Abstract
Background
Angiogenesis and bone formation are vital for fracture healing. Nerve growth factor (NGF) not only promotes neuronal survival but also enhances the proliferation and differentiation of osteoblasts. Vascular endothelial growth factor (VEGF) plays an important role in angiogenesis. However, the potential correlation of NGF and VEGF levels with fracture healing in patients with traumatic brain injury (TBI) remains unclear.
Methods
This study enrolled 22 patients with clavicle fracture and concomitant TBI (CFT group) and 25 patients with clavicle fracture alone (CF group). Serum NGF levels were measured with ELISA. The expressions of NGF, VEGF, and CD31 in callus tissues were measured with immunohistochemistry.
Results
The fracture healing time in CFT group (82.22±13.61 days) was significantly shorter than that in CF group (127±25.05 days; P<0.001). The expression of CD31, marker of blood vessels, in callus tissues of CFT group was higher compared with that of CF group. Serum NGF levels and the expression of NGF in callus tissues of CFT group were higher than those in CF group (P<0.01). The expressions of CD31, NGF, and VEGF are correlated with shorter fracture healing time.
Conclusion
The formation of blood vessels was increased in CFT group compared with CF group. NGF and VEGF levels were higher in CFT group than in CF group and correlated with shorter fracture healing time. Accelerated fracture healing in patients with TBI may be due to NGF- and VEGF-mediated angiogenesis at the fracture site.
Introduction
Traumatic brain injury (TBI) is defined as damage to the brain caused by external mechanical force.Citation1 Previous studies have shown an association between TBI and accelerated fracture healing.Citation2–Citation4 Angiogenesis and bone formation are vital for fracture healing. During fracture healing process, immune cells are rapidly recruited to the injury sites for initiating inflammatory response.Citation5,Citation6 Thus, mesenchymal and hematopoietic cells are recruited and activated to promote angiogenesis and bone formation.Citation7–Citation10 However, the mechanism of the angiogenesis and bone formation in patients with TBI remains elusive.
Fracture healing process may require a variety of cytokines, such as bone morphogenetic protein, transforming growth factor-β, fibroblast growth factor-2, and platelet-derived growth factors. Nerve growth factor (NGF) is a well-studied protein that is involved in the regulation of growth, proliferation, maintenance, and survival of certain target neurons.Citation11,Citation12 NGF has been shown to promote peripheral nerve regeneration in rats model.Citation13 NGF has also been shown to accelerate wound healing.Citation14 NGF expression has been detected in bone marrow mesenchymal stem cells of both human and rodents.Citation15,Citation16 The expression of NGF and its receptor in the regional callus tissue were found to be increased.Citation17 NGF has been shown to play a vital role in osteogenesis process. NGF-TrkA signaling mediates the communication between osteoblasts and sensory nerves, which is essential for bone formation in mice.Citation18 NGF treatment promotes osteogenesis and bone formation.Citation19,Citation20
Vascular endothelial growth factor (VEGF) is a signal protein that is critical for the formation of new blood vessels during embryonic development and post injury.Citation21,Citation22 VEGF and its corresponding receptors levels are rapidly upregulated after traumatic injury of the central nervous system,Citation23 suggesting that VEGF could promote angiogenesis after TBI. In addition, VEGF could be released in rheumatoid arthritis in response to TNF-α, thus enhancing endothelial permeability and swelling, thereby stimulating angiogenesis.Citation24
However, there has been no study about the association of NGF and VEGF with accelerated fracture healing in TBI patients so far. Therefore, the present study aims to address the potential role of both NGF and VEGF in the process of fracture healing in clavicle fracture patients concomitant with TBI compared to patients with clavicle fracture alone.
