Abstract
Background
To examine the outcomes of multidirectional percutaneous drilling and autologous concentrated bone marrow (BM) transplantation for atrophic femoral diaphyseal nonunion characterized by intact hardware and mechanical stability at the nonunion site.
Methods
Fourteen patients (22–63 years of age) were admitted to our hospital with atrophic femoral diaphyseal nonunion. All patients were treated with a combination of multidirectional percutaneous drilling and autologous concentrated BM transplantation. Radiographic evaluation was conducted every month after transplantation until bone healing was achieved.
Results
Bony union was achieved in 13 of the 14 patients (92.9%) after an average of 3.9 months (range: 2.5–6 months). The average radiographic union scale in tibial (RUST) scale score improved significantly from the preoperative period (6.15±1.21) to follow-up (11.23±0.73; P<0.05). The mean follow-up after transplantation was 31.4±9.5 months (range: 18–50 months). At the final follow-up, the quality of function had improved significantly, allowing a return to normal activities.
Conclusion
Combined multidirectional percutaneous drilling and autologous concentrated BM transplantation is an easy, safe, inexpensive, and efficacious method to treat atrophic femoral diaphyseal nonunion characterized by intact hardware and mechanical stability at the nonunion site.
Trial registration number: ISRCTN29808592
Introduction
Diaphyseal femoral fractures occur in both the young and the elderly. Their causes are diverse, as are the treatment options used to treat both the fractures and the associated injury.Citation1 Current methods include intramedullary nailing, plate fixation, and external fixation. While the results are generally favorable, delayed union or nonunion occurs in some cases. Böstman et al reported a 4% delayed or nonunion rate in fractures treated with locking plate fixation.Citation2 Reamed interlocking intramedullary nailing was shown to yield better clinical results than plate fixation, with a nonunion rate of 0.9–1.1%.Citation3,Citation4 However, despite these improved outcomes, patients with nonunion of the diaphyseal femur will inevitably suffer serious complications, including limb deformity or shortening, degeneration of neighboring joints, and local pain.
The treatment of nonunion is a challenge to orthopedic surgeons. In patients with nonunion of diaphyseal femoral fractures, various treatment strategies based on the above-described methods have been proposed. A prerequisite is careful classification of the nonunion, as this will guide the treatment choice. For patients with atrophic femoral diaphyseal nonunion, autologous cancellous bone is a fairly common practice, with the most common autologous graft donor site being the iliac crest.Citation5 Autologous bone grafts, analogous bone grafts, and bone substitutes are additional therapeutic options.Citation6 The complications that follow treatment include donor site pain, blood loss, increased operative time, and a risk of infection.Citation7,Citation8 The incidence of morbidities related to bone grafts is as high as 22%, with 12-month donor site pain occurring in up to 45% of patients.Citation9,Citation10
To minimize the drawbacks associated with traditional bone grafting, percutaneous approaches have been developed for harvesting autologous bone marrow (BM).Citation11–Citation13 These approaches are based on osteo-inducing cells harvested from the iliac crest and injected directly into the fracture site. BM grafted using this method is easy, safe, and relatively non invasive and can be performed under local anesthesia with minimal secondary local damage. However, not all patients who undergo this procedure experience satisfactory healing, and the incidence of nonunion after BM injection continues to be high, at 18–37%.Citation14–Citation16
One difficulty in treating nonunion is that the involved site is stiff and not easily handled. In standard bone-grafting procedures, resection of nonviable bone and the intervening nonunion tissue is necessary.Citation17 However, in previously reported percutaneous BM grafting procedures, no attempts were made to remove the intervening callus or fibrous tissue from the nonunion site.Citation13,Citation16,Citation18–Citation20 This omission would make it difficult to inject the entire volume of BM, and the injected marrow may leak through the gap between bone and soft tissue. We, therefore, hypothesized that multidirectional percutaneous drilling of the nonunion site would produce an ideal incubation bed for fixing the BM graft in position while simultaneously promoting the revascularization of intervening fibrous tissue and inducing osteoinductive factors locally in the bone matrix, thus enhancing bone healing.
