switched to other AEDs without any further delay which affected the compliance and well-being of patients to seizure control. There were three patients that dropped out due to Steven–Johnson syndrome.
Inclusion criteria
Epileptic patients in whom CBZ as monotherapy was advised were enrolled in the study. Patients’ willingness was checked before entering them into the study. The aim of the study in the context of local language was also explained to them. The seizure-provoking factors (sleep deprivations, drugs, etc) were controlled from date of enrollment to the end of the follow-up period. As epilepsy has multivariate etiology, we have tried to control the confounding factors that affect the clinical response.
Exclusion criteria
Patients who were not willing to participate in the study or were suffering from comorbidities were excluded from the study.
Blood sampling
Blood samples from each patient were taken for plasma therapeutic drug monitoring at end of 3rd and 6th month of the CBZ therapy. Timing of the blood sampling was 3 hours after administration of morning oral dose. We focused on 3rd and 6th month as we were also focusing on possible shift in other biochemical aspects, which may change during the course of therapy. Briefly, blood samples from the targeted patients were taken in gel tubes. The plasma was separated and stored for further analysis. For genotyping, blood samples were taken in EDTA tubes, stored at 8°C till used for DNA extraction and subsequent genotyping.
Genotyping of SCN1A (rs2298771) and SC2A (rs17183814) genes
Genomic DNA was extracted using kit method (NucleoSpin® Blood, MACHEREY-NAGEL GmbH & Co., Düren, Germany). Briefly, the extraction of DNA was carried out through a series of reactions which included deproteination of blood (proteinase K), lysis of blood cell (lysis buffer), purification of DNA (washing buffer), and finally elution of DNA (through elution buffer) according to standard protocols provided by the manufacturer. The extracted DNA was stored at −20°C for further analysis. SCN1A (c.3184 A>G) and SCN2A (c.56G>A) genes were amplified using gradient thermocycler. After amplification, the PCR products were run on 2% agarose gel and their sizes were assessed with a 50 bp ladder. SCN1A (c.3184 A>G) gene was amplified by using primer 5-TGCACAAAGGAGTAGCTTATG-3 and primer 5-AGTCAAGATCTTTCCCAATTTCAG-3. The amplified product was digested using restriction enzyme PvuII. Similarly, SCN2A (c.56G>A) gene was amplified by using primer 5-AATCACCTTTTATTCTAATGGTC-3 and primer 5-CAGTGAAGGCAACTTGACTAAGA-3. The resulting product was digested using restriction enzyme ScrFI. The digested products of each amplified compound were run on 2% agarose gel, and respective fragments sizes were assessed with 50 bp ladder.Citation22
Analysis of plasma levels of CBZ
Plasma level of CBZ was determined on adjusted dose in epileptic patients at the 3rd and 6th month of therapy. Plasma from the centrifuged sample (0.2 mL) in gel tubes were added to 50 µL of 25% ammonium hydroxide (BDH Laboratory Supplies, Poole, England) and 5 mL of chloroform (high-performance liquid chromatography grade) (Scharlab S>L., Barcelona, Spain). The samples were mixed in a mechanical shaker for 20 minutes and were centrifuged (PLC-05, Taiwan) at 3,000 rpm for 10 minutes. After centrifugation, the organic (chloroform) layer was transferred to another glass tube and evaporated by keeping the tube at 50°C in a water bath. The dry extract was reconstituted in 10 mL mobile phase. The extracted CBZ was analyzed using reverse-phase HPLC (RP-HPLC) (HPLC LC-20AT Shimadzu, Kyoto, Japan) coupled with UV detector SPD-20A/20AV (Shimadzu) with a slight modifications in method of Dordević et al.Citation23 In short, the mobile phase was a mixture of methanol (Chromasolv HPLC grade, Cerritos, CA, USA): demineralized water: glacial acetic acid 100% (Merck KGaA, Darmstadt, Germany) at a ratio of 65:34:1 (v/v/v) with a pH 5.6. The flow rate was 0.8 mL/min on a C18 column (SEA 18, 5 µm 25×0.46 Mediterranean) with a load volume of 20 µL of sample. Detection of CBZ was performed in the UV range at λmax 220 nm.Citation23 The validation parameters such % CV, precision, % accuracy, and % recovery were tested and validated on an intra- and interday basis (unpublished data).
Measurement of patients’ seizure control
Seizure control was recorded in the form of reduction of frequencies of seizures using a standardized proforma. The numbers of seizures per week of the enrolled patients were recorded at baseline at the time of initiation of CBZ therapy (Tegral 200 and 400 mg), followed by evaluation of seizure control at the 3rd and 6th month of the CBZ monotherapy. The patient compliance to the medication was checked by counting the pills remaining in the strip, and feedback from the patients was taken on a standardized patients’ response sheet designed for this purpose in local language. Prognosis was determined as freedom from seizures after initiation of CBZ therapy and evaluated by time to time for clinical response. However, we present here prognosis at 3rd and 6th month of the therapy so as to correlate prognosis with plasma levels of CBZ therapy. Hence, the number of seizures was recorded in term of “number of seizures per week” on a prescribed proforma designed and standardized for the local context.