Materials and methods
Study subjects
This study was approved by the Ethical Review Committee of Liuzhou General Hospital and conducted under the guidance of the Declaration of Helsinki. From January 2009 to July 2017, there were 47 patients with clavicle fracture enrolled in Liuzhou General Hospital. All subjects were fully informed, and they provided written consent prior to participation. Among these 47 patients, there were 25 patients with clavicle fracture alone (CF group) and 22 patients with clavicle fracture and concomitant TBI (CFT group). Patients in CF group underwent surgery within 1 or 2 weeks post injury. Patients in CFT group include 12 patients with TBI treated with open reduction and internal fixation of the clavicle within 1 week post injury and 10 patients underwent surgery between 8 days and 14 days after brain injury. On admission, both CF and CFT groups were imaged by conventional X-rays at the clavicle fracture and diagnosed with the Glasgow Coma Scale (GCS) system. The GCS score of CFT group was <8 points, while the GCS score of CF group was >13 points. Callus volume was preoperatively measured with length, width, and height obtained from CT scan images. The following formula was used to calculate the callus volume: volume=length × width × height × π /6. The width and height in the formula were subtracted from the width and height of clavicle bone, respectively. The brain injuries were clinically defined as loss of consciousness, post-traumatic amnesia, disorientation, confusion and/or neurological deficit after injuries including cerebral concussion, cerebral contusion, laceration, subarachnoid hemorrhage, subdural hemorrhage, and intracerebral hemorrhage. Participants with any forms of prior nervous system or bone-related diseases, malignant disease, multiple fractures, diabetes, autoimmune disease, rheumatoid arthritis, other chronic inflammation diseases as well as nonsteroidal antiinflammatory drugs, immunosuppressant, history of long-time steroid, survived <1 year after surgery or bisphosphonate therapy were excluded.
Follow-up and assessment of fracture healing
Both CF and CFT groups were followed up for at least 6 months post surgery. The follow-up assessment was based on examinations at 1, 2, 4, 8, 12, 16, 20, and 24 week post injury. The fracture was recorded as clinical heal when the clinical indications as follow were achieved: 1) the patient had no tenderness and no pain in the surgical site; 2) the patient could lift a 1 kg weight for 1 minute with the affected side; and 3) the fracture line was blurred and continuous epiphyseal based on the X-ray images. The healing time was independently evaluated by two radiologists who were blinded to the presence or absence of TBI.
Immunohistochemistry
The anti-NGF/P75 (Boster Biological Technology, cat#: M01187), anti-VEGF (Boster Biological Technology, cat#: P802), and anti-CD31 (Boster Biological Technology, cat#: BM4213) were used for immunohistochemistry for measuring corresponded proteins expressions at the callus tissue between fractures. To block the endogenous peroxidase, H2O2 was added to the section after antigen repair and incubated for 10 minutes at room temperature and then washed with PBS buffer for 3 minutes three times and incubated with 100 µL of the blocking normal calf serum working solution for 15 minutes at room temperature. Then, it was incubated with 100 µL of primary antibody overnight at 4°C after removing the serum. On the second day, it was washed with PBS buffer for 3 minutes three times after being incubated at room temperature for 15 minutes. Then, it was incubated with100 µL biotin-labeled goat anti-mouse/rabbit IgG polymer at room temperature for 13 minutes and washed with PBS buffer for 3 minutes three times. It was then incubated with 100 µL of horseradish peroxidase-labeled streptolysin working solution at room temperature for10 minutes and washed with PBS buffer for 3 minutes three times. Appropriate amount of 3,3′-Diaminobenzidine (DAB) was freshly prepared and incubated for 5–8 minutes. Hematoxylin and eosin stain was incubated for 20 seconds and tap water was used for counterstaining.
ELISA
The procedure of ELISA for detecting serum NGF levels was followed with manufacturer’s instruction (EK0471, Boster Biological Technology). Briefly, the serum and standard solution were added to each well. The reactions were incubated for 2.5 hours at room temperature. After that, the wells were washed with washing buffer and then incubated with anti-NGF antibody for 1 hour at room temperature. After washing, streptavidin solution was added and incubated for 45 minutes and then substrate reagent was incubated for 30 minutes at room temperature. Finally, stop solution was added to stop the reaction. SPECTROstar Nano plate reader (BMG Labtech) was used to measure the value of OD 450 nm. NGF concentrations were calculated by comparing the value of the serum to the standard.
Image analysis
Scanning was performed on each slice, and the top left, top right, bottom right, bottom left, and the middle region were selected for five fields of view, which were imaged with the Minmei microscope digital imaging system (V9.5.2). Image-pro plus 6.0 (Media Cybernetics, Rockville, MD, USA) was used to analyze the images. In order to train the software, positive cells were manually selected in several images. After that, the sensitivity was set to level 4, and all positive cells were automatically counted and processed by the software. The percentage of blood vessels in each visual field (CD31 positive area) was calculated as a percentage of the total visual field area with the Image pro software. The following formula was used to calculate vessel density: vessel density =vessel area/total area*100%.
Statistical analysis
Statistical Package for Social Sciences software (SPSS Inc., Chicago, IL, USA) version 16.0 for Windows was used for statistical analysis. The mean SD or median (interquartile range) was used for presenting the data. Data normality was analyzed by Kolmogorov–Smirnov test. The significance in clinical characteristics between CF and CFT groups was assessed by Student’s t-test, Mann–Whitney U test, or chi-squared test. The significance of healing time difference between CF and CFT group was assessed by clinical variables Pearson correlation. Statistical significance and correlations were defined as P<0.05.
Results
Baseline characteristics of the study groups
This study includes 22 patients with fracture and concomitant TBI (CFT group) and 25 patients with fracture alone (CF group). In , the demographics and clinical profile of patients in CF and CFT groups, including age, gender, fracture time from injury to surgery, are not significantly different between CF and CFT groups (for all, P>0.05). CFT group has a significantly lower GCS scores (11.5 vs 15; ), shorter healing time (82.2 vs 127.0 days; ), and larger fracture callus volume (21.9 vs 8.5 cm3; ) compared with CF group (for all, P<0.001). Callus volume is a very important indicator of bone fracture healing. Based on our clinical experience, the larger the callus volume, the shorter will be the fracture healing time. The representative, longitudinal chest radiographs from the same patients showed fractures before surgery and the fixation at around 4 and 12 weeks after surgery (). Healed clavicle fracture was observed at around 12 weeks after surgery in the patients of CFT group, rather than the CF group (). In CFT group, there were eleven patients with a combined cerebral contusion, laceration, and subdural hemorrhage, seven patients with cerebral contusion and laceration only, one patient with cerebral contusion and subdural hematoma, one patient with subdural hematoma only, and two patients with cerebral concussion.
Figure 1 Fracture healing time (A) and callus volume (B) in clavicle fracture alone group and clavicle fracture and concomitant TBI group were based on clinical and radiological examinations at 1, 2, 4, 8, 12, 16, 20, and 24 weeks after trauma.
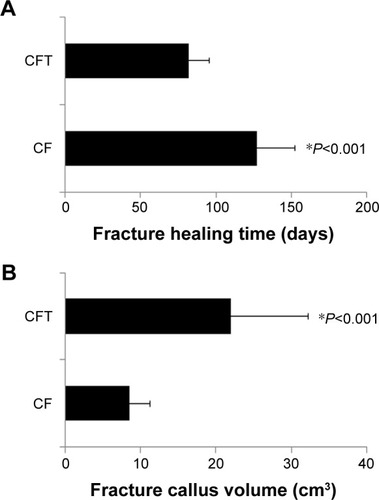
Figure 2 The representative, longitudinal chest radiographs of the clavicle fracture alone group (upper panels) and clavicle fracture and concomitant TBI group (lower panels) were collected at the time points of pre-operation, 4 and 12 weeks post operation from the same patients.
Abbreviations: CF, clavicle fracture alone; CFT, clavicle fracture and concomitant TBI; TBI, traumatic brain injury.
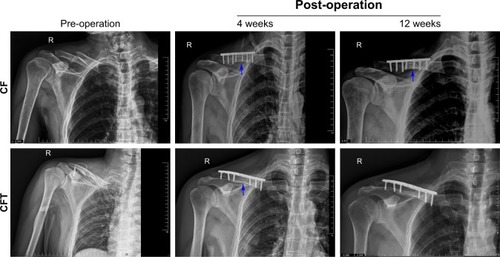
Table 1 The demographics of clinical profile of patients in CF and CFT groups
The serum NGF levels increased during fracture healing process
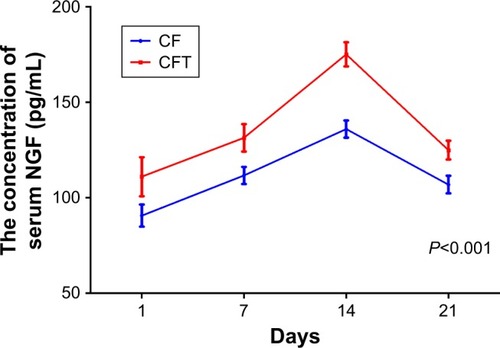
To characterize the relationship between NGF levels and fracture healing, we measured the serum NGF levels of each patient with ELISA at 1, 7, 14, and 21 days after surgery, respectively. The concentration of serum NGF in both CF and CFT groups increases with time and reaches the peak at 14 days after surgery ().
Figure 3 The concentrations of serum NGF in patients with clavicle fracture alone (CF group) and in patients with clavicle fracture and concomitant traumatic brain injury (CFT group) were measured at 1, 7, 14, and 21 days after surgery.
Abbreviations: CF, clavicle fracture alone; CFT, clavicle fracture and concomitant traumatic brain injury; NGF, nerve growth factor.
The NGF and VEGF levels are higher in CFT group compared with CF group
The concentration of serum NGF in CFT group is significantly higher than that in CF group at 1, 7, 14, and 21 days after surgery (P<0.001; ). CD31 is specifically expressed in endothelial cells, and its expression level represents the intensity of angiogenesis in callus tissues. Interestingly, the representative immunohistochemistry images showed that the expression of CD31 in callus tissues in CFT group is significantly higher compared with CF group (). Furthermore, we measured the NGF and VEGF levels in callus tissues in both CFT and CF groups with immunohistochemistry. The representative immunohistochemistry images showed that NGF and VEGF levels in CFT group are significantly higher compared with CF group (). To solidify this observation, we analyzed the images with Image-pro plus 6.0 and calculated the density of CD31, NGF, and VEGF positive cells, which are higher in CFT group compared with CF group (for all, P<0.001; ). In addition, the density of NGF positive cells in callus tissues is correlated with serum NGF levels at 1, 7, 14, and 21 days after surgery (P<0.01; ).
Figure 4 Expression of NGF, VEGF, and CD31 in callus tissue of clavicle fracture.
Abbreviations: CF, clavicle fracture alone; CFT, clavicle fracture and concomitant TBI; NGF, nerve growth factor; TBI, traumatic brain injury; VEGF, vascular endothelial growth factor.
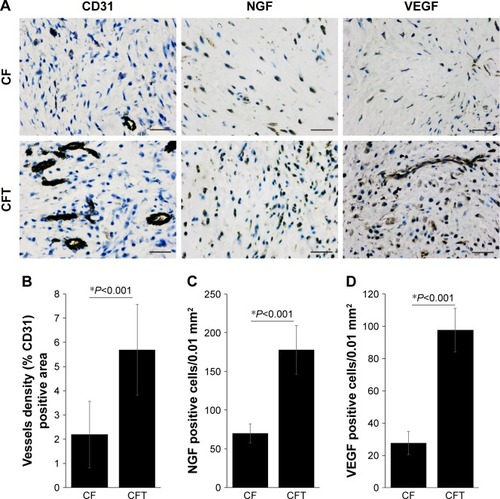
Table 2 The density of NGF+ cells in callus tissues is correlated with serum NGF levels (n=47)
Higher NGF and VEGF levels predict shorter fracture healing time
To analyze the correlation among healing time and clinical variables, we performed partial correlation analysis by using surgery time post injury as control variable, because the expressions of NGF, VEGF, and CD31 might be affected by the surgery time post injury. Consistent with previous results, there is a significant negative correlation between fractures healing time and injury type (r=−0.747, P<0.001) or callus volume (r=−0.632, P<0.001). Furthermore, the fracture healing time is also negatively correlated with the density of NGF (r=−0.837, P<0.001), VEGF (r=−0.831, P<0.001), and CD31 (r=−0.847, P<0.001) positive cells, but not with age and gender (for both, P>0.05; ).
Table 3 Partial correlations among healing time and clinical variables by using surgery time post injury as control variable (n=47)
Discussion
Bone fracture patients with contaminant TBI including subarachnoid hemorrhage, subdural hemorrhage, and intracerebral hemorrhage present accelerated fracture healing process compared with the patients with bone fracture alone,Citation3,Citation4 and the types of brain injury did not show significant difference of effect on fracture healing.Citation25 During fracture healing process, NGF promotes regeneration of injured nerves after bone fracture,Citation26 while VEGF stimulates the formation of blood vessels.Citation21 However, the relative importance of NGF and VEGF levels during the fracture healing process in patients with TBI remains poorly understood.