Materials and methods
Study population and protocol
This study was a single-center, single-group interventional study conducted at our hospital, a university teaching hospital, from January 2009 to September 2013. Patients aged >18 years with atrophic nonunion, and who underwent percutaneous autologous BM grafting with multidirectional percutaneous pre drilling procedures, were voluntarily enrolled in the study. Patients with local angular deformity, shortening, fixation problems, or mental comorbidities resulting in poor compliance were excluded. Thus, the final study population consisted of 14 patients with atrophic femoral diaphyseal nonunion who agreed to undergo this novel procedure. The characteristics of the patients are listed in . The nine males and five females had a mean age of 33.8 years (range: 22–63 years). Eight nonunions involved the left femur and six the right femur. All patients had previously undergone plate or intramedullary nail fixation. Atrophic nonunion of a diaphyseal femoral fracture (International Classification of Diseases 10th edition [ICD-10] code M84.151) was diagnosed according to clinical signs and radiographic examinations. In all of the patients, >9 months had passed since the initial fixation. No visible progressive signs of healing at the fracture site had appeared during the 3 previous consecutive months, thus fulfilling the Food and Drug Administration’s definition of nonunion. All data in this study were collected prospectively. Informed consent was obtained from all individual participants included in the study.
Table 1 Patient characteristics
BM aspiration and concentration
BM was harvested according to a previously published method.Citation21 Briefly, after the patients were placed under general anesthesia, a BM aspiration needle was inserted into the posterior iliac crest. The needle was reoriented repeatedly within the iliac crest to optimize the number of BM cells harvested. Samples obtained from each puncture point were pooled into a BM collection bag containing anticoagulant. Erythrocytes were removed by centrifugation for 10 mins at 1200 g. The remaining aggregates were then centrifuged for 10 mins at 3000 g to remove supernatant plasma and anticoagulants. Finally, a concentrate of BM aspirate (30–40 mL) was collected in a syringe for injection.
Multidirectional percutaneous drilling at the nonunion site and BM grafting
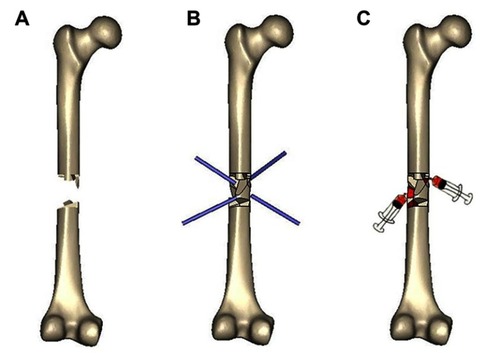
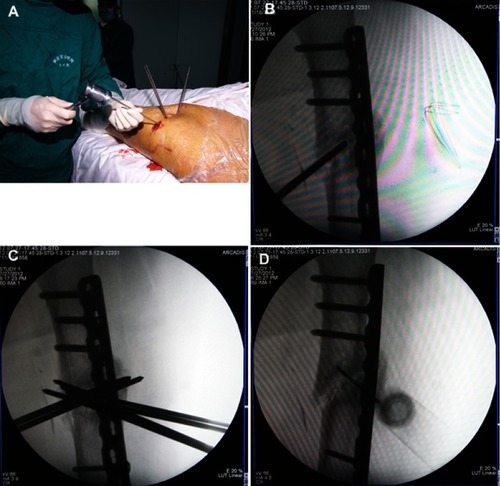
Under fluoroscopic guidance, multiple 4.0-mm steinmann pins were drilled into the nonunion site in various directions to push the intervening soft tissue away. After repeated drilling, a concentrated BM syringe needle was placed in the nonunion gap; the BM was slowly injected for 1 min, and the needle was gradually withdrawn. shows the steps of the procedure, and provides intraoperative photographs of a patient with femoral shaft nonunion.
Radiographic evaluation
Radiographic evaluation (plain anteroposterior and lateral radiographs) was conducted every month for 1 year after transplantation until bone healing was achieved. Healing at the four cortices (medial/lateral/anterior/posterior) was evaluated using the Radiographic Union Scale for Tibial fractures (RUST) classification system,Citation22 which employs 4- to 12-point scales and sums the score for each of the four cortices. Three senior orthopedists judged the healing independently, with the final diagnosis based on the majority opinion.
Statistical analyses
All data were expressed as mean and SD of the mean using SPSS 22.0 software (IBM Corporation, Armonk, NY, USA). The univariate significance of the comparison between the preoperative and postoperative of RUST score was established through a independent-samples t-test.
Results
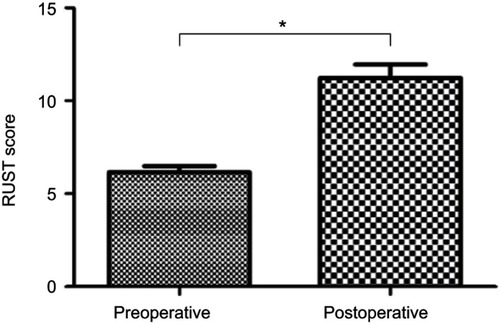
All patients reported slight discomfort at the donor site for the first 2 days postoperatively, but the pain was managed sufficiently with oral pain killers administered during the first 24 hrs. No major complications were identified. Of the 14 patients, bony union was achieved in 13 (92.9%) after an average of 3.9 months (range: 2.5–6 months) (, ). Radiographic union was diagnosed by a RUST score ≥10, and the results were confirmed by clinical examination. The average RUST score improved significantly, from 6.15±1.21 preoperatively to 11.23±0.73 at follow-up (P=0.000, P<0.05) (). The mean final follow-up duration was 31.4±9.5 months after transplantation (range: 18–50 months). By the final follow-up examination, the patients’ quality of life had significantly improved and a return to normal activities was possible. Only one patient failed to achieve bony union without signs of healing 3 months after BM injection. This patient received an additional plate and autogenous bone grafting at 3 months and achieved bony union within 6 months after the latter procedure.
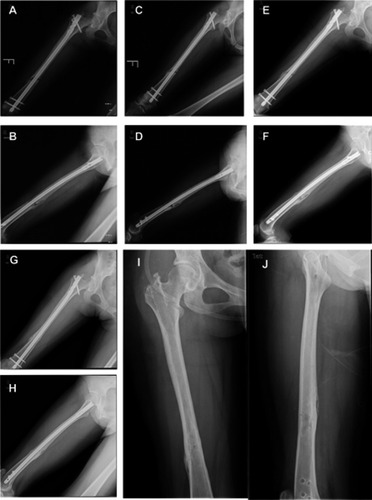
Figure 3 BM transplantation of a 44-year-old male patient. (A and B) Postoperative result immediately after multidirectional percutaneous drilling and autologous concentrated bone marrow (BM) transplantation in a 44-year-old male patient; (C and D) 1 month postoperatively; (E and F) bony union, achieved 3 months postoperatively; (G and H) complete remodeling of the callus obtained at 19 months postoperatively.
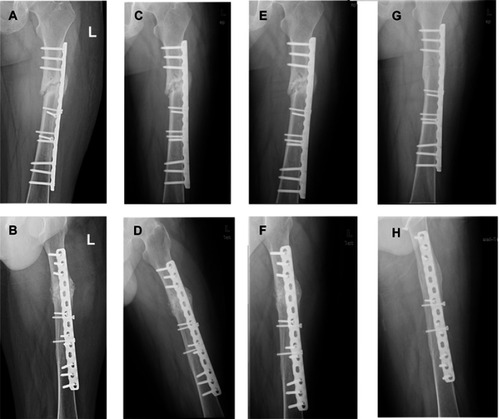
Figure 4 Bone marrow (BM) transplantation of a 25-year-old female patient. (A and B) Radiographs of femoral shaft nonunion in a 25-year-old female patient; (C and D) postoperative result immediately after multidirectional percutaneous drilling and autologous concentrated BM transplantation; (E and F) 1 month postoperatively; (G and H) bony union, achieved 4 months postoperatively; (I and J) radiological results after removal of the intramedullary nail.
Discussion
Femoral diaphyseal nonunion has long been a challenging clinical problem. Effective surgical treatment of these patients requires a detailed understanding of the pathophysiology and classification of nonunion.Citation23 Fracture union mainly depends on adequate mechanical stabilization and the biological environment (pro-osteogenic cells, growth factors, and vascularization) of the fracture site.Citation24,Citation25 Hypertrophic nonunion is considered to arise from insufficient stability; in these cases, treatment primarily involves stabilization of the fracture. However, atrophic nonunion has a primarily biological, rather than mechanical, etiologyCitation26 and thus requires a biological approach to treatment. In this study, the mechanical properties of the nonunion were carefully evaluated by radiographic examination. There were no fixation problems, and all cases were identified as atrophic nonunion. The lack of an appropriate biological stimulus was thus hypothesized to be a major factor leading to nonunion in the study patients.
The standard treatment for nonunion is autologous bone grafting, which is performed to accelerate osteogenesis by stimulating the local microenvironment at the nonunion site. However, the autogenous bone originates mainly from the iliac spine and is harvested during a procedure that is relatively invasive and may cause pain, neurovascular injury, infection, or other complications at the donor site.
To minimize the drawbacks associated with traditional bone grafting, autologous BM grafting for nonunion has been employed, with mixed results. The first report of percutaneous BM grafting of delayed union and nonunion was by Healey et al, in 1990. Their patients had undergone lower-extremity resections for sarcomas affecting bone. Bony union was finally achieved in five of the eight patients (63%).Citation14 With gradual improvements to the technique, bony re union rates after autologous BM grafting have reached 76–82% according to recent studies,Citation15,Citation16 but there remains considerable room for improvement.
Prior to this study, we carefully assessed the drawbacks associated with previously reported autologous BM grafting procedures.Citation13,Citation16,Citation18–Citation20,Citation27,Citation28 A major problem was that in none of them, an attempt had been made to remove the intervening calluses or fibrous tissue from the nonunion site. We, therefore, designed a new procedure that combined the advantages of multidirectional percutaneous drilling with minimally invasive percutaneous supplementation of autologous concentrated BM, thus providing an ideal incubation bed and a strong biological stimulus. Patients treated by this technique achieved a bone union rate of 92.9% (13/14).
BM contains osteogenic progenitors, and its implantation can lead to effective bone regeneration. Both preclinical investigations and clinical studies have demonstrated that BM cells can differentiate into osteoblasts.Citation29–Citation33 In clinical practice, autologous BM is harvested from the iliac crest and implanted at the fracture site. Previous studies have shown a link between the number of progenitors in the graft and the volume of the callus around the nonunion site. In failed cases, the number of transplanted BM stem cells was low.Citation34 In our approach, with the aim of grafting as many osteogenic stem cells as possible, we grafted concentrated aspirates. Hernigou et al demonstrated that mononuclear cells could be concentrated by centrifugation of the BM aspirate, resulting in improved osteogenesis.Citation27 They also hypothesized that the concentrated BM includes stem and other mononuclear cells with osteogenic or angiogenic properties that influence the clinical outcome. Although culture-expanded human BM stromal cells have been used successfully for enhanced fracture healing in nonunion,Citation35 they must be expanded in vitro for several weeks to achieve a sufficient number of cells for transplantation. This delays treatment and increases both the culture-related contamination risk and therapy cost. By contrast, BM concentration is a simple, safe, clean, and low-cost procedure that allows the harvest and transplantation of cells shortly after their aspiration. This advantage recommends the use of BM cell concentrates, instead of expanded purified BM stromal cells, for bone cell therapy.
Resection of the scar and fibrous tissue at the nonunion site is considered essential for the treatment of atrophic nonunion.Citation17 However, for percutaneous cellular transplantation, additional surgery to refresh the nonunion site may disrupt already compromised tissues and increase the risk of continued nonunion, or even lead to further complications such as infection. Moreover, a previous study in humans showed that nonunion tissue at the fracture gap contains osteogenic or chondrogenic progenitor cells.Citation17 Three factors are thought to be essential to the healing process: an adequate number of osteoprogenitor cells, an appropriate scaffold (cell incubation bed), and biologically active molecules.Citation28 In nonunion treated with BM, the marrow is injected both into the gap in the nonunion and around the bones. However, it may be difficult to inject the entire graft and the BM may leak through the gap between the bone and soft tissue. Therefore, the existing fibrous tissue in the fracture gap should be removed, by chipping or drilling, to obtain an ideal cell incubation bed for placement of the concentrated BM. In our procedure, multidirectional percutaneous drilling was performed to cause local damage (microfracture), promote the revascularization of fibrous tissue, and accelerate the inflow of BM cells or growth factors from normal peripheral bone. In addition, hematomas caused by local microfracture help restart the healing process, while cells in the concentrated BM secrete angiogenic cytokines, which promote angiogenesis and thus improve osteogenesis.Citation36
The best indication for the combined use of multidirectional percutaneous drilling and autologous concentrated BM is the treatment of nonunion, especially oligotrophic or atrophic nonunion, which are less biologically active. In our study, this approach led to complete healing in 92.9% of the patients, all of whom had been diagnosed with atrophic nonunion. While the infection may be considered the main risks of the procedure, our study showed that it is safe, simple, and less invasive, and that local or systemic complications did not develop in any of the treated patients. Hernigou et al reported the clinical results of >1000 patients who underwent BM aspiration and none developed complications.Citation19 There are also no reports of infection among any of the published studies on autologous BM grafting for nonunion.Citation11–Citation13,Citation16,Citation18–Citation20,Citation27–Citation29,Citation35 Additionally, because the material injected is autologous BM, there is no risk of disease transmission or an immune reaction.
Conclusion
The therapy proposed in this study combined two simple techniques: multidirectional percutaneous drilling and autologous concentrated BM transplantation under fluoroscopy. Together, they constitute a simple, minimally invasive, and inexpensive method to successfully treat femoral diaphyseal nonunion. Because the results were at least as good as those achieved with other methods, our study demonstrates the utility of this combined technique for treating aseptic nonunion characterized by intact hardware and mechanical stability at the nonunion site.
Ethical approval and informed consent
Ethical approval was obtained by the ethics committee of the Ethics Review Board of Chinese PLA General Hospital. Written informed consent was obtained from all participants.
Acknowledgment
This work was supported by the Medical Big Data Center of PLA General Hospital (2017MBD-014).
Disclosure
The authors report no conflicts of interest in this work.
References
- Neumann MV, Sudkamp NP, Strohm PC. Management of femoral shaft fractures. Acta Chir Orthop Traumatol Cech. 2015;82(1):22–32.25748658
- Bostman O, Varjonen L, Vainionpaa S, Majola A, Rokkanen P. Incidence of local complications after intramedullary nailing and after plate fixation of femoral shaft fractures. J Trauma. 1989;29(5):639–645. doi:10.1097/00005373-198905000-000192724381
- Wolinsky PR, McCarty E, Shyr Y, Johnson K. Reamed intramedullary nailing of the femur: 551 cases. J Trauma. 1999;46(3):392–399. doi:10.1097/00005373-199903000-0000710088839
- Winquist RA, Hansen ST Jr., Clawson DK. Closed intramedullary nailing of femoral fractures. A report of five hundred and twenty cases. J Bone Joint Surg Am. 2001;83–A(12):1912. doi:10.2106/00004623-200112000-00021
- Niu Y, Bai Y, Xu S, et al. Treatment of lower extremity long bone nonunion with expandable intramedullary nailing and autologous bone grafting. Arch Orthop Trauma Surg. 2011;131(7):885–891. doi:10.1007/s00402-010-1226-921165632
- Nauth A, Lane J, Watson JT, Giannoudis P. Bone graft substitution and augmentation. J Orthop Trauma. 2015;29(Suppl 12):S34–S38. doi:10.1097/BOT.000000000000046426584264
- Kumar G, Narayan B. Morbidity at bone graft donor sites In: Banaszkiewicz PA, Kader DF, editors. Classic Papers in Orthopaedics. London: Springer; 2014:503–505.
- Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45(Suppl 6):S116–S120. doi:10.1016/j.injury.2014.10.03425457330
- Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine (Phila Pa 1976). 2001;26(13):1473–1476. doi:10.1097/00007632-200107010-0001811458153
- Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi:10.1097/00003086-199706000-00011
- Galois L, Bensoussan D, Diligent J, et al. Autologous bone marrow graft and treatment of delayed and non-unions of long bones: technical aspects. Biomed Mater Eng. 2009;19(4–5):277–281. doi:10.3233/BME-2009-059220042794
- Goel A, Sangwan SS, Siwach RC, Ali AM. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36(1):203–206. doi:10.1016/j.injury.2004.01.00915589942
- Singh AK, Shetty S, Saraswathy JJ, Sinha A. Percutaneous autologous bone marrow injections for delayed or non-union of bones. J Orthop Surg (Hong Kong). 2013;21(1):60–64. doi:10.1177/23094990130210011623629990
- Healey JH, Zimmerman PA, McDonnell JM, Lane JM. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990;256:280–285.
