Abstract
Background
Wells and Geneva scores are widely used in the assessment of pretest probability of pulmonary embolism (PE).
Objective
The objective of this study was to examine the hypothesis that mean platelet volume (MPV) may better predict PE than the clinical prediction rules.
Methods
A study was performed among patients with PE. Baseline characteristics and complete blood counts including MPV were prospectively recorded upon admission. To assess clinical probability in patients with PE risk, we used Wells and Geneva scores.
Results
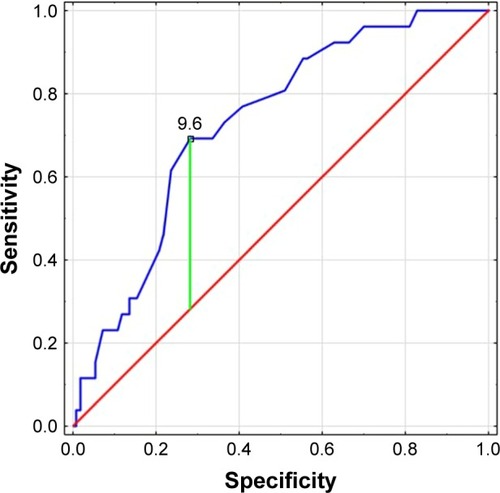
Data records of 136 patients (males: 44%) with median age of 66 years (interquartile range [IQR] 57.5–78.0) diagnosed with PE at the Intensive Cardiac Therapy Clinic in Lodz (Poland) were analyzed. Baseline characteristics indicate that patients suffered from arterial hypertension (65%), obesity (32%), and diabetes mellitus (24%). Furthermore, they reported active smoking (21%), prolonged immobilization (20%), major surgery (21%), pregnancy (4%), and oral contraceptives (9%). Patients presented with various symptoms. The MPV, plateletcrit, and D-dimer values on admission were respectively as follows: 10.71 (IQR 3.29–13.67), 0.2 (IQR 0.15–0.24), and 9.23 (IQR 8.5–9.85). The study revealed that Wells score correlated significantly with an elevated MPV value (P<0.05) per contra to Geneva score (P>0.05). According to our results, there is a lack of coherence between Wells and Geneva scores (P>0.05). Finally, we determined that the optimum MPV level cutoff point for PE on admission with reference to the original Wells score is 9.6 fL.
Conclusion
MPV may be considered useful as an adjunctive or independent predictive marker for PE used in lieu of clinical prediction rules.
Introduction
Over the past years, pulmonary embolism (PE) has been consistently associated with a high mortality rate of 15%–20%, and it consequently places a heavy burden on economics in multiple countries.Citation1 Despite a great progress in PE management in the past few decades, there is still room for a predictive evaluation improvement. Both European and US guidelines recommend more aggressive treatment for high-risk patients for early mortality.Citation2 Therefore, searching for new predictive markers for PE is essential.
The clinical manifestation of PE is often ambiguous; therefore, clinical physicians should particularly pay attention to suspected PE and use validated scores to assess pretest probability in patients with PE risk. The diagnostic algorithm should always be preceded by one of the following clinical scoring systems: original and modified Wells scoring systems or revised Geneva score ().Citation2 The Wells score is the most widely used clinical prediction rule to suspect PE and is considered the most accurate one.Citation3,Citation4 However, we should bear in mind that both the scores are based on various clinical variables, each with different points assigned, and therefore miscalculations may occur frequently.
