Abstract
Cerebral palsy (CP) is the main cause of physical disability in childhood and is an important health issue that has a strong socioeconomic impact. There is no effective treatment for CP and therapeutic approaches report only partial benefits for affected people. In this study we assessed the effects of growth hormone (GH) treatment combined with psychomotor and cognitive stimulation in the neurodevelopment of children with CP and GH deficiency (GHD). The study was carried out in 11 patients (7 boys and 4 girls; 4.12 ± 1.31 years) with GHD and CP who were treated with recombinant GH (rGH) and psychomotor and cognitive stimulation during 2 months. Battelle Developmental Inventory Screening Test (BDIST) was performed 2 months before commencing GH treatment, just before commencing GH administration, and after 2 months of combined treatment involving GH and cognitive stimulation. Psychomotor and cognitive status did not change during the period in which only cognitive stimulation was performed; however, significant improvements in personal and social skills, adaptive behavior, gross motor skills and total psychomotor abilities, receptive and total communication, cognitive skills and in the total score of the test (P < 0.01), and in fine motor skills and expressive communication (P < 0.02) were observed after the combined treatment period. Therefore, GH replacement together with psychomotor and cognitive stimulation seem to be useful for the appropriate neurodevelopment of children with GHD and CP.
Introduction
Nervous system plasticity and regeneration of the brain in response to both neurological injury and repetitive practice of different neurological stimulations have been widely demonstrated.Citation1 However, achievements obtained with such stimulations usually are incomplete. Results are better when brain injuries occur at an early age and rehabilitation intervention begins promptly after the injury, since there is a greater plasticity when the central nervous system (CNS) is developing.Citation2
Cerebral palsy (CP) is the most common cause of physical disability in childhoodCitation3 and the estimated prevalence of CP in the general population is 2/1000.Citation4 CP is defined as a persistent but not progressive disorder of posture and movement system, associated with functional activity limitations and sensorial, cognitive, communications problems, epilepsy, and musculoskeletal system problems.Citation4 Major causes for CP involve prematurity (40% to 50% of cases of CP), abnormal intrauterine developments due to fetal–maternal infections, asphyxia during delivery, brain trauma during labor, and delivery and complications in the perinatal period.Citation5 Currently there is no cure for CP and therapeutical approaches show only partial benefits for affected individuals.Citation1
It has been shown that most CP children have poor linear growth during childhood resulting in a diminished final adult height.Citation6 We demonstrated that in a population of CP children, 70% showed impaired GH secretion.Citation5 This phenomenon, could have a great importance since the GH-plasma insulin-like growth factor (IGF-1) system plays an important role during CNS development, and also in inducing neurogenesis and increasing brain plasticity.Citation1,Citation7–Citation9 In this sense, we demonstrated that combined treatment involving GH replacement and adequate physical or cognitive stimulation is effective in the motor rehabilitation of children with CP or adult patients with cognitive disorders after traumatic brain injury, respectively.Citation1,Citation10
This pilot study assessed the effects of GH replacement combined with psychomotor and cognitive stimulation in the cognitive rehabilitation of children with CP and GHD.
Method
Participants
Eleven patients with GHD and CP admitted for cognitive and motor rehabilitation were included in the study (7 males and 4 females; 4.12 ± 1.31 years; range: 3 to 7 years old). According to the Gross Motor Function Classification System (GMFCS) patients’ levels ranged from I to V.Citation11 shows the main characteristics of patients studied.
Table 1 Main characteristics of the patients studied
GHD
The existence of GHD was detected previously in the CP children included in the study.Citation5 The existence of GHD was established according to auxological parameters and the GH peak response to the insulin-induced hypoglycemia test. IGF-1 and plasma insulin-like growth factor binding protein 3 (IGFBP-3) plasma levels were measured by a solid-phase, enzyme-labeled chemiluminiscent immunometric assay (Inmulite 2000, Siemens). Other pituitary hormone deficiencies were excluded by chemiluminiscent immunometric assays (data not shown). Routine blood analyses (hematology and chemistry) were carried out at 2-month intervals (Coulter HmX, Beckman and AU400, Beckman, respectively).