Statistical analysis
Possible changes in plasma levels of CBZ were compared after 3 and 6 months of the CBZ therapy among patients with different genotypes of SCN1A (c.3184 A>G) and SCN2A (c.56G>A) genes using one-way ANOVA followed by Tukey’s test. Changes in plasma levels within different genotypes were also compared by using unpaired Student’s t-test with Welchs’ correction for high difference in SD. Differences in duration and frequencies of seizures in CBZ-resistant patients were determined using unpaired Student’s t-test with Welchs’ correction for high difference in SD. Association of variant genotypes of SCN1A (c.3184 A>G) and SCN2A (c.56G>A) genes with patients with poor response to CBZ therapy and those with response to CBZ were determined using Chi-squared test with Yates’ correction at 95% CI, P≤0.05 for small sample size. All data were analyzed using Graph Pad Prism 6 (GraphPad Software, La Jolla, CA, USA) and G.Power 3.1 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany).
Results
Demographic data and treatment
Patients’ demographic data and types of epilepsies are shown in . Mean age of the patients was 18.6±9.3 at the 3rd month and 18.7±9.5 at the 6th month. Generalized tonic–clonic seizures were the most frequently (62%) reported type of epilepsy (). The initial baseline mean dose was 468±19.8 mg/d, and the mean increased dose was 516±21.2 mg/d for better management of seizures at the 3rd month. Similarly, mean uptitrated dose was 520±21.4 mg/d at 6th month of the therapy (). CBZ concentration-to-dose ratio (C/D) was 0.021±0.003 at 3rd month and 0.024±0.04 at 6th month of the therapy ().
Table 1 Demographic features of the enrolled patients
Table 2 Dose and frequency of CBZ as monotherapy
Plasma levels of CBZ vs genotypes of SCN1A and SCN2A genes
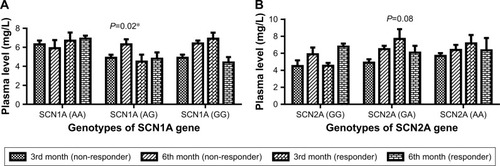
The plasma level of CBZ was compared among different genotypes of SCN1A c.3184 A>G and SCN2A c.56G>A genes in nonresponders and responders at 3rd and 6th month of therapy (). The difference in plasma levels of CBZ was significant (P=0.004) between 3rd and 6th month of the study among different genotypes of SCN1A c.3184 A>G genes in nonresponder and responder patients (). However, no significant (P>0.05) changes were found in plasma levels of CBZ between 3rd and 6th month of the study among different genotypes of SCN2A c.56G>A genes (). There were 18 patients whose plasma level was below the therapeutic range at the 3rd month of the therapy (). However, the number of patients reduced to 12 whose plasma level was below the therapeutic range at 6th month of the therapy (). The validation parameters of method development for the analysis of plasma level of CBZ were found within the recommended range on both interday and intraday basis (unpublished data).
Control of seizures at 3rd month of the therapy
After 3 months of CBZ therapy, 51 patients were found with poor response to CBZ. The epileptic patients’ seizure control for 3 months is presented in . It has been observed that poor seizure-controlled patients were more likely to have variants (3184AG and 3184GG) of SCN1A gene than patients in whom seizures were controlled (χ2=6.9, P=0.003) (). Similarly, poor seizure-controlled patients were also more likely to have variants (56GA, 56AA) of SCN2A gene than patients in whom seizures were controlled (χ2=14.4, P=0.0007) ().
Table 3 Mean plasma levels of CBZ in patients with SCN (1A and 2A) gene polymorphisms with its relative frequencies in poor seizure-controlled and seizure-controlled patients at 3rd month of CBZ therapy
Control of seizures at 6th month of the therapy
Following 6 months of CBZ therapy, it was evident that 34 patients were found with poor response to CBZ therapy (). Poor seizure-controlled patients were more likely to have variant (3184AG and 3184GG) genotypes of SCN1A gene than controlled patients (χ2=9.1, P=0.01) (). Similarly, poor seizure-controlled patients were more likely to have variant genotypes (56GA, 56AA) than in controlled patients (χ2=13.8, P=0.001) (). It has been found that variant genotypes (3184AG, 3184GG) of SCN1A gene and (56GA and 56AA) of SCN2A gene in resistant patients at 3rd month of the therapy also remained resistant at the 6th month of CBZ therapy. Differences in frequency of seizures per week were not significant (P>0.05) in CBZ-nonresponsive patients in comparison of baseline with 3rd as well as baseline with 6th month of the therapy, especially in those with variant genotypes of SCN1A and SCN2A genes (). This means that frequency of seizures per week was not affected in those patients with variant genotypes of SCN1A and SCN2A genes.
Figure 4 Duration of seizures per week in nonresponder patients to CBZ on 3rd and 6th month of the therapy.
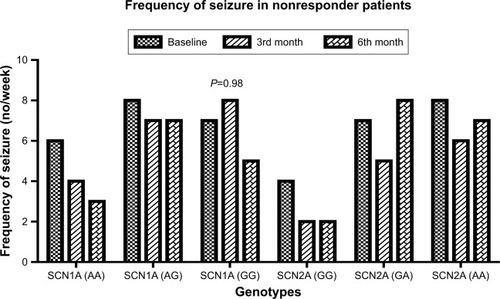
Table 4 Mean plasma level of CBZ in patients with SCN (1A and 2A) gene polymorphisms with its relative frequencies in poor seizure-controlled and seizure-controlled patients at 6th month of CBZ therapy
Discussion
It has been found that generalized tonic–clonic seizure was the most frequent type of seizure among the enrolled patients. It was obvious that CBZ is the drug of choice for simple partial, complex partial, and also generalized tonic–clonic seizures in most parts of the world.Citation2 Epilepsy incidence was higher in male patients as compared with female patients. SCN1A and SCN2A genes polymorphisms have an association with pharmacodynamics but not with pharmacokinetics and plasma levels of CBZ.Citation19 We found that the shift in the plasma levels of CBZ at 3rd month of the therapy was significant however, difference was not significant among the wild (3184AA and 56GG) of SCN1A and SCN2A genes in responder and nonresponder patients. The changes in the plasma levels of CBZ in variant genotypes (3184AG, 3184GG) of SCN1A gene were significant in responders and nonresponders. However, changes in the plasma levels of CBZ were not significant in responders and nonresponders in variant genotypes (56GA, 56AA) of SCN2A gene. So, it has been suggested that a shift in plasma level of CBZ is not consistent among different genotypes of SCN1A and SCN2A genes at 3rd and 6th month of the therapy. Ebid et alCitation24 reported that therapeutic steady state did not guarantee clinical effectiveness of a drug. It has been found that variant genotypes (3184AG, 3184GG and 56GA, 56AA) of SCN1A and SCN2A genes were associated with poor response to CBZ at 3rd as well as 6th month of the therapy. However, there are controversies with regard to clinical outcomes with respect to variant genotypes (3184AG, 3184GG and 56GA, 56AA) of SCN1A and SCN2A genes.Citation25 However, Abe et alCitation19 have shown that variant genotypes of 3184AG, 3184GG and 56GA, 56AA of SCN1A and SCN2A genes, respectively, are associated with poor response to CBZ. Recently, a common polymorphism in SCN1A was shown to be significantly associated with maximum doses of CBZ serum concentrations at maintenance dose in English and Chinese patient cohorts.Citation18,Citation26 SCN1A and SCN2A gene polymorphisms have been associated with drug-resistant epilepsy, but these reports have not been consistent so far in many studies. Such discrepancies are also related to the other factors apart from genes polymorphisms. It has been found that there was no significant reduction in the number of seizures per week in poor seizure-control patients who have variant genotypes of SCN1A and SCN2A polymorphisms despite the fact that all seizure-provoking factors (deprivation of sleep, anxiety, seizure inducing drugs, etc) were strictly controlled for. This was done because these factors affect the clinical response of AEDs and lead to bias in the conclusion of the study. However, quite a high reduction in the number of seizures was found in those with wild genotypes of SCN1A and SCN2A.
Conclusion
Our study suggested that variant genotypes of SNC1A (3184AG and 3184GG) and SCN2A (56AG and 56GG) genes are associated with poor response to CBZ monotherapy despite the fact that the patients’ plasma levels were in the therapeutic range, which may point to possible pharmacodynamic interactions and are not related to a major shift in plasma levels of the drug in mean dose of 520±21.4 mg/kg/d.
Consent for publication
The patients gave consent to publish their data. Their confidentiality was maintained.
Data sharing statement
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
HRN carried out experimental work as a PhD research scholar. SU and HRN prepared the first draft of manuscript. NA extensively revised the manuscript. He also designed the study and wrote the research project. All authors contributed equally during experiment, data analysis and writing the manuscript. All authors approved the final version of manuscript and agree to be accountable for all aspects of the work.
Acknowledgments
The authors also thank Professor Dr Adnan Khan and the Chief Executive of Govt. Lady Reading Hospital Peshawar for providing the opportunity to carry out this research work. The authors thank the Higher Education Commission of Pakistan (grant no 20-4092/NRPU/R&D/HEC) for providing funds for the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChadwickDValproate in the treatment of partial epilepsiesEpilepsia199435Suppl 5S96S988039481
- KenyonKMintzerSNeiMCarbamazepine treatment of generalized tonic-clonic seizures in idiopathic generalized epilepsySeizure201423323423624370318
- KwanPBrodieMJEarly identification of refractory epilepsyN Engl J Med2000342531431910660394
- CardarelliWJSmithBJThe burden of epilepsy to patients and payersAm J Manag Care20101612 SupplS331S33621517648
- Emich-WideraELikusWKazekBCYP3A5*3 and C3435T MDR1 polymorphisms in prognostication of drug-resistant epilepsy in children and adolescentsBiomed Res Int2013201317
- KumariRLakhanRGargRKKalitaJMisraUKMittalBPharmacogenomic association study on the role of drug metabolizing, drug transporters and drug target gene polymorphisms in drug-resistant epilepsy in a north Indian populationIndian J Hum Genet201117Suppl 1S3221747585
- BrodieMEngelJLeePde BoerHEuropean white paper on epilepsy-Call to actionEpilepsia200344412790881
- KwanPBrodieMJEffectiveness of first antiepileptic drugEpilepsia200142101255126011737159
- KasperaviciuteDSisodiyaSMEpilepsy pharmacogeneticsPharmacogenomics200910581783619450130
- CatterallWAStructure and function of voltage-gated sodium channels at atomic resolutionExp Physiol2014991355124097157
- CatterallWAChannelsSSodium channels, inherited epilepsy, and antiepileptic drugsAnnu Rev Pharmacol Toxicol20145431733824392695
- ParkerDSandersEJBurghardtKJPharmacogenetics of antiepileptic drugs: A brief reviewMent Health Clin201661283429955444
- KwanPPoonWSNgHKMultidrug resistance in epilepsy and polymorphisms in the voltage-gated sodium channel genes SCN1A, SCN2A, and SCN3A: correlation among phenotype, genotype, and mRNA expressionPharmacogenet Genomics2008181198999818784617
- BaumLHaerianBSNgHKCase-control association study of polymorphisms in the voltage-gated sodium channel genes SCN1A, SCN2A, SCN3A, SCN1B, and SCN2B and epilepsyHum Genet2014133565165924337656
- WangPZhouQShengYTangBLiuZZhouBAssociation between two functional SNPs of SCN1A gene and efficacy of carbamazepine monotherapy for focal seizures in Chinese Han epileptic patientsZhong Nan Da Xue Xue Bao Yi Xue Ban201439543344124921390
- LakhanRKumariRMisraUKKalitaJPradhanSMittalBDifferential role of sodium channels SCN1A and SCN2A gene polymorphisms with epilepsy and multiple drug resistance in the north Indian populationBr J Clin Pharmacol200968221422019694741
- EbrahimiAHoushmandMTonekaboniSHFallah Mahboob PassandMSZainaliSMoghadasiMTwo novel mutations in SCN1A gene in Iranian patients with epilepsyArch Med Res201041320721420682179
- TateSKDepondtCSisodiyaSMGenetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoinProc Natl Acad Sci U S A2005102155507551215805193
- AbeTSeoTIshitsuTNakagawaTHoriMNakagawaKAssociation between SCN1A polymorphism and carbamazepine-resistant epilepsyBr J Clin Pharmacol200866230430718489610
- MargariLLegrottaglieARVincentiAAssociation between SCN1A gene polymorphisms and drug resistant epilepsy in pediatric patientsSeizure201855303529353705
- BaoYLiuXXiaoZAssociation between two SCN1A polymorphisms and resistance to sodium channel blocking AEDs: a meta-analysisNeurol Sci20183961065107229582177
- DeeparaniTPillaiMRElavazhaganTDetection of MTHFR C677T and A1298C gene polymorphism in congenital heart diseaseMiddle-East J Scientific Res200942127132
- DordevićSKilibardaVStojanovićTDetermination of carbamazepine in serum and saliva samples by high performance liquid chromatography with ultraviolet detectionVojnosanit Pregl200966534735219489468
- EbidAHAhmedMMMohammedSATherapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective studyTher Drug Monit200729330531217529887
- HaerianBSBaumLKwanPTanHJRaymondAAMohamedZSCN1A, SCN2A and SCN3A gene polymorphisms and responsiveness to antiepileptic drugs: a multicenter cohort study and meta-analysisPharmacogenomics201314101153116623859570
- TateSKSinghRHungCCA common polymorphism in the SCN1A gene associates with phenytoin serum levels at maintenance dosePharmacogenet Genomics2006161072172617001291