Abstract
Purpose
Hemophilia care in China is characterized by widespread use of on-demand regimens and low-dose prophylaxis. With a limited number of approved recombinant factor VIII (FVIII) products, the incidence of arthropathy and disability in hemophilia patients remains high in China. The purpose of this trial was to evaluate the safety and efficacy of turoctocog alfa for prophylaxis and treatment of bleeding episodes in patients from China with severe hemophilia A across all age groups.
Patients and Methods
In this Phase 3, open-label trial, previously treated males of all ages with severe hemophilia A from China received turoctocog alfa for prophylaxis or on-demand treatment of bleeds. The primary endpoint was hemostatic effect for the treatment of bleeds during the main phase of the trial. Secondary endpoints included annualized bleeding rate during prophylaxis and the frequency of FVIII inhibitor development.
Results
Overall, 42 pediatric patients (age <12 years) and 26 adolescent/adult patients (≥12 years) were dosed with turoctocog alfa; 51 patients initiated treatment with prophylaxis, while 17 patients initiated on-demand treatment. During the main phase of the trial (6 months), hemostatic success was 95.1%. During the full trial (up to 24 months), hemostatic success was 95.4%; the overall median ABR was 1.18 bleeds/patient/year for prophylaxis patients; and 25 (51.0%) of 49 patients with target joints at baseline had all target joints resolved. No FVIII inhibitors (≥0.6 BU) were reported.
Conclusion
Turoctocog alfa was safe and effective for prophylaxis and treatment of bleeding episodes and for surgery in patients from China with severe hemophilia A across all ages.
Introduction
Hemophilia A is a congenital bleeding disorder characterized by a deficiency in coagulation factor VIII (FVIII) that results in increased tendency for spontaneous or traumatic bleeding events.Citation1 The international standard of care for hemophilia management is prophylactic administration of clotting factor concentrates, which aim to prevent and treat bleeding, and reduce the risk of hemophilic arthropathy.Citation1–Citation4 Despite recent advances in care with the development of non-factor replacement products and gene therapy, prophylaxis with clotting factor concentrates remains the cornerstone of hemophilia treatment in many parts of the world, including China.Citation3–Citation5
Historically, the implementation of effective hemophilia care in China has been challenging, due to healthcare infrastructure, affordability of treatment and relatively low disease awareness.Citation6 Due to resource constraints, hemophilia A treatment in China is characterized by widespread use of on-demand treatment.Citation7,Citation8 As the incidence of arthropathy and disability remains high in hemophilia patients from China, low-dose prophylaxis regimens are available mainly for younger patients.Citation7,Citation9,Citation10 Plasma-derived concentrates are the mainstay of treatment, and there is a limited number of approved recombinant FVIII (rFVIII) products and prospective trials in this population in recent years.Citation8,Citation11
Turoctocog alfa (NovoEight®, Novo Nordisk A/S, Bagsværd, Denmark) is a third-generation, B domain-truncated, human coagulation, rFVIII molecule approved for the prophylaxis and treatment of bleeding episodes in hemophilia A; the molecule design and characterization have been discussed in detail elsewhere.Citation12–Citation14 Turoctocog alfa demonstrated favorable efficacy and safety in the long-term guardian 2 extension trial in previously treated adults and children with severe hemophilia A.Citation15 In a population of previously untreated pediatric patients, turoctocog alfa was also shown to be effective at preventing and controlling bleeding episodes.Citation16 It is the first rFVIII molecule to demonstrate stability and be approved for storage at up to 40°C,Citation14,Citation17 offering patients greater flexibility to store their factor replacement without refrigeration, which is associated with fewer restrictions on daily activities and increased patient satisfaction.Citation18
The aim of guardian 7 was to evaluate the safety, efficacy and pharmacokinetics (PK) of turoctocog alfa for prophylaxis, treatment of bleeding episodes and for surgery in previously treated patients (PTPs) of all ages from China with severe hemophilia A. At the time of the investigation, the trial was the first in China to prospectively investigate the efficacy of rFVIII prophylaxis in hemophilia patients from China of all ages, designed in accordance with European Medicines Agency (EMA) guidelines for the investigation of FVIII products.Citation19 It also had the longest treatment duration (up to 24 months) and the largest sample size of any phase 3 trial of its kind. (Watch video abstract in Chinese).