- Braly HL, O’Connor DP, Brinker MR. Percutaneous autologous bone marrow injection in the treatment of distal meta-diaphyseal tibial nonunions and delayed unions. J Orthop Trauma. 2013;27(9):527–533. doi:10.1097/BOT.0b013e31828bf07723443050
- Sugaya H, Mishima H, Aoto K, et al. Percutaneous autologous concentrated bone marrow grafting in the treatment for nonunion. Eur J Orthop Surg Traumatol. 2014;24(5):671–678. doi:10.1007/s00590-013-1369-924275891
- Iwakura T, Miwa M, Sakai Y, et al. Human hypertrophic nonunion tissue contains mesenchymal progenitor cells with multilineage capacity in vitro. J Orthop Res. 2009;27(2):208–215. doi:10.1002/jor.2073918752274
- Guimaraes JA, Duarte ME, Fernandes MB, et al. The effect of autologous concentrated bone-marrow grafting on the healing of femoral shaft non-unions after locked intramedullary nailing. Injury. 2014;45(Suppl 5):S7–S13. doi:10.1016/S0020-1383(14)70013-0
- Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87(7):896–902. doi:10.1302/0301-620X.87B7.1628915972899
- Kassem MS. Percutaneous autogenous bone marrow injection for delayed union or non union of fractures after internal fixation. Acta Orthop Belg. 2013;79(6):711–717.24563979
- Hernigou P, Mathieu G, Poignard A, Manicom O, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):322–327. doi:10.2106/JBJS.F.0020316951103
- Whelan DB, Bhandari M, Stephen D, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68(3):629–632. doi:10.1097/TA.0b013e3181a7c16d19996801
- Megas P. Classification of non-union. injury. 2005;10:8.
- Portal-Nunez S, Lozano D, Esbrit P. Role of angiogenesis on bone formation. Histol Histopathol. 2012;27(5):559–566. doi:10.14670/HH-27.55922419020
- Berner A, Reichert JC, Muller MB, et al. Treatment of long bone defects and non-unions: from research to clinical practice. Cell Tissue Res. 2012;347(3):501–519. doi:10.1007/s00441-011-1184-821574059
- Dickson K, Katzman S, Delgado E, Contreras D. Delayed unions and nonunions of open tibial fractures. Correlation with arteriography results. Clin Orthop Relat Res. 1994;302:189–193.
- Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi:10.2106/JBJS.D.0221515995108
- Scaglione M, Fabbri L, Dell’Omo D, Gambini F, Guido G. Long bone nonunions treated with autologous concentrated bone marrow-derived cells combined with dried bone allograft. Musculoskelet Surg. 2014;98(2):101–106. doi:10.1007/s12306-013-0271-223700322
- Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64(6):671–672.8291415
- Leonardi E, Devescovi V, Perut F, Ciapetti G, Giunti A. Isolation, characterisation and osteogenic potential of human bone marrow stromal cells derived from the medullary cavity of the femur. Chir Organi Mov. 2008;92(2):97–103. doi:10.1007/s12306-008-0057-018791684
- Paley D, Young MC, Wiley AM, Fornasier VL, Jackson RW. Percutaneous bone marrow grafting of fractures and bony defects. An experimental study in rabbits. Clin Orthop Relat Res. 1986;208:300–312.
- Phedy P, Dilogo IH, Jusuf AA, Kholinne E, Efendi Z. Iliac crest and femoral bone marrow as the source of plastic-adherent cells. Med J Indonesia. 2011. doi:10.13181/mji.v20i2.436
- Tiedeman JJ, Connolly JF, Strates BS, Lippiello L. Treatment of nonunion by percutaneous injection of bone marrow and demineralized bone matrix. An experimental study in dogs. Clin Orthop Relat Res. 1991;268:294–302.
- Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79(11):1699–1709. doi:10.2106/00004623-199711000-000129384430
- Centeno CJ, Schultz JR, Cheever M, Freeman M, Robinson B, Faulkner SJ. A case series of percutaneous treatment of non-union fractures with autologous, culture expanded, bone marrow derived, mesenchymal stem cells and platelet lysate. J Bioeng Biomed Sci. 2011;01(S2). doi:10.4172/2155-9538.S2-007
- Hernigou P. Growth factors released from bone marrow are promising tools in orthopedic surgery. Rev Rhum Engl Ed. 1998;65(2):79–84.9540115