Clinical assessments
To assess neurodevelopment in patients under study we used the Battelle Developmental Inventory Screening Test (BDIST).Citation12 The BDIST assesses key developmental skills in children from 6 months to 8 years of age. The reliability and validity of the BDIST has been previously demonstrated.Citation13 The test comprises 96 items assessing the following 5 domains: personal and social skills (including adult interaction, expression of feelings, self-concept, peer interaction, copying, and social roles), adaptive behavior (including attention, eating, dressing, toileting, and personal responsibility), psychomotor ability, communication and cognition (including perceptual discrimination, memory, reasoning and academic skills, and conceptual development). Subdomain scores can be computed for psychomotor ability (fine motor skills and gross motor skills) and communication (expressive and receptive communication). Scores for each domain are expressed as age-equivalent scores that indicate the age (in months) at which a raw score is average. Total test score is obtained after combining the scores of all domains.
Study timing
BDIST was during 2 study periods: first, a pretreatment period (from 2 months before starting GH treatment until just before commencing it); second, a treatment period (from just before the beginning of GH treatment until 2 months after GH administration commenced) ().
Figure 1 Study timing.
During the pretreatment period only psychomotor and cognitive stimulation was performed. During the treatment period all patients received GH replacement together with psychomotor and cognitive stimulation.
It is remarkable that before admission at our center all these CP children had been intensively stimulated in other centers, most of them since they were 1 year old.
Intervention
GH treatment
The patients were treated with recombinant human GH (rhGH; Omnitrope, Sandoz) (subcutaneously; 30 μg/kg/day, 5 days/week, during 2 months; after 2 months of psychomotor and cognitive stimulation). Fasting blood samples were analyzed for routine hematology and chemistry parameters at 2-month intervals and plasma thyroid stimulating hormone (TSH), IGF-I, and IGFPB3 levels were also measured every 2 months.
Psychomotor and cognitive stimulation
Patients received psychomotor and cognitive stimulation; these therapies were carried out for 45 minutes per day, 5 days per week during 4 months.
Psychomotor and cognitive stimulation were adapted to the specific needs of each patient. Psychomotor stimulation involved tasks aimed at improving tonic–postural control, laterality, breathing and relaxation, static and dynamic balance, motor coordination and dissociation, body image, oculomotor coordination, spatial and temporal orientation, and gross and fine motor skills. Cognitive stimulation involved tasks directed at improving interaction with the environment, communication, attention, perception, memory, reasoning, and concept learning.
Data analysis
Data from each BDIST assessment were compared using a nonparametric test for 2 related samples (Wilcoxon signed-rank test). The analysis was done by comparing data obtained before and after the 2 study periods (pretreatment and treatment periods). The improvement means relative to baseline during the pretreatment period and during the treatment period were compared in the same way (Wilcoxon signed-rank test) (**P < 0.02 and ***P < 0.01). Similar statistical analysis was used for evaluating plasma changes in IGF-I, IGFBP3 changes, total cholesterol, triglycerides, and glucose levels. Results are shown as the mean ± standard deviation (SD) of the mean.
Results
No undesirable side-effects were observed during or after the study. Plasma cholesterol and triglyceride values decreased significantly at the end of treatment period (); plasma IGF-I and IGFBP3 increased significantly (), but their values were not higher than the mean for the patient’s age (data not shown). No changes were observed in plasma glucose levels () or in plasma TSH levels (data not shown).
Table 2 Main plasma chemistry parameters analyzed
Patients did not improve their psychomotor and cognitive status during the pretreatment period, during which only psychomotor and cognitive stimulation were performed. However, significant improvements in all BDIST domains were observed when GH was administered together with psychomotor and cognitive stimulation; specifically, patients improved in personal and social skills, adaptive behavior, gross motor skills and total psychomotor abilities, receptive and total communication, cognitive skills, and in the total score of the scale (P < 0.01), and in fine motor skills and expressive communication (P < 0.02) ( and ).