Materials and Methods
Trial Design
Guardian 7 was a Phase 3, multicenter, open-label, non-randomized safety and efficacy trial (NCT02938585). PTPs with severe hemophilia A (FVIII ≤1%) of all ages were enrolled across 10 sites in mainland China (Supplementary material). The trial was conducted between 12 December 2016 (first patient first visit) and 12 December 2018 (last patient last visit) and consisted of a main phase and extension phase. Unless otherwise stated, data reported here are the combined results from both phases. The duration of participation in the trial was ~6 months (equaling ~50 exposure days [EDs] for prophylaxis patients) in the main phase and ≤18 months in the extension phase. Optional PK sessions took place for selected patients during the main phase. Patients who underwent surgery could receive treatment with turoctocog alfa before, during and after surgery according to the standard of practice at the trial site. The trial was approved by all relevant independent ethics committees and institutional review boards. All patients or their representatives provided written informed consent to participate in the trial, which was conducted in accordance with the Declaration of HelsinkiCitation20 and Good Clinical Practice.Citation21
Participants
Male patients with severe congenital hemophilia A, ≥50 EDs (patients <12 years of age) or ≥100 EDs (≥12 years) to any FVIII concentrates, Asian ethnicity and residency in China were enrolled. Patients with a known history or presence of inhibitor (≥0.6 Bethesda Units [BU]), or immunocompromised patients (CD4+ T lymphocyte count ≤200 μL) were not eligible.
Treatment
Turoctocog alfa prophylaxis was administered every other day (EOD) or three times weekly as an intravenous (iv) injection. The prophylaxis dose for patients ≥12 years of age was 20–40 IU/kg (of body weight) EOD or 20–50 IU/kg three times weekly; patients <12 years were dosed with 25–50 IU/kg EOD, or 25–60 IU/kg three times weekly. These regimens were consistent with prescribing information,Citation14 with the individual regimen and dose chosen by the investigator. For the treatment of bleeding episodes, investigators determined the individual doses based on recommendations from the World Federation of Hemophilia (WFH).Citation1 Treatment could be administered at home, at the trial site, or at another clinic.
For PK assessment, a dose of 50 ± 5 IU/kg was administered iv as a single bolus injection. The washout period before the PK session was dependent on the patient’s age. Dosing guidance for surgery was provided in the trial protocol and followed WFH guidelines.Citation1 For prevention of surgical bleeding, the recommended FVIII level was 30–60 IU/dl (%) for minor surgery (including tooth extractions) and 80–100 IU/dL (%) for major surgery.
Study Endpoints and Clinical Assessments
The primary endpoint was the hemostatic effect of turoctocog alfa for treatment of bleeding episodes during the main phase. Secondary endpoints included hemostatic effect, annualized bleeding rate (ABR), consumption, incidence rate of inhibitory antibodies against FVIII (≥0.6 BU) and frequency of adverse events (AEs), all of which were assessed during both the main phase and combined main and extension phases. PK endpoints included incremental recovery (IR) of FVIII, area under the curve (AUC), half-life (t½), clearance (CL) and highest measured FVIII activity (Cmax). Patient-reported outcomes included the change in total scores for health-related quality of life (HRQoL) parameters. Plasma FVIII activity (one-stage clotting assay), FVIII inhibitor testing, genotyping and clinical laboratory tests were performed in China at the central laboratory (Quintiles Limited, United Kingdom [now IQVIA]). Testing of FVIII activity with chromogenic assays and FVIII binding antibody analysis was performed in a specialized laboratory outside of China.
Statistical Methods
The evaluation of data was based mainly on descriptive statistics. Hemostatic responses rated as “good” or “excellent” were classified as successful, and responses rated as “moderate”, “none” or “missing” were classified as failure. ABR was analyzed using a negative binomial model; as a sensitivity analysis, a Poisson model for over-dispersion was applied. Two separate criteria (study protocol and a more recent 2014 International Society on Thrombosis and Haemostasis Scientific and Standardization Committee [ISTH SSC] communication) were applied to define target joints and target joint resolution ().Citation22 The incidence rate of FVIII inhibitors (≥0.6 BU) was calculated with a one-sided 97.5% upper confidence limit based on an exact calculation for a binomial distribution. Exceptional outlier PK profiles and/or individual plasma concentrations could be excluded. Only one PK datapoint was excluded in patients with several other data points missing. There was no tabulation of PK parameters, including outliers. Disease- and age-specific HRQoL data were collected using HAEM-A-QOL (adults) and HAEMO-QOL (children/adolescents and their parents) questionnaires.