Table 1 Clinical prediction rules for PE
On account of a growing need for the additional predictive markers for PE, an interest in platelet indices has aroused, particularly with regard to mean platelet volume (MPV). It may better reflect the platelet function when compared to the platelet count itself. Elevation of MPV may indicate increase in platelet production and activation. Furthermore, larger platelets are younger, contain more granules, and have a greater thrombogenic potential. They produce more prothrombotic substances such as thromboxane A2, serotonin, b-thromboglobulin, p-selectin, and glycoprotein IIIa, which cumulatively can hyperactivate platelets and subsequently accelerate their turnover.Citation5,Citation6 Prior studies proved the association between increased MPV level and coronary artery disease (CAD) risk factors (eg, smoking, hypertension, diabetes mellitus, and dyslipidemia), renal failure, and acute myocardial infarction.Citation7–Citation9 The best solution would be to discover the ideal predictive marker for PE. Undoubtedly, MPV is not specific for diagnosing PE; however, it may be useful in the initial estimation of PE risk.
Literature evidence lacks the MPV predictive value in PE. Therefore, the objective of this study was to determine whether MPV may be used as an equivalent of clinical prediction rules or rather an adjunctive predictive marker for PE. We believe that it may be a great chance for patients suffering from PE to improve survival rate. Perhaps, MPV may become an easy and fast predictive tool which in the future may be a part of the standard protocol for patients with PE.
Methods
Study design and population
A single-center, prospective study was based on the 136 consecutive patients with PE. To enroll the patients in our study, the diagnosis of PE had to be confirmed with computerized tomography angiography (CTA) which is considered a noninvasive gold standard in PE detection. Patients were hospitalized at the Intensive Cardiac Therapy Clinic in Lodz, Poland. Due to the fact that patients suffering from PE may present with various symptoms, we decided not to establish any other stringent selection criteria.
All blood samples were collected at admission in standardized dipotassium ethylenediaminetetraacetic acid tubes and tested within 2 hours to minimize variations due to sample aging. The blood counts were determined using automated hematology analyzers (analyzer XN 1000; Sysmex, Kobe, Japan).
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Bioethics Committee of Medical University of Lodz. Written informed consent was obtained from all the patients before participating in the study.
Definitions and analyses
PE refers to the most serious clinical manifestation of deep venous thromboembolism (DVT) and is defined as an obstruction of a pulmonary artery or one of its branches. Pressure overload may lead to right ventricular (RV) failure which is considered the primary cause of death in PE.Citation2
Wells and Geneva scores are the clinical prediction rules frequently used for a PE clinical probability assessment. Both the scores are calculated from points assigned to each of the criteria and allow physicians to delineate level of PE risk.Citation2
Baseline characteristics such as gender, age, main symptoms, and risk factors but also complete blood counts including MPV and D-dimer were prospectively recorded upon admission and subsequently collected in a dedicated database. For the pretest probability assessment of PE, we used dichotomized Wells and Geneva scores. PE diagnosis was considered likely in case of original Wells score ≥5, modified Wells score ≥2, and revised Geneva score ≥3. We evaluated MPV and D-dimer values in all patients and correlated them with PE risk according to Wells or Geneva scores. Furthermore, we checked the coherence between Wells score (original and modified) and revised Geneva score. Additionally, we determined the best MPV value cutoff point for high-risk PE.
Statistical analysis
Categorical variables were summarized as frequencies with percentage. Shapiro–Wilk test was used to assess the normal distribution of variables. Non-parametric statistics were used when variables had other than normal distribution. Continuous variables with normal distribution were expressed as mean ± SD, whereas those with other than normal distribution were expressed as medians with inter-quartile range (IQR). Correlations were assessed by using Spearman’s rank correlation coefficient. Differences between continuous variables were compared by using Mann–Whitney U test, whereas differences between categorical variables were compared by chi-squared test with Yates’s correction for continuity. To assess the suitability of MPV values in PE probability estimation, the receiver operating characteristic (ROC) curve analysis was performed.
All statistical analyses were performed using STATISTICA 12.5 (StatSoft Inc., Tulsa, OK, USA). A P-value <0.05 was considered statistically significant.
Results
Patients were admitted via PE protocol to Intensive Cardiac Therapy Clinic in Lodz, Poland. After considering the inclusion criterion, which is a positive CTA scan, we established a PE diagnosis and enrolled 136 patients in our study. Based on the pretest probability assessment of PE, complete blood counts, and D-dimer, we focused on the correlation of MPV value with PE risk evaluated previously using Wells and Geneva scores.
presents the baseline characteristics. Our study included 76 women (56%) and 60 men (44%) with median age of 66 years (IQR 57.5–78.0). Patients suffered from arterial hypertension (65%) and diabetes mellitus (24%) and one-third were found to be obese (32%). Furthermore, patients reported active smoking (21%), prolonged immobilization (20%), and recent major surgery (21%). Pregnancy and oral contraceptives use were confirmed in 4% and 9% of women, respectively.
Table 2 Baseline characteristics of patients with PE
Patients with confirmed PE developed various symptoms. The most common were fatigue (73%), dyspnea (60%), chest pain (44%), and syncope (24%); however, cough (17%), pleuric pain (3%), and hemoptysis (2%) were observed as well ().
Table 3 Symptoms at admission of patients with PE
As anticipated, patients presenting with PE had an elevated D-dimer value on admission with a median of 10.71 (IQR 3.29–13.67). Moreover, we observed an increase in MPV values (9.23, IQR 8.5–9.85), whereas the plateletcrit was normal (0.2, IQR 0.15–0.24) ().
Table 4 Clinical variables
In our investigation, we not only determined the relationship between the original and modified Wells score and MPV value but also explored the relationship between revised Geneva score and MPV value. The study reveals that both the versions of Wells score correlated significantly with an elevated MPV value (P<0.05) per contra to Geneva score ().
Figure 1 Correlation between mean platelet volume value and (A) Wells rule (original version), (B) Wells rule (simplified version), and (C) revised Geneva score (original version).
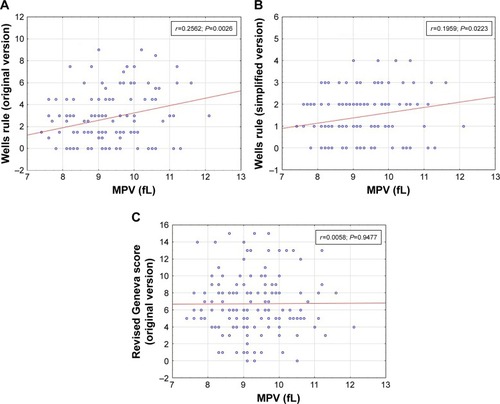
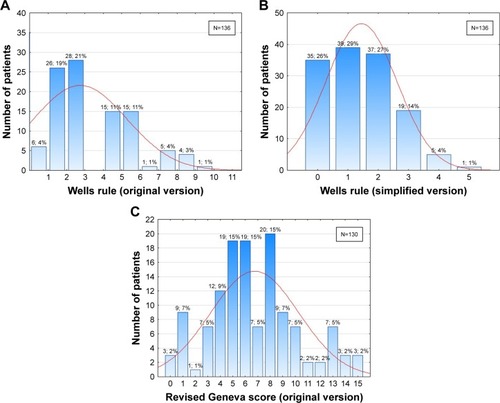
The distribution analysis of the individual values of clinical prediction rules used to suspect PE showed that the distribution varied substantially depending on the clinical scoring system. Our results plausibly indicated lack of coherence between clinical prediction rules ().
Figure 2 The distribution of (A) Wells scores (original version), (B) Wells scores (simplified version), and (C) revised Geneva scores (original version) in patients with pulmonary embolism.
As for the MPV value with reference to the original Wells score, ROC curve analysis showed that the area under the ROC curve of the MPV value was 0.73 (95% CI: 0.632–0.828, P<0.00) and the optimal cutoff point value was 9.6 fL (sensitivity: 69.2%; specificity: 71.8%; Youden’s index: 0.41) ().
Figure 3 Receiver operating characteristic curve and the optimal cutoff point of MPV (9.6 fL) for pulmonary embolism at admission with reference to the original Wells score (area under curve: 0.73; sensitivity: 69.2%; specificity: 71.8%; Youden’s index: 0.41).
Discussion
Little is known about the importance of MPV in patients suffering from PE. To the author’s best knowledge, this is the first study investigating MPV correlation with scoring systems used to predict PE. Our in-depth analysis of patients suffering from PE unequivocally suggests that MPV may be considered a predictive marker of PE. MPV was demonstrated to be significantly correlated with Wells score which is acknowledged to be currently the most reliable clinical prediction rule for PE. Although the Wells score appears to be simple and easy to obtain, physicians are overwhelmed with various information and may struggle to remember another important score in the face of high stress and difficult triage decisions at the emergency department. Furthermore, a complete medical history may not always be taken on account of a frequently confirmed severe health condition of a patient. Therefore, we suggest using MPV in lieu of scoring systems or at least consider MPV a red flag to suspect PE. It may be a great chance to improve the survival and even raise the quality of life among patients presenting with PE.
Among cardiovascular diseases, PE is the third most common cause of death.Citation10 According to the European guidelines, PE should be suspected when the patients present with the following symptoms: dyspnea, chest pain, pre-syncope or syncope, and/or hemoptysis.Citation2 Its clinical manifestation is rather non-specific. Patients may either develop mild symptoms with normal vital signs or experience a life-threatening hemodynamic instability. Delay in PE diagnosis may result in disability and death, even among the hemodynamically stable patients. On account of the fact that PE may escape prompt diagnosis, we still seek an effective method to predict it and urgently provide the patients with an appropriate therapy. PE is considered to be very often misdiagnosed and discovered incidentally at autopsy.Citation11,Citation12
Planquette et alCitation13 conducted a study among 155 general practitioners who had been in practice for more than 20 years (76%) to evaluate their state of knowledge of the PE diagnostic algorithm. Disappointingly, the study revealed that only 55% of GPs were aware of clinical probability scores for PE.Citation13 Although the predictive value of Wells score is considered good, assessment of seven parameters is clinically difficult. MPV is a simple and cost-effective measurement and can be easily achieved even before patient is presented to the emergency department. We hypothesize that its implementation into a clinical practice may consequently contribute to further diagnostic accuracy improvement.
On account of MPV widespread availability, it is an attractive index for research. Recently we observe a growing interest in MPV. Current publications indicate that MPV has many potential applications and its increase is associated with arterial and venous diseases such as myocardial infarction or venous thromboembolism.Citation14,Citation15 Moreover, an elevated MPV value was found in various diseases, for example, infective endocarditis, rheumatoid arthritis, and gestational diabetes.Citation16,Citation17 Gawlita et al pointed out that in patients with stable CAD, an elevated MPV correlates with the severity of disease, whereas in myocardial infarction, high value of MPV may be a hallmark of increased in-hospital and long-term mortality.Citation18
Several publications indicate that MPV may be used for mortality prediction among PE patients. According to Kostrubiec et al,Citation19 MPV is not only a significant predictor of increased early death in PE but is also associated with RV dysfunction and myocardial injury. MPV >10.9 fL on admission was considered a predictor of 30-day mortality, especially 7-day mortality.Citation19 Another study highlights the correlation between increased values of MPV and RV dysfunction and clinical severity in PE. The MPV cutoff value in the prediction of RV dysfunction was 7.85 fL.Citation20 Akgüllü et al suggested using a new model that is superior to the simplified PE severity index. It includes troponin I, creatinine, MPV, neutrophil to lymphocyte ratio, QTcd, and Pd. The MPV cutoff value in the prediction of early death in PE was >9.7 fL.Citation21
Tajarernmuang et alCitation22 reported that the sequential monitoring of changes in MPV may be more significant than a single measurement. They showed that the gradual increase of MPV during hospitalization was related to an increased in-hospital mortality.Citation22 Furthermore, there are a few medical investigations proving that increasing MPV after admission was observed in the nonsurvivors group.Citation23–Citation26 Other studies indicated a remarkable correlation between an elevated MPV and short-term mortality.Citation23,Citation24 Sezgi et al concordantly demonstrated that among nonsurvivors, MPV value on admission was lower than that at the time of discharge, whereas it was reported to be decreasing in the survivors group.Citation27 Biino et al discovered that the longer the time since arterial thrombosis, the lower the MPV level.Citation28 Interestingly, Sevuk et al investigated the value of MPV and platelet distribution width (PDW) to predict the occurrence of PE in patients presenting with DVT. They found that MPV and PDW values were significantly higher among patients suffering from DVT and concomitant PE compared with DVT patients. Also, they demonstrated that MPV and PDW were independent risk factors for PE in patients with DVT.Citation29 We look forward to larger studies that concern especially patients with PE.
We hypothesize that, even if not independently, an elevated MPV value may be an important element of clinical prediction rules. Huang et al suggested that the combined application of MPV might enhance the value of D-dimer used to exclude PE. D-dimer is a widely used biomarker, however, on account of its poor specificity its role is limited.Citation30
The clinical prediction rules for PE do not include any of the laboratory parameters. Assuming D-dimer value is unambiguous, it would be, without a doubt, already incorporated in one of the scoring systems for PE. However, an increased D-dimer neither confirms a PE diagnosis nor defines the extent of the disease. On the other hand, thus far the MPV-based prediction for PE has not been practiced, even if an elevated MPV correlates significantly with Wells score. Therefore, we encourage to regard MPV as an independent or at least adjunctive predictive marker for PE that, for instance, may be combined with widely used scoring system such as Wells score.
Study limitations
Our study had certain limitations. There were multiple conditions that may have affected the platelet volume.Citation22 Furthermore, the sample size was relatively small, but in spite of everything, to our knowledge this is the first study investigating MPV correlation with scoring systems used to assess the clinical probability of PE. Moreover, MPV measurement may have been associated with technical problems. Finally, the widespread usefulness of MPV was limited since the MPV cutoff value has not been established yet.
Conclusion
In our study, we established a positive correlation between an elevated MPV and increased PE risk according to the Wells score and determined the best MPV value cutoff point for PE. MPV is easy to obtain, inexpensive, and reliable parameter. Despite the limited evidence, we suggest considering MPV either an adjunctive or an independent predictive marker for PE used in lieu of clinical prediction rules. Further larger studies are necessary to confirm our finding. Moreover, in future studies, it would be valuable to assess the correlation between MPV value and other predictive markers for PE to subsequently enhance a diagnostic accuracy. We hypothesize that combining MPV and D-dimer with scoring systems for PE might be highly beneficial.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoldhaberSZVisaniLde RosaMAcute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER)Lancet199935391621386138910227218
- KonstantinidesSVTorbickiAAgnelliGESC guidelines on the diagnosis and management of acute pulmonary embolismEur Heart J201435433033307325173341
- PenalozaAMelotCMotteSComparison of the Wells score with the simplified revised Geneva score for assessing pretest probability of pulmonary embolismThromb Res20111272818421094985
- WongDDRamaseshanGMendelsonRMComparison of the Wells and Revised Geneva Scores for the diagnosis of pulmonary embolism: an Australian experienceIntern Med J201141325826320214691
- ThompsonCBJakubowskiJAQuinnPGDeykinDValeriCRPlatelet size as a determinant of platelet functionJ Lab Clin Med198310122052136822760
- ThompsonCBEatonKAPrinciottaSMRushinCAValeriCRSize dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and functionBr J Haematol19825035095197066203
- GasparyanAYAyvazyanLMikhailidisDPKitasGDMean platelet volume: a link between thrombosis and inflammation?Curr Pharm Des2011171475821247392
- UçarHGürMKoyunseverNYMean platelet volume is independently associated with renal dysfunction in stable coronary artery diseasePlatelets201425427427823772896
- Kiliçli-CamurNDemirtunçRKonuralpCEskiserABaşaranYCould mean platelet volume be a predictive marker for acute myocardial infarction?Med Sci Monit2005118CR387CR39216049381
- GoldhaberSZBounameauxHPulmonary embolism and deep vein thrombosisLancet201237998281835184622494827
- SweetPHArmstrongTChenJMasliahEWituckiPFatal pulmonary embolism update: 10 years of autopsy experience at an academic medical centerJRSM Short Rep201349204253331348982424040503
- McdonaldCHernandezMGofmanYSucheckiSSchreierWThe five most common misdiagnoses: a meta-analysis of autopsy and malpractice dataThe Internet Journal of Family Practice200872
- PlanquetteBMauriceDPeronJKnowledge of the diagnostic algorithm for pulmonary embolism in primary careEur J Intern Med2015261182225498510
- ChuSGBeckerRCBergerPBMean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysisJ Thromb Haemost20108114815619691485
- JensvollHBlixKBrækkanSKHansenJBPlatelet count measured prior to cancer development is a risk factor for future symptomatic venous thromboembolism: the Tromsø StudyPLoS One201493e9201124642868
- YaziciSYaziciMErerBThe platelet indices in patients with rheumatoid arthritis: mean platelet volume reflects disease activityPlatelets201021212212520050760
- BozkurtNYilmazEBiriATanerZHimmetoğluOThe mean platelet volume in gestational diabetesJ Thromb Thrombolysis2006221515416786233
- GawlitaMWasilewskiJOsadnikTRegułaRBujakKGoneraMMean platelet volume and platelet-large cell ratio as prognostic factors for coronary artery disease and myocardial infarctionFolia Cardiol201610418422
- KostrubiecMŁabykAPedowska-WłoszekJMean platelet volume predicts early death in acute pulmonary embolismHeart201096646046519910287
- YardanTMericMKatiCCelenkYAticiAGMean platelet volume and mean platelet volume/platelet count ratio in risk stratification of pulmonary embolismMedicina201652211011527170484
- AkgüllüÇÖmürlüİKEryılmazUPredictors of early death in patients with acute pulmonary embolismAm J Emerg Med201533221422125499176
- TajarernmuangPPhrommintikulALimsukonAPothiratCChittawatanaratKThe Role of Mean Platelet Volume as a Predictor of Mortality in Critically Ill Patients: A Systematic Review and Meta-AnalysisCrit Care Res Pract20162016437083426966574
- BecchiCAl MalyanMFabbriLPMarsiliMBoddiVBoncinelliSMean platelet volume trend in sepsis: is it a useful parameter?Minerva Anestesiol200672974975616871155
- KitazawaTYoshinoYTatsunoKOtaYYotsuyanagiHChanges in the mean platelet volume levels after bloodstream infection have prognostic valueIntern Med201352131487149323812196
- ZampieriFGRanzaniOTSabatoskiVAn increase in mean platelet volume after admission is associated with higher mortality in critically ill patientsAnn Intensive Care201442025520853
- KimCHKimSJLeeMJAn increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shockPLoS One2015103e011943725742300
- SezgiCTaylanMKayaHAlterations in platelet count and mean platelet volume as predictors of patient outcome in the respiratory intensive care unitClin Respir J20159440340824725778
- BiinoGPortasLMurgiaFA population-based study of an Italian genetic isolate reveals that mean platelet volume is not a risk factor for thrombosisThromb Res20121294e8e1322137741
- SevukUBahadirMVAltindagRValue of serial platelet indices measurements for the prediction of pulmonary embolism in patients with deep venous thrombosisTher Clin Risk Manag2015111243124926316769
- HuangJChenYCaiZChenPDiagnostic value of platelet indexes for pulmonary embolismAm J Emerg Med20153381093109425937379