Figure 2 Clinical data from the BDIST during pre-treatment and treatment periods.
Notes: A) Pre-treatment period; (B) Treatment period. Horizontal axis: the different domains of the BDIS T and total score of it (TOTAL) are described. Vertical axis: score achived. Notice that different scales are used for (A) and (B). A) means and SD of the mean before (white bars) and after (grey bars) for each specific assessment. B) means and SD of the mean before (white bars) and after (grey bars) treatment period for each specific assessment. Statistical significance was calculated by comparing the data from the BDIST before and after the pre-treatment period (A) and the treatment period (B) (Wilcoxon signed-rank test). **P < 0.02 and ***P < 0.01.
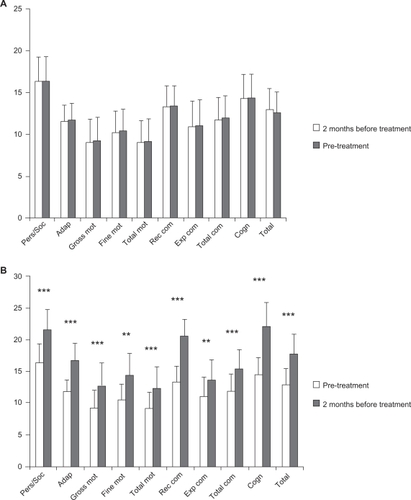
Figure 3 Comparison of improvements during pre-treatment and treatment periods.
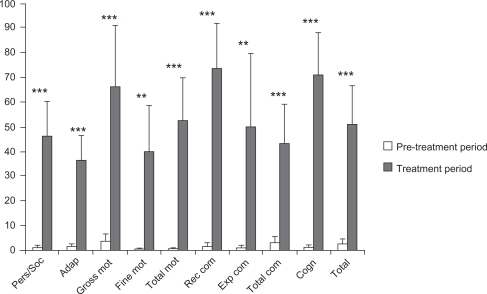
Table 3 Results obtained from the Battelle Developmental Inventory Screening Test (BDIST)
Discussion
Results from this study show that the combined treatment involving rhGH treatment and psychomotor and cognitive stimulation led to significant improvements in all BDIST domains during the treatment period. This indicates that the combined treatment clearly improved the neurodevelopment process of CP children by increasing their psychomotor and cognitive capacities and abilities. Specifically, the patients improved their potential to interact with their environment (both with adults and with their peers), affectivity, self-concept, collaboration, and understanding of their social role. Moreover, patients improved their attention and abilities to carry out their daily living activities. In the motor area patients specifically improved their motor control, body coordination, locomotion, performing precision activities, and, in general, their movement functionality. Patients increased their communication too (both receptive and expressive) and their cognitive abilities and capacities (perceptual discrimination, memory, reasoning, and concept development).
Although we did not use any control group to compare the results of the treatment used, we think that the pretreatment period can be considered as a control, despite the limitations of this strategy. Moreover, patients had received intense stimulation before admission, without any significant achievements.