Table 1 Definition of Target Joints
Results
Patient Disposition and Baseline Characteristics
In total, 42 pediatric patients (<12 years) and 26 adolescent/adult patients (≥12 years) received treatment with turoctocog alfa during the combined main and extension phase, for a cumulative total of 99.7 patient years and 15,240 EDs. Of these, 51 patients initiated the trial with prophylaxis, while 17 patients initiated the trial with on-demand treatment (). Of the 17 patients treated on-demand, one withdrew from the main phase and the majority (n=11) switched to prophylaxis at the start of the extension phase. The remaining five patients continued with on-demand treatment in the extension period, of which one also later switched to prophylaxis.
Figure 1 Participant flow.
Notes: a3 screening failures: 1 patient did not meet the inclusion criteria (severe congenital hemophilia A with FVIII ≤1%) and 2 patients had inhibitors to FVIII (≥0.6 BU) at screening. b2 patients (one from each regimen) withdrew at will from the main phase. c2 patients in the prophylaxis regimen withdrew before starting the extension phase (1 due to refusal to participate in the extension and the other for personal reasons). d2 patients withdrew during the extension phase (1 due to medical insurance and the other for personal reasons) and the remaining 62 patients completed the extension period. A total of 5 patients were switched from their original prophylaxis dosing frequency during the trial: 1 small child, 1 older child and 1 adult switched from three times weekly to EOD, and 1 older child and 1 adult switched from EOD to three times weekly.
Abbreviations: BU, Bethesda Units; EOD, every other day; PK, pharmacokinetics.
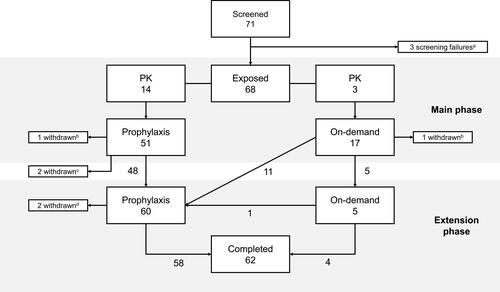
Overall, mean exposure was 63.6 EDs (range: 2–136) in the main phase, and 224.1 EDs (range: 2–345) in the combined main and extension phase. A total of 66 patients completed the main phase. Of the 64 patients who entered the extension phase, two patients withdrew and the remaining 62 patients completed the extension phase.
Baseline demographics are shown in . The overall mean (SD) age was 13.9 (11.0) years. In the year prior to trial participation, 40 patients reported receiving prophylactic treatment at some time, and 32 patients reported receiving intermittent on-demand treatment at some time, with either recombinant or plasma-derived FVIII used. Dosing schedules for previous prophylaxis varied in frequency and only 14/40 (35.0%) patients reported receiving standard prophylaxis (three times weekly or more) in the previous year. The mean (SD) historical weekly prophylaxis dose was 33.0 (15.4) IU/kg. Based on the reported bleeding frequency, the mean historical ABR was 24.57 bleeds/patient/year and 53.67 bleeds/patient/year for patients receiving prophylaxis or on-demand treatment, respectively.
Table 2 Demographics and Baseline Characteristics
Treatment of Bleeding Episodes
A total of 611 bleeds that required treatment were reported in 47 patients during the main phase; the success rate for the treatment of bleeds was 95.1% (n=581), including missing values (n=1) as failure. A total of 925 bleeds that required treatment were reported in 54 patients in the combined main and extension phase (); the success rate for the treatment of bleeds was 95.4% (n=882), including missing values (n=1) as failure. Of the 925 bleeds that required treatment, 693 (74.9%) were reported in 17 patients treated on-demand, and 232 (25.1%) in 44 patients exposed to prophylaxis. The majority (98.3%) of the 925 bleeds were classed as mild/moderate. All severe bleeds (n=16, 1.7%) were joint bleeds, of which 14 involved target joints. Joint bleeds accounted for the majority of bleeding episodes in older children (64.2%), adolescents (92.5%) and adults (80.1%). A total of 872 (94.3%) bleeds were resolved with 1–2 infusions of turoctocog alfa (). The mean (SD) number of injections used to treat a bleed was 1.3 (1.0).
Table 3 Details of Bleeding Episodes and Hemostatic Response to Turoctocog Alfa Treatment (Combined Main and Extension Phase)
Prevention of Bleeding Episodes
In total, 63 patients were treated prophylactically during the trial, of which the majority (n=57; 90.5%) initiated treatment three times weekly. Of these 63 patients, 19 (30.2%) had no bleeds that required treatment while receiving prophylaxis. Overall, the Poisson estimate of ABR was lower for patients receiving prophylaxis versus on-demand treatment (2.62 vs 65.75 bleeds/patient/year, respectively). The overall median ABR for prophylaxis patients was also lower compared with those treated on-demand (1.18 vs 74.40 bleeds/patient/year, respectively) ().
Table 4 Annualized Bleeding Rates
Resolution of Target Joints
A total of 49 patients had target joints at trial baseline, with up to 6 target joints observed in patients. Per the protocol definition, 25 (51.0%) of these 49 patients had all target joints resolved at the end of the trial. Of the 63 patients exposed to prophylaxis, a total of 111 target joints were reported by 50 patients at prophylaxis baseline (defined as the start of the prophylaxis regimen in the main or extension phase). During prophylaxis treatment, 26 (52.0%) of these 50 patients and 78 (70.3%) of the 111 target joints at prophylaxis baseline had no bleeds that required treatment. A total of 36 (72.0%) patients had ≥1 baseline target joint resolved and 24 (48.0%) had all baseline target joints resolved. The Poisson estimate of ABR for target joints was 1.32 bleeds/patient/year.
Per the 2014 ISTH definition, 36 (73.5%) of the 49 patients with target joints at trial baseline had all baseline target joints resolved during the trial. During prophylaxis treatment, 45 (90.0%) of the 50 patients with target joints at prophylaxis baseline had ≥1 baseline target joint resolved and 38 (76.0%) had all baseline target joints resolved.
For patients treated on-demand, 35 target joints were reported in 11 patients at baseline. Per the protocol definition, two (18.2%) patients had no target joint bleeds. Per the protocol definition and ISTH definition, one (9.1%) patient had all baseline target joints resolved.
Consumption of Turoctocog Alfa
The mean prophylaxis dose of turoctocog alfa was 40.5 IU/kg; the mean annual and monthly consumptions for prophylaxis were 6288 IU/kg/patient/year and 524.0 IU/kg/month/patient, respectively. The mean (SD) on-demand dose of turoctocog alfa for treatment of bleeds was 27.8 (13.2) IU/kg; the annual and monthly consumptions used for on-demand treatment of bleeds were 2999 IU/kg/patient/year and 249.9 IU/kg/month/patient, respectively. Overall, the mean (SD) consumption of turoctocog alfa for the treatment of a bleed across both regimens was 30.95 (13.1) IU/kg.
Surgery
Turoctocog alfa was used for bleeding prevention in four major and five minor surgeries that took place during the trial (). Hemostatic response to turoctocog alfa was successful (“excellent” and/or “good”) during and after all eight surgeries for which responses were recorded, and none of the patients received a blood transfusion.
Table 5 Hemostatic Response and Surgery Details
Pharmacokinetics
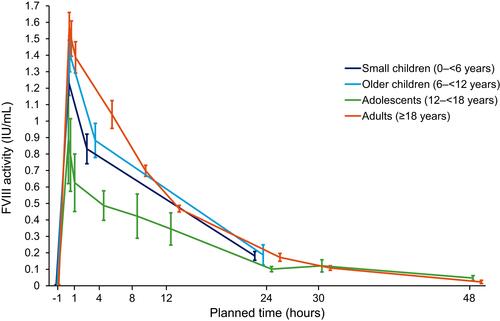
A total of 17 patients were included in the PK assessment (age range: 3 to 44 years). PK parameters based on FVIII activity measured using a chromogenic assay are presented (; ). The 17 patients evaluated had a mean (SD) IR of 0.023 (0.006) (IU/mL)/(IU/kg) at the time of the assessment. The t½ of turoctocog alfa was similar in children <12 years and adults; the overall mean (SD) terminal t½ was 9.0 (2.4) hours.
Table 6 Pharmacokinetic Parameters
Figure 2 Mean FVIII activity profile following administration of turoctocog alfa, by age group.
Notes: Chromogenic assay (linear scale), excluding outliers; bars show the standard error of the mean. A total of 17 patients were included in the PK assessment (small children, n=4; older children, n=6; adolescents, n=3; adults, n=4). Sampling frequency per patient for PK assessment varied between age groups (0–11 years, n=5 samples; ≥12 years, n=11 samples).
Abbreviation: PK, pharmacokinetic.
Safety
The safety analysis set included all 68 patients exposed to turoctocog alfa. No FVIII inhibitors (≥0.6 BU) were reported. A total of 143 AEs were reported in 46 (67.6%) patients. The majority (99.3%) of events were of mild to moderate severity. The most commonly reported AEs were upper respiratory tract infections (27.9% of patients), nasopharyngitis (14.7%), pyrexia (8.8%) and diarrhea (7.4%). Four serious AEs (lung infection, asthma, femur fracture and hand-foot-and-mouth disease) were reported. The serious AEs all occurred in three patients on prophylaxis and were evaluated as unlikely related to turoctocog alfa. The investigator evaluated two AEs of acne in one adolescent patient as possibly related to turoctocog alfa. The dose was not changed as a result of these AEs and both were resolved. No patients were withdrawn due to AEs, and no fatalities, hypersensitivity reactions or allergic reactions occurred during the trial.
Patient-Reported Outcomes
Improvements in total HAEM-QOL scores (demonstrated by a negative change in value) from baseline to the end of trial were reported across all age groups. Changes in total HAEM-QOL scores were –10.1 (8–12 years), –4.9 (13−16 years), and –6.3 (≥17 years), as reported by patients. Patients ≥17 years of age reported the greatest improvement in HAEM-A-QOL scores for “physical health” (–23.1) and “work and school” (–11.3). Patients 13–16 years of age reported the greatest improvement in HAEMO-QOL scores for “future” (–18.8) and “physical health” (–14.9). Patients 8–12 years of age reported the greatest improvement in HAEMO-QOL scores in “physical health” (–22.3) and “sports and school” (–13.5).
Discussion
Guardian 7 is the first prospective, confirmatory safety and efficacy trial conducted in PTPs from China in accordance with EMA guidelines for the investigation of FVIII products,Citation19 and the first trial to have investigated the efficacy of standard prophylaxis (three times weekly or more) at full dose (up to 50 or 60 IU/kg) in patients from China of all age groups. It comprised a robust study design with a larger patient population and longer duration of follow-up compared with previous studies of rFVIII products in patients from China. Furthermore, it included the assessment of a relatively high number of pediatric hemophilia A patients (<12 years, n=42) to demonstrate the safety and efficacy of turoctocog alfa as primary and secondary prophylaxis.
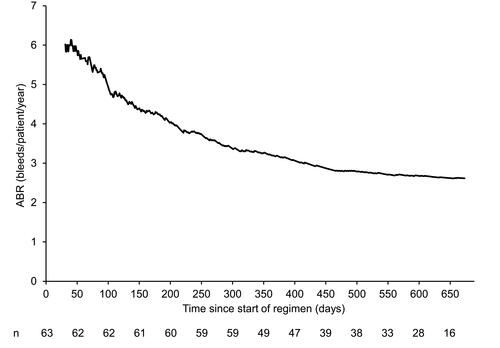
Haemophilia treatment in China is characterised by suboptimal treatment with low-dose, low-frequency prophylaxis regimens,Citation7,Citation10,Citation23 which was observed in the guardian 7 patient population in the year prior to joining the trial, where the mean reported ABR for patients with prophylaxis treatment was 24.57 bleeds/patient/year, and only 35.0% patients reported receiving standard prophylaxis. During the trial, patients who received prophylaxis demonstrated a lower ABR compared with those treated on demand. A reduction in ABR over time for patients on standard prophylaxis was observed across all age groups (), and the resulting Poisson estimate of ABR and mean ABR for prophylaxis patients was 2.62 and 2.91 bleeds/patient/year, respectively. These findings suggest that long-term treatment with standard prophylaxis offers improved ABR outcomes compared with those observed with the low-dose, low-frequency regimens frequently used in China.Citation9,Citation24
Figure 3 Development of individual ABR over time for patients in the prophylaxis regimen.
Notes: Only treatment-requiring bleeds and periods in which a patient was at risk are included. Results for the first month and periods where there are less than 10 patients on a regimen have been excluded for stability purposes.
Abbreviations: ABR, annualized bleeding rate; n, number of patients.
Guardian 7 results suggest a benefit of standard prophylaxis for reducing joint bleeds and resolving target joints following long-term treatment, which is in agreement with conclusions from other studies.Citation8,Citation25,Citation26 The limited use of primary prophylaxis in China suggests that many adult patients would have received turoctocog alfa as tertiary prophylaxis treatment.Citation7,Citation27 Considering up to 70% of hemophilia patients in China are expected to develop arthropathy in adulthood,Citation28 the reduction of joint bleeds and resolution of target joints observed during guardian 7 suggests that even tertiary prophylaxis with standard doses and frequency can help to rehabilitate joints.
Hemostatic success of turoctocog alfa for the treatment of bleeds during guardian 7 was comparable with other turoctocog alfa trials investigating PTPