Abstract
Objective
To identify risk factors for surgical site infection (SSI) in patients who had undergone lumbar spinal surgery.
Methods
Studies published in PubMed, Web of Science, and Embase were systematically reviewed to determine risk factors for SSI following lumbar spinal surgery. Results are expressed as risk ratios (RRs) with 95% CIs and weighted mean difference (WMD) with 95% CI. A fixed-effect or random-effect model was used to pool the estimates according to heterogeneity among the studies included.
Results
Sixteen studies involving 13,393 patients were included in this meta-analysis. Pooled estimates suggested that diabetes (RR 2.19, 95% CI 1.43–3.36; P<0.001), obesity (RR 2.87, 95% CI 1.62–5.09; P<0.001), BMI (WMD 1.32 kg/m2, 95% CI 0.39–2.25; P=0.006), prolonged operating time (WMD 24.96 minutes, 95% CI 14.77–35.15; P<0.001), prolonged hospital stay (WMD 2.07 days, 95% CI 0.28–3.87; P=0.024), hypertension (RR 1.28, 95% CI 1.08–1.52; P=0.005), and previous surgery (RR 2.06, 95% CI 1.39–3.06; P<0.001) were independent risk factors for SSI in patients who had undergone lumbar spine surgery. Current smoking (RR 0.89, 95% CI 0.75–1.06; P=0.178), American Society of Anesthesiologists grade >2 (RR 2.63, 95% CI 0.84–8.27; P=0.098), increased age (WMD 1.43 years, 95% CI −1.15 to 4.02; P=0.278), COPD (RR 1.21, 95% CI 0.68–2.17; P=0.521), cardiovascular disease (RR 1.63, 95% CI 0.40–6.70; P=0.495), rheumatoid arthritis (RR 1.76, 95% CI 0.53–5.90; P=0.359), and osteoporosis (RR 1.91, 95% CI 0.79–4.63; P=0.152) were not risk factors for postoperative SSI.
Conclusion
Our results identified several important factors that increased the risk of postoperative SSI. Knowing these risk factors, surgeons could adequately analyze and evaluate risk factors in patients and then develop prevention measurements to reduce the rate of SSI.
Introduction
Surgical site infection (SSI) is one of the most serious complications following lumbar spine surgery during the early postoperative stage. SSI rates have been reported to be 0.7%–12.0%.Citation1,Citation2 Despite several interventions in clinical practice, including the use of prophylactic antibiotics, improvements in surgical techniques, and postoperative care, SSI continues to affect patients after lumbar surgery.Citation3,Citation4 SSI usually requires surgical debridement, which leads to higher postoperative morbidity and mortality.Citation5–Citation7 This would increase the duration of hospital stay, reoperation rates, and additional treatment costs.Citation5–Citation7 Therefore, determining risk factors for postoperative SSI and seeking methods to reduce SSI rates are very necessary.
There have been several studies to investigate postoperative SSI risk factors, such as increased age,Citation8 obesity,Citation9,Citation10 diabetes,Citation8,Citation10 smoking,Citation10 previous infection,Citation11 prolonged operating time,Citation12 prolonged hospital stay,Citation13 and admission from a health care facility.Citation14 However, the results of these studies were inconsistent. In order to systematically assess the most important risk factors for SSI following lumbar spinal surgery, we conducted this meta-analysis. Based upon identified risk factors, we can deduce preventive strategies to reduce the risk for SSI, thereby decreasing the morbidity, mortality, and health care costs.
Methods
Search strategy
This meta-analysis was performed according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement.Citation15 We did a comprehensive search on PubMed, Embase, and Web of Science from their inception to May 11, 2018. Search items were ((“lumbosacral region” [MeSH terms] OR (“lumbosacral” [all fields] AND “region” [all fields]) OR “lumbosacral region” [all fields] OR “lumbar” [all fields]) AND (“surgery” [subheading] OR “surgery” [all fields] OR “surgical procedures, operative” [MeSH terms] OR (“surgical” [all fields] AND “procedures” [all fields] AND “operative” [all fields]) OR “operative surgical procedures” [all fields] OR “surgery” [all fields] OR “general surgery” [MeSH terms] OR (“general” [all fields] AND “surgery” [all fields]) OR “general surgery” [all fields])) AND (“surgical wound infection” [MeSH terms] OR (“surgical” [all fields] AND “wound” [all fields] AND “infection” [all fields]) OR “surgical wound infection” [all fields] OR (“surgical” [all fields] AND “site” [all fields] AND “infection” [all fields]) OR “surgical site infection” [all fields]) AND (“risk factors” [MeSH terms] OR (“risk” [all fields] AND “factors” [all fields]) OR “risk factors” [all fields] OR (“risk” [all fields] AND “factor” [all fields]) OR “risk factor” [all fields]). There was no limitation on language or publication type. Moreover, we also manually searched the references of the studies and reviews included to identify other potentially eligible studies.
Inclusion criteria
Two independent investigators performed the literature search, literature review (title/abstract review, full-text review, and included eligible studies). Any disagreement between them was resolved by discussion and consensus. All studies that investigated risk factors for postoperative SSI after lumbar spinal surgery were considered eligible for data analysis. We included the studies that met inclusion criteria of randomized controlled trial, cohort study, or case–control study, adult patients who had undergone lumbar spinal surgery, and presence of risk factors for postoperative SSI.
Data extraction and quality assessment
Two independent investigators performed the data extraction. Data extracted included country of study, number of patients in SSI group and non-SSI group, baseline characteristics, and outcomes. We used the modified Newcastle–Ottawa Scale (NOS)Citation16 to evaluate the quality of observational studies (cohort study, case–control study). This method consists of three items: patient selection, comparability of experimental and control groups, and assessment of outcomes of interest.Citation16 The total score is 9, and higher scores indicate better quality. Any study is considered of high quality if the NOS score is >5 points.Citation16
Statistical analysis
Dichotomous variables are expressed as RRs with 95% CIs and continuous variables weighted mean difference (WMD) with 95% CIs. We used a fixed-effect model (Mantel–Haenszel method)Citation17 or random-effect model (DerSimonian–Laird method)Citation18 to pool all data according to heterogeneity across the included studies. Heterogeneity among the studies was assessed using the I2 statistic,Citation19 where I2>50% was considered substantial heterogeneity.Citation19 When significant heterogeneity was identified, sensitivity analysis was performed to explore the potential source of heterogeneity. Publication bias was evaluated by Begg’sCitation20 and Egger’sCitation21 test. We considered P<0.05 statistically significant, except where otherwise specified. All statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX, USA).
Results
Study identification and selection
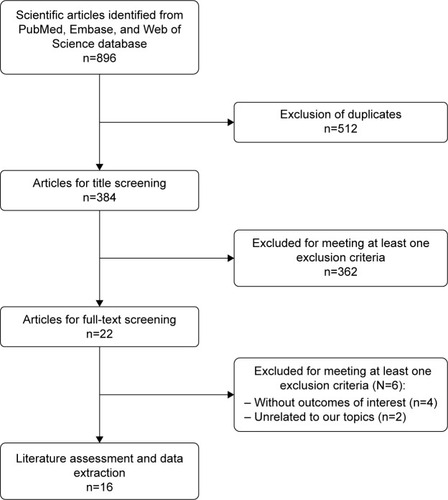
shows the article-screening and -selection process for inclusion in this study. The initial search yielded 896 studies. Of these, 512 were excluded for duplicate records and 362 excluded after the review of title/abstract. Then, 22 studies were left for full-text review. Among these, six were excluded: four for not providing eligible data,Citation22–Citation25 and two that were unrelated to our topic.Citation26,Citation27 Finally, 16 studiesCitation28–Citation43 met the inclusion criteria and were included in this meta-analysis.
Study characteristics and quality assessment
The main characteristics of included studies are presented in . These were published between 2003 and 2018. The total sample size was 13,393, of which 704 were in the SSI group and 12,689 the non-SSI group. Among these studies, nineCitation28,Citation31,Citation32,Citation35–Citation37,Citation40,Citation41,Citation43 were conducted in the US, two in China,Citation33,Citation42 and one each in South Korea,Citation29 Norway,Citation30 Japan,Citation34 Brazil,Citation38 and the Netherlands.Citation39 Most studies were performed with a retrospective case–control design, except three, which were prospectiveCitation34,Citation38 or retrospectiveCitation41 cohort design. All patients had undergone lumbar fusion surgery or posterior lumbar spinal surgery. NOS scores ranged from 5 to 7, which indicated that these studies were of high quality.
Table 1 Baseline characteristics of patients in trials included
Risk factors
Sex
The most important risk factors for SSI are presented in . Eleven studies investigated the relationship between sex and postoperative SSI.Citation28–Citation31,Citation33,Citation34,Citation36–Citation39,Citation43 The pooled estimate showed that males had a significantly lower risk of developing postoperative SSI compared with females (RR 0.88, 95% CI 0.80–0.97; P=0.008; ). There was no significant heterogeneity among the studies (I2=49.2%, P=0.032).
Figure 2 Forest plot showing the relationship between male sex and postoperative surgical site infection.
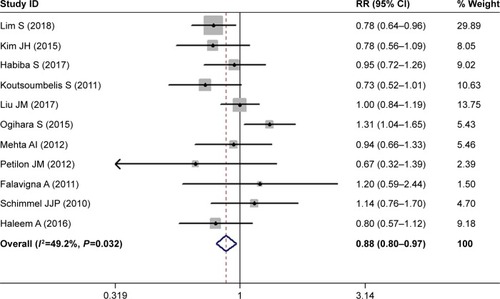
Table 2 Pooled estimates of RR (WMD)Table Footnotea obtained from meta-analysis of risk factors of SSI following lumbar spine surgery
Increased age
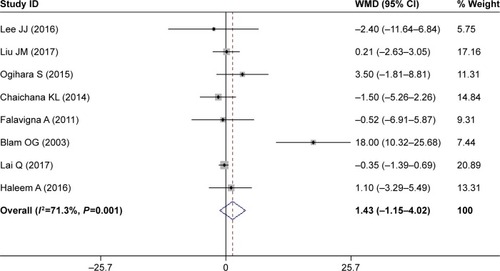
Eight studies assessed the relationship between increased age and postoperative SSI.Citation32–Citation35,Citation38,Citation41–Citation43 The pooled result suggested that patients with SSI were older than those without (WMD 1.43 years, 95% CI 1.15–4.02; ); however, this difference was not significant (P=0.2777). This indicated that increased age was not a significant risk factor for SSI.
Diabetes
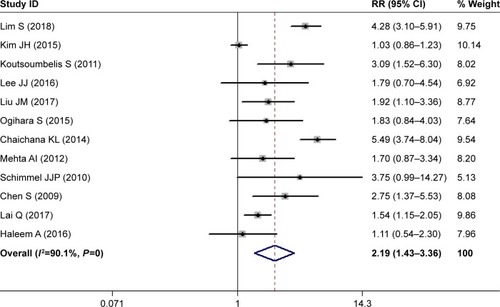
Twelve studies investigated the relationship between diabetes and postoperative SSI.Citation28,Citation29,Citation31–Citation36,Citation39,Citation40,Citation42,Citation43 The pooled estimate suggested that diabetes patients had a 2.19-fold increased risk of developing SSI compared with those without diabetes (RR 2.19, 95% CI 1.43–3.36; P<0.00; ). Heterogeneity was significant (I2=90.1%, P<0.001), and thus we conducted sensitivity analysis. When we excluded a study with a relatively small sample (n=149),Citation32 the pooled estimate of the remaining studies did not change substantially (RR 2.23, 95% CI 1.71–3.43; P<0.001), but heterogeneity was still present (I2=89.5%, P<0.001). Furthermore, we excluded studies one at a time, and overall estimates changed slightly, but heterogeneity was still observed.
Current smoking
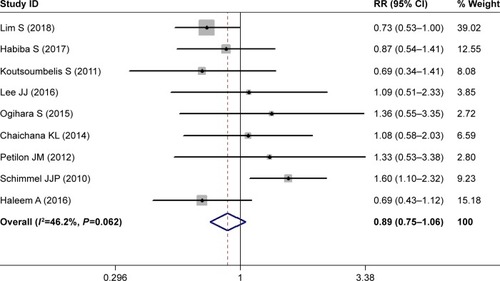
Nine studies investigated the relationship between current smoking and postoperative SSI.Citation28,Citation30–Citation32,Citation34,Citation35,Citation37,Citation39,Citation43 Pooled estimates showed that current smokers had a comparable rate of postoperative SSI than nonsmokers (RR 0.89, 95% CI 0.75–1.06; P=0.178; ). This indicated that current smoking did not increase the risk of postoperative SSI in patients with lumbar spine surgery. Heterogeneity was not significant (I2=46.2%, P=0.062).
Obesity
Six studies investigated the relationship between obesity and postoperative SSI.Citation30–Citation32,Citation35,Citation36,Citation43 The pooled result showed that obesity patients had a 2.87-fold increased risk of SSI than those of normal weight (RR 2.87, 95% CI 1.62–5.09; P<0.001). This indicated that obesity was a significant risk for SSI. There was no significant heterogeneity among the studies (I2=43.4%, P=0.078).
ASA grade >2
Four studies investigated the relationship between American Society of Anesthesiologists (ASA) grade and postoperative SSI.Citation28,Citation30,Citation34,Citation43 Pooled estimates suggested that patients with ASA grade >2 had a similar rate of postoperative SSI compared with those with ASA grade 1–2 (RR 2.63, 95% CI 0.84–8.27; P=0.098). This demonstrated that ASA grade >2 did not increase the risk of postoperative SSI. Heterogeneity was not significant (I2=45.6%, P=0.073).
BMI
Nine studies investigated the relationship between body-mass index (BMI) and postoperative SSI.Citation30,Citation32,Citation34,Citation36,Citation37,Citation39,Citation41–Citation43 Pooled estimates suggested that patients with high BMI values had a higher risk of developing SSI than those with normal BMI (WMD 1.32 kg/m2, 95% CI 0.39–2.25; P=0.006). This indicated that BMI was a significant risk factor for postoperative SSI. Heterogeneity was not significant (I2=48.3%, P=0.067).
Duration of surgery
Ten studies investigated the relationship between duration of surgery and postoperative SSI.Citation29–Citation33,Citation37,Citation39,Citation41–Citation43 Pooled estimates suggested that patients with longer surgeries were more likely to develop SSI (WMD 24.96 minutes, 95% CI 14.77–35.15; P<0.001). This indicated that prolonged surgery was an (I2=33.9%, P=0.267).
Duration of hospital stay
Five studies investigated the relationship between duration of hospital stay and postoperative SSI.Citation30,Citation31,Citation35,Citation37,Citation41 Pooled estimates suggested that patients with longer hospital stay had a higher risk of SSI (WMD 2.07 days, 95% CI 0.28–3.87; P=0.024). This indicated that prolonged hospital stays increased the risk of SSI. Heterogeneity was not significant (I2=29.6%, P=0.384).
Estimated blood loss
Four studies investigated the relationship between estimated blood loss and postoperative SSI.Citation33,Citation37,Citation41,Citation42 The pooled result showed that patients with greater blood loss had a higher risk of SSI (WMD 106.9 mL, 95% CI 65.14–278.53); however, this was not significant (P=0.224). This indicated that increased blood loss was not a significant risk factor for SSI in patients who had undergone lumbar spine surgery. Heterogeneity was not significant (I2=44.2%, P=0.0698).
Chronic obstructive pulmonary disease
Five studies investigated the relationship between COPD and postoperative SSI.Citation28,Citation31,Citation39,Citation42,Citation43 Pooled estimates suggested that patients with COPD had a similar rate of SSI as those without (RR 1.21, 95% CI 0.68–2.17; P=0.521). This indicated that COPD was not a significant risk factor for SSI in patients who had undergone lumbar spine surgery. Heterogeneity was not significant (I2=13.8%, P=0.292).
Publication bias
Assessment of publication bias using Begg’s and Egger’s tests showed that there was no potential publication bias across the included studies (Egger’s test, P=0.473; Begg’s test, P=0.527).
Discussion
The present study was a meta-analysis of eligible studies with the objective of identifying risk factors for SSI following lumbar spinal surgery. Our study suggested that female sex, diabetes, obesity, BMI, pronged operation time, prolonged hospital stay, hypertension, and previous surgery were risk factors for SSI in patients who had undergone lumbar spinal surgery, whereas, current smoking, ASA grade >2, increased age, COPD, cardiovascular disease, rheumatoid arthritis, and osteoporosis were not.
To the best of our knowledge, this is the first comprehensive meta-analysis to investigate risk factors for SSI in patients who have undergone lumbar spinal surgery. Our study indicated that patients with diabetes had a 2.19-fold increased risk of developing postoperative SSI compared with those without. Findings from the present study were consistent with most of the studies included, except three,Citation29,Citation32,Citation34 which found that diabetes was not a risk factor for SSI. Lee et alCitation32 retrospectively analyzed 149 adult patients who had undergone lumbar spine surgery with a midline posterior approach. Among these patients, 15 experienced postoperative SSI and 134 had no infection.Citation32 The prevalence of patients with diabetes in the SSI and non-SSI groups was 26.7% (four of 15) and 14.9% (20 of 134), respectively, which were not significant (P=0.249).Citation32 Similarly, Kim et alCitation29 undertook a review of a case series to identify risk factors for SSI in posterior lumbar interbody fusion, and they also reported a negative relationship between diabetes and SSI. In that study, 80% (24 of 30) of patients in the SSI group had diabetes compared with 77.8% (1,401 of 1,801) in non-SSI group.Citation29 However, in another retrospective study of 2,715 patients investigating risk factors for SSI following posterior lumber spinal surgery, the authors suggested that diabetes was an independent risk factor for SSI.Citation33 The rate of diabetes in SSI and control groups was 25% (16 of 64) and 13% (25 of 192), respectively, which demonstrated that diabetes patients were at higher risk of developing SSI.Citation33 The inconsistent results of these three studies are difficult for us to explain, since they all had large samples and used multivariate logistic regression analyses to reduce the influences of selection bias in retrospective studies.
In the present study, we found that obesity was a significant risk factor for SSI in patients who had undergone lumbar spinal surgery. These results were in line with previous studies.Citation31,Citation32,Citation36 Koutsoumbelis et al collected 3,218 patients who had undergone posterior lumbar instrumented arthrodesis,Citation31 and found that 42.9% (36 of 84) of them who developed SSI had obesity compared with 7.1% (12 of 168) of patients who had no SSI.Citation31 The OR for obesity was 9.75 (95% CI 4.70–20.21, P<0.001), indicating that patients with obesity had 9.75-fold increased risk of developing SSI than those without. Consistent with these results, Lee et alCitation32 reported that obesity was associated with a 4.09-fold increased risk of SSI (OR 4.09, 95% CI 1.32–12.7; P=0.015). In that study, the obesity rate in the SSI and non-SSI groups was 66.7% (ten of 15) and 32.8% (44 of 134), respectively, which indicated that obese patients were more likely to develop SSI than normal patients.Citation32 When obese patients are undergoing surgery, it is necessary to cut through a large amount of oily liquid. The surgical incision is filled with sterile gauze, and bacteria can become embedded in the incision.Citation42 This increases the risk of infection. Moreover, previous studiesCitation2,Citation44 have demonstrated that BMI is a risk factor for postoperative complications: when BMI is increased by 5 kg/m2, the risk of postoperative SSI is accordingly increased by 10%.
Consistently with prior studies, prolonged operations were significantly associated with postoperative SSI.Citation29,Citation31,Citation33 Kim et alCitation29 analyzed 1,831 patients who had undergone posterior lumbar interbody fusion, and found that SSI patients had had longer surgery than those in the non-SSI group. In that study, operation times in SSI and non-SSI groups were 195.3 minutes and 177.1 minutes (P=0.008), respectively,Citation29 suggesting that prolonged surgery increased the risk of SSI. Similar results were found in another study, which assessed risk factors for SSI among patients with posterior lumbar instrumented arthrodesis.Citation31 In that study, the duration of surgery in SSI and non-SSI groups was 373.1±167.1 minutes and 291.6±130.7 minutes, respectively.Citation31 The difference between them was significant (P<0.001), which confirmed the role of prolonged surgery in postoperative SSI. However, in another case–control study,Citation37 a negative relationship was found between duration of surgery and SSI. In that study, the authors performed a propensity-score-matched case–control study of 60 patients who had undergone instrumented lumbar fusion.Citation37 The operating time for SSI patients was less (259.27 minutes) than non-SSI patients (288.17), and the difference between them was not significant (P=0.298).Citation37 The negative result might be explained by the small sample.
Previous surgery was another risk factor for SSI, and this result was comparable to previous studies.Citation35,Citation39 Ogihara et al performed prospective multicenter surveillance to determine the risk factors for SSI in adult patients who had undergone lumbar spinal surgery.Citation34 They enrolled 2,736 patients, and 24 (0.9%) developed SSI.Citation34 The prevalence of patients who had had previous surgery in deep SSI and nondeep SSI groups was 29.2% and 15.5%, respectively, suggesting that previous surgery was an increased risk for postoperative SSI.Citation34 Chaichana et al performed a study with 817 consecutive cases, and found previous surgery was associated with 2.994-fold increased risk of SSI (RR 2.994, 95% CI 1.26–9.35; P=0.009).Citation35 It was assumed that patients who had had previous lumbar spine surgery typically had longer surgeries, which increased procedural complexity and propensity for durotomies, thereby increasing the risk of SSI.Citation45
Limitations
This study has several potential limitations. First, in some outcomes, substantial heterogeneity was identified among the included studies. Despite sensitivity analysis being performed to detect potential sources of heterogeneity, no valuable information was found. Second, most of the studies were conducted with a retrospective design, and their results might be biased by the inherent disadvantages. This may have had a potential impact on our pooled estimates.
Conclusion
Our study indicates that female sex, diabetes, obesity, BMI, prolonged operation, prolonged hospital stay, hypertension, and previous surgery are independent risk factors for SSI following lumbar spine surgery, whereas, current smoking, ASA grade >2, increased age, COPD, cardiovascular disease, rheumatoid arthritis, and osteoporosis are not. Knowing these risk factors, surgeons could adequately analyze and evaluate risk factors in patients, and then develop prevention measurements to reduce the rate of SSI.
Disclosure
The authors report no conflicts of interest in this work.
References
- FeiQLiJLinJRisk Factors for Surgical Site Infection After Spinal Surgery: A Meta-AnalysisWorld Neurosurg20169550751526054871
- OlsenMANeppleJJRiewKDRisk factors for surgical site infection following orthopaedic spinal operationsJ Bone Joint Surg Am20089016269
- WangTYBackAGHompeEWallKGottfriedONImpact of surgical site infection and surgical debridement on lumbar arthrodesis: A single-institution analysis of incidence and risk factorsJ Clin Neurosci20173916416928202380
- Pull Ter GunneAFMohamedASSkolaskyRLvan LaarhovenCJCohenDBThe presentation, incidence, etiology, and treatment of surgical site infections after spinal surgerySpine201035131323132820150831
- CalderoneRRGarlandDECapenDAOsterHCost of medical care for postoperative spinal infectionsOrthop Clin North Am19962711711828539047
- de LissovoyGFraemanKHutchinsVMurphyDSongDVaughnBBSurgical site infection: incidence and impact on hospital utilization and treatment costsAm J Infect Control200937538739719398246
- VeeravaguAPatilCGLadSPBoakyeMRisk factors for postoperative spinal wound infections after spinal decompression and fusion surgeriesSpine200934171869187219644339
- SatakeKKanemuraTMatsumotoAYamaguchiHIshikawaYPredisposing factors for surgical site infection of spinal instrumentation surgery for diabetes patientsEur Spine J20132281854185823612899
- JiangJTengYFanZKhanSXiaYDoes obesity affect the surgical outcome and complication rates of spinal surgery? A meta-analysisClin Orthop Relat Res2014472396897524146361
- MengFCaoJMengXRisk factors for surgical site infections following spinal surgeryJ Clin Neurosci201522121862186626282155
- FangAHuSSEndresNBradfordDSRisk factors for infection after spinal surgerySpine200530121460146515959380
- SassoRCGarridoBJPostoperative spinal wound infectionsJ Am Acad Orthop Surg200816633033718524984
- ClassenDCEvansRSPestotnikSLHornSDMenloveRLBurkeJPThe timing of prophylactic administration of antibiotics and the risk of surgical-wound infectionN Engl J Med199232652812861728731
- LeeJSingletaryRSchmaderKAndersonDJBolognesiMKayeKSSurgical site infection in the elderly following orthopaedic surgery. Risk factors and outcomesJ Bone Joint Surg Am20068881705171216882891
- MoherDLiberatiATetzlaffJAltmanDGPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- WellsGSheaBO’ConnellDPetersonJWelchVThe Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses3rd Symposium on Systematic Reviews: Beyond the BasicsJul 3–5. 2000Oxford UK
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- DersimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- KimBDHsuWKde OliveiraGSSahaSKimJYOperative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: an analysis of 4588 surgical casesSpine201439651052024365901
- KlemencsicsILazaryASzoverfiZBozsodiAEltesPVargaPPRisk factors for surgical site infection in elective routine degenerative lumbar surgeriesSpine J201616111377138327520077
- TsubouchiNFujibayashiSOtsukiBRisk factors for implant removal after spinal surgical site infectionEur Spine J Epub2017914
- AsomughaEUMillerJAMclainRFSurgical Site Infections in Posterior Lumbar Surgery: A Controlled-Cohort Study of Epidural Steroid PasteSpine2017421636927135641
- GolubovskyJLIlyasHChenJTanenbaumJEMrozTESteinmetzMPRisk factors and associated complications for postoperative urinary retention after lumbar surgery for lumbar spinal stenosisSpine J20181891533153929447854
- GlassmanSCarreonLYAndersenMPredictors of Hospital Readmission and Surgical Site Infection in the United States, Denmark, and Japan: Is Risk Stratification a Universal Language?Spine201742171311131528146028
- LimSEdelsteinAIPatelAAKimBDKimJYSJysKRisk Factors for Postoperative Infections After Single-Level Lumbar Fusion SurgerySpine201843321522225271498
- KimJHAhnDKKimJWKimGWParticular Features of Surgical Site Infection in Posterior Lumbar Interbody FusionClin Orthop Surg20157333734326330956
- HabibaSNygaardØPBroxJIHellumCAustevollIMSolbergTKRisk factors for surgical site infections among 1,772 patients operated on for lumbar disc herniation: a multicentre observational registry-based studyActa Neurochir201715961113111828424918
- KoutsoumbelisSHughesAPGirardiFPRisk factors for postoperative infection following posterior lumbar instrumented arthrodesisJ Bone Joint Surg Am201193171627163321915578
- LeeJJOdehKIHolcombeSAFat Thickness as a Risk Factor for Infection in Lumbar Spine SurgeryOrthopedics2016396e1124e112827575036
- LiuJMDengHLChenXYRisk Factors for Surgical Site Infection After Posterior Lumbar Spinal SurgerySpine2018431073273728922276
- OgiharaSYamazakiTMaruyamaTProspective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adultsJ Orthop Sci2015201717725366698
- ChaichanaKLBydonMSantiago-DieppaDRRisk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive casesJ Neurosurg Spine2014201455224206038
- MehtaAIBabuRKarikariIO2012 Young Investigator Award winner: The distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infectionsSpine201237191652165622146285
- PetilonJMGlassmanSDDimarJRCarreonLYClinical outcomes after lumbar fusion complicated by deep wound infection: a case–control studySpine201237161370137422261633
- FalavignaARighessoOTraynelisVCTelesARda SilvaPGEffect of deep wound infection following lumbar arthrodesis for degenerative disc disease on long-term outcome: a prospective study: clinical articleJ Neurosurg Spine201115439940321682558
- SchimmelJJHorstingPPde KleuverMWondersGvan LimbeekJRisk factors for deep surgical site infections after spinal fusionEur Spine J201019101711171920445999
- ChenSAndersonMVChengWKWongworawatMDDiabetes associated with increased surgical site infections in spinal arthrodesisClin Orthop Relat Res200946771670167319225851
- BlamOGVaccaroARVanichkachornJSRisk factors for surgical site infection in the patient with spinal injurySpine200328131475148012838110
- LaiQSongQGuoRRisk factors for acute surgical site infections after lumbar surgery: a retrospective studyJ Orthop Surg Res201712111628724387
- HaleemAChiangHYVodelaRRisk Factors for Surgical Site Infections Following Adult Spine OperationsInfect Control Hosp Epidemiol201637121458146727573067
- CollinsIWilson-MacdonaldJChamiGThe diagnosis and management of infection following instrumented spinal fusionEur Spine J200817344545018075763
- WimmerCGluchHFranzrebMOgonMPredisposing factors for infection in spine surgery: a survey of 850 spinal proceduresJ Spinal Disord19981121241289588468