It has been demonstrated that early intervention in low-birth-weight (LBW) premature infants leads to significant improvements in diverse cognitive parameters only in the heavier LBW stratum.Citation14,Citation15 Moreover, a systematic review of the literature shows that early interventions for preterm infants have a positive influence on cognitive outcomes in the short to medium term but not on motor outcomes.Citation16 Few studies have described the effects of GH replacement in GHD CP children and most report only growth data without assessing any possible neurologic functional recovery.Citation6,Citation17,Citation18 GH replacement therapy in GH-deficient children has been reported recently to significantly improve some areas such as the motor-component scale and performance IQ.Citation19 This agrees with our data in this study and with our previous report demonstrating that the combined treatment involving GH replacement and adequate physical therapy is effective in the gross motor function rehabilitation of patients with CP and GHD.Citation1
The neurotrophic role of GH is now well known.Citation7,Citation8,Citation20–Citation22 Moreover, the GH–IGF-I axis is involved in the regulation of brain growth, development, and metabolism.Citation23 Recent data from our group demonstrate that exogenous growth hormone may combine with locally produced GH in increasing the proliferative response of hippocampal progenitors to brain injury in rats.Citation9 Thus the possibility exists that most of the changes we observed in our study are a consequence of GH inducing newborn neural cells. However, some of these changes seem not to be related to this putative effect of GH, but to an enhanced or modified metabolic turnover of exciters neurotransmitters, such as noradrenaline (NA) and dopamine (DA). The prompt appearance of some positive responses in our CP children treated with GHD, such as alertness and increased interaction with the environment, cannot be explained by a putative neural stem cell proliferation induced by the hormone. GH neuroregulation is complex and a number of neurotransmitters participate in it. The pituitary release of the hormone is tonically inhibited by hypothalamic somatostatin (SS).Citation24 In turn, NA negatively controls hypothalamic SS release.Citation25 We previously demonstrated that GH administration increases hypothalamic SS release, which in turns triggers cerebral NA and DA synthesis and release for negatively controlling SS.Citation24 Thus, it is tempting to speculate that in the absence of a pituitary GH secretion, as occurs in GH deficiency, hypothalamic SS secretion is weak or also deficient. The exogenous administration of the hormone would lead to increased SS tone, therefore to increased DA and NA turnover for maintaining the physiological feedback circuits involved in GH control.Citation24 This might explain some of the early responses observed after GH administration in this study.
The effect of GH on plasma lipids profile is well known. GHD patients usually display an abnormal lipid profile characterized by elevated total cholesterol, elevated low-density lipoprotein cholesterol, and elevated triglycerides.Citation26 GHD adolescents present an abnormal fasting and postprandial lipid profile that, in addition to increased fibrinogen and homocysteine levels, suggest the accumulation of cardiovascular risk factors early in life.Citation27 In our study, GH replacement therapy significantly decreased fasting plasma total cholesterol and triglyceride levels, in agreement with that observed in other studies.Citation26 Fasting glycemia was not significantly modified after 2 months of GH administration. As expected, plasma IGF-1 and IGFBP3 significantly increased too after GH treatment, indicating that GHD in our patients did not occur as a consequence of any mutation in GH receptor, but as a result of an altered hypothalamic control of pituitary GH release. This agrees with the fact that no clear anatomical abnormalities were observed in hypothalamic and pituitary MRI studies carried out before admission.
In summary, we have demonstrated that the combined therapy involving GH replacement and psychomotor and cognitive stimulation seems to be useful for the appropriate neurodevelopment of children with GHD and CP. Since these children had received an intensive neurostimulation without significant improvements before being treated with GH, it seems to be clear that the hormone, and/or IGF-1, was the main factor responsible for the results obtained. Although this was a pilot study, and new and larger studies are needed to understand more precisely the effects of GH treatment in patients with CP and GHD, accumulating evidence (this and our previous studies) shows that GH administration improves both motor and cognitive abilities in GHD CP children. Since exogenous GH combines with locally produced GH for repairing brain injuries,Citation9 it is feasible to assume that GH treatment may be used too in CP children without GHD. Because GH treatments last a few months, and GH doses are not higher than those used for treating GH deficiency, no significant risks seem to be associated with GH treatment in CP children in whom GH secretion is normal.
Acknowledgements
This study was supported by Fundación Foltra and Fundación Repsol.
Disclosure
The authors report no conflicts of interest in this research.
References
- ReimundePRodicioCLopezNAlonsoADevesaPDevesaJEffects of recombinant growth hormone replacement and physical rehabilitation in recovery of gross motor function in children with cerebral palsyTher Clin Risk Manag2010658559221151628
- JohnstonMVPlasticity in the developing brain: implications for rehabilitationDev Disabil Res Rev20091529410119489084
- Krageloh-MannICansCCerebral palsy updateBrain Dev200931753754419386453
- Kerem GunelMRehabilitation of children with cerebral palsy from a physiotherapist’s perspectiveActa Orthop Traumatol Turc200943217318019448358
- DevesaJCasteleiroNRodicioCLopezNReimundePGrowth hormone deficiency and cerebral palsyTher Clin Risk Manag2010641341820856687
- ShimMLMoshangTJrOppenheimWLCohenPIs treatment with growth hormone effective in children with cerebral palsy?Dev Med Child Neurol200446856957115287249
- AbergNDBryweKGIsgaardJAspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brainScientific World Journal2006186538016432628
- DevesaJDevesaPReimundePGrowth hormone revisitedMed Clin (Barc)20101351466567020045134
- DevesaPReimundePGallegoRDevesaJArceVMGrowth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injuryBrain Inj201125550351021456999
- ReimundePQuintanaACastanonBEffects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injuryBrain Inj2011251657321117918
- PalisanoRRosenbaumPWalterSRussellDWoodEGaluppiBDevelopment and reliability of a system to classify gross motor function in children with cerebral palsyDev Med Child Neurol19973942142239183258
- NewborgJStockJRWnekLSpanish adaptationCruz LópezMVGonzález CriadoMBattelle: Inventario de desarrollo1st edMadridTEA Ediciones1996
- NewborgJStockJRWnekLBattelle Developmental Inventory with Recalibrated Technical Data and Norms: Screening Test Examiner’s Manual2nd edAllen, TXDLM, Inc1988
- Brooks-GunnJMcCartonCMCaseyPHEarly intervention in low-birth-weight premature infants. Results through age 5 years from the Infant Health and Development ProgramJAMA199427216125712627933370
- McCartonCMBrooks-GunnJWallaceIFResults at age 8 years of early intervention for low-birth-weight premature infants. The Infant Health and Development ProgramJAMA199727721261328990337
- SpittleAJOrtonJDoyleLWBoydREarly developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infantsCochrane Database Syst Rev200722CD00549517443595
- ConiglioSJStevensonRDGrowth hormone deficiency in two children with cerebral palsyDev Med Child Neurol19953711101310158566448
- AliOShimMFowlerEGrowth hormone therapy improves bone mineral density in children with cerebral palsy: a preliminary pilot studyJ Clin Endocrinol Metab200792393293717179200
- Puga GonzálezBFerrández LongásAOyarzábalMNosasRGrupo Colaborativo EspañolThe effects of growth hormone deficiency and growth hormone replacement therapy on intellectual ability, personality and adjustment in childrenPediatr Endocrinol Rev20107432833820684123
- ScheepensAWilliamsCEBreierBHGuanJGluckmanPDA role for the somatotropic axis in neural development, injury and diseaseJ Pediatr Endocrinol Metab200013Suppl 61483149111202225
- AbergNDJohanssonIAbergMAPeripheral administration of GH induces cell proliferation in the brain of adult hypophysectomized ratsJ Endocrinol2009201114115019171566
- ChristophidisLJGorbaTGustavssonMGrowth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: A potential role for growth hormone in injury-induced neurogenesisGrowth Horm IGF Res200919649750619524466
- GasperiMCastellanoAEGrowth hormone/insulin-like growth factor I axis in neurodegenerative diseasesJ Endocrinol Invest201033858759120930497
- DevesaJLimaLTresguerresJANeuroendocrine control of growth hormone secretion in humansTrends Endocrinol Metab19923517518318407098
- DevesaJArceVLoisNTresguerresJALimaLAlpha 2-adrenergic agonism enhances the growth hormone (GH) response to GH-releasing hormone through an inhibition of hypothalamic somatostatin release in normal menJ Clin Endocrinol Metab1990716158115881977761
- GleesonHBarretoESSalvatoriRMetabolic effects of growth hormone (GH) replacement in children and adolescents with severe isolated deficiency due to a GHRH receptor mutationClin Endocrinol (Oxf)200766446647417371461
- LanesRPaoliMCarrilloEVillaroelOPalaciosACardiovascular risk of young growth-hormone-deficient adolescents. Differences in growth-hormone-treated and untreated patientsHorm Res200360629129614646407