In this study, we find that the expression of CD31 in callus tissues in CFT group is significantly higher compared with CF group, suggesting that the angiogenesis is enhanced in CFT group. Furthermore, we find that serum NGF levels increased with time and reached the peak at 14 days after surgery in both CF and CFT groups. This finding is consistent with previous observation that the peak of NGF mRNA expression occurred 10 days after distraction, and therefore, NGF may promote the development of nerves around bone tissue.Citation27 Beside its expression in bone marrow mesenchymal stromal cells (MSCs), NGF expression was also detected in osteogenic cells, periosteal mesenchymal osteoprogenitor cells, superficial osteocytes, and osteoblastic progenitor cells and upregulated during the proliferation phase.Citation28 VEGF expression is not limited in endothelial cells and is also detected in various nonvascular cells, such as type 2 pneumocytes, monocytes, megakaryocytes, dendritic cells, hematopoietic stem cells, lens epithelial cells, and a variety of neural cells.Citation29 Hypoxia is one of the most important stimuli of VEGF expression and beneficial for fracture healing.Citation30,Citation31 After bone injury, peripheral blood enters the fracture site and forms a hematoma, which involves cells from both peripheral and intramedullary blood, as well as bone marrow cells. At this early stage, M1 macrophages, neutrophil granulocytes, and CD8+ cytotoxic T cells release proinflammatory cytokines to attract further immune cells and CD34+ endothelial progenitors. Subsequently, the expression of proangiogenic factors, such as VEGF, drive angiogenic processes and a first wave of revascularization. Furthermore, fibroblasts and MSCs migrate to the fracture site from peripheral blood and surrounding tissue for facilitating callus progression.Citation6 In the CFT group, the accelerated healing is probably caused by enhanced angiogenesis process, as expression of VEGF increased in callus tissue. Since the NGF and VEGF levels are higher in CFT group compared with CF group, it will be of future interest to investigate the regulatory mechanism of the elevated levels of NGF and VEGF in patients with clavicle fracture and concomitant TBI. Interestingly, we showed that higher NGF and VEGF levels predict shorter fracture healing time. It could be supported by the results that NGF and VEGF promote the healing of fractures in animal models.Citation32,Citation33 During the inflammatory phase of bone fracture healing, NGF upregulates the expression of adhesion molecules and stimulates the proliferation of endothelial cells.Citation34,Citation35 Combining the correlation between NGF and VEGF levels, there is a possibility that NGF induces VEGF expression and thus enhances the proliferation of endothelial cells. NT-3, another member of NGF protein family, has been shown to induce VEGF expression at the injured site and promote angiogenesis in vitro.Citation36 The upregulated NGF and VEGF levels may promote fracture healing through enhancing angiogenesis at the fracture site.
Although this study is the first report that NGF and VEGF levels are correlated with accelerated fracture healing in patients with TBI, however, there are several limitations. Firstly, the power to elucidate association between the NGF and VEGF levels and clavicle fracture and concomitant TBI was limited by small number subjects (n=47). Secondly, fracture and concomitant TBI animal model is needed to validate the role of NGF and VEGF in fracture process. It will be of future interest to perform prospective studies based on the results from large samples.
Conclusion
Serum NGF levels increased during repairing stage in both CF and CFT groups. NGF and VEGF levels are higher in CFT group compared with CF group correlated with a shorter fracture healing time. To our knowledge, this is the first report that NGF and VEGF levels are associated with accelerated fracture healing in patients with TBI. However, the role of NGF and VEGF levels in fracture and concomitant TBI must be carefully validated in animal models. Although NGF and VEGF levels predict shorter fracture healing time in 47 samples, prospective studies are required based on the results from larger samples.
Acknowledgments
The study was supported by the Science and Technology Program of Liuzhou (No 2015 J030524).
Disclosure
The authors report no conflicts of interest in this work.
References
- MaasAIStocchettiNBullockRModerate and severe traumatic brain injury in adultsLancet Neurol20087872874118635021
- LocherRJLünnemannTGarbeATraumatic brain injury and bone healing: radiographic and biomechanical analyses of bone formation and stability in a combined murine trauma modelJ Musculoskelet Neuronal Interact201515430931526636276
- BajwaNMKesavanCMohanSLong-term consequences of traumatic brain injury in bone metabolismFront Neurol2018911529556212
- SeemannRGraefFGarbeALeptin-deficiency eradicates the positive effect of traumatic brain injury on bone healing: histological analyses in a combined trauma mouse modelJ Musculoskelet Neuronal Interact2018181324129504576
- BahtGSViLAlmanBAThe role of the immune cells in fracture healingCurr Osteoporos Rep201816213814529508143
- SchlundtCEl KhassawnaTSerraAMacrophages in bone fracture healing: their essential role in endochondral ossificationBone2018106788926529389
- LienauJSchmidt-BleekKPetersADifferential regulation of blood vessel formation between standard and delayed bone healingJ Orthop Res20092791133114019274756
- PajarinenJLinTGibonEMesenchymal stem cell-macrophage crosstalk and bone healingBiomaterials pii: S0142-9612(17)30834-7
- ZhengYHuangCLiuFReactivation of denervated Schwann cells by neurons induced from bone marrow-derived mesenchymal stem cellsBrain Res Bull201813921122329524470
- PengSCaoLHeSAn overview of long noncoding RNAs involved in bone regeneration from mesenchymal stem cellsStem Cells Int20182018827364829535782
- MinnoneGde BenedettiFBracci-LaudieroLNGF and its receptors in the regulation of inflammatory responseInt J Mol Sci2017185E102828492466
- Bracci-LaudieroLde StefanoMENGF in early embryogenesis, differentiation, and pathology in the nervous and immune systemsCurr Top Behav Neurosci20162912515226695167
- SunWSunCLinHThe effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury modelBiomaterials200930274649465619573907
- KawamotoKMatsudaHNerve growth factor and wound healingProg Brain Res200414636938414699974
- BrohlinMKinghamPJNovikovaLNNovikovLNWibergMAging effect on neurotrophic activity of human mesenchymal stem cellsPLoS One201279e4505223028757
- TaghiGMGhasem Kashani MaryamHTaghiLLeiliHLeylaMCharacterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factorsCell Biol Int201236121239124922994924
- MajutaLALongoGFealkMNMcCaffreyGMantyhPWOrthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factorPain2015156115716525599311
- TomlinsonRELiZLiZMinichielloLRiddleRCVenkatesanANGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in miceProc Natl Acad Sci U S A201711418E3632E364128416686
- JinPYinFHuangLZhengLZhaoJZhangXGuangxi cobra venom-derived NGF promotes the osteogenic and therapeutic effects of porous BCP ceramicExp Mol Med2017494e31228386125
- MatsumotoKEmaMRoles of VEGF-A signalling in development, regeneration, and tumoursJ Biochem2014156111024839295
- CaoJWangLLeiDLLiuYPDuZJCuiFZLocal injection of nerve growth factor via a hydrogel enhances bone formation during mandibular distraction osteogenesisOral Surg Oral Med Oral Pathol Oral Radiol20121131485322677691
- WangHQiuLMaYNaoxintong inhibits myocardial infarction injury by VEGF/eNOS signaling-mediated neovascularizationJ Ethnopharmacol2017209132328669772
- MecollariVNieuwenhuisBVerhaagenJA perspective on the role of class III semaphorin signaling in central nervous system traumaFront Cell Neurosci2014832825386118
- ZhangJLiCZhengYLinZZhangYZhangZInhibition of angiogenesis by arsenic trioxideOncotarget2017843735297354629088724
- YangTYWangTCTsaiYHHuangKCThe effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaftJ Bone Joint Surg Br201294222723022323691
- SangQSunDChenZZhaoWNGF and PI3K/Akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat modelsBiomed Pharmacother20181031146115329715758
- AigaAAsaumiKLeeYJExpression of neurotrophins and their receptors tropomyosin-related kinases (Trk) under tension-stress during distraction osteogenesisActa Med Okayama200660526727717072373
- SuYWZhouXFFosterBKGrillsBLXuJXianCJRoles of neurotrophins in skeletal tissue formation and healingJ Cell Physiol201823332133214528370021
- D’AmorePAVascular endothelial cell growth factor-a: not just for endothelial cells anymoreAm J Pathol20071711141817591949
- ClarkinCEGerstenfeldLCVEGF and bone cell signalling: an essential vessel for communication?Cell Biochem Funct201331111123129289
- WilsonSSWongAToupadakisCAYellowleyCEExpression of angiopoietin-like protein 4 at the fracture site: regulation by hypoxia and osteoblastic differentiationJ Orthop Res20153391364137325864912
- BeiCLinZYangZStudy on effect of NGF on fracture healingZhongguo Xiu Fu Chong Jian WaiKe ZaZhi2009235570576
- FreySPJansenHRaschkeMJMeffertRHOchmanSVEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit modelClin Orthop Relat Res2012470123607361422806260
- KolostovaKTaltynovOPinterovaDTissue repair driven by two different mechanisms of growth factor plasmids VEGF and NGF in mice auricular cartilage: regeneration mediated by administering growth factor plasmidsEur Arch Otorhinolaryngol201226971763177022072234
- RaychaudhuriSKRaychaudhuriSPWeltmanHFarberEMEffect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cellsArch Dermatol Res2001293629129511480588
- SuYWChungRRuanCSNeurotrophin-3 induces BMP-2 and VEGF activities and promotes the bony repair of injured growth plate cartilage and bone in ratsJ Bone Miner Res20163161258127426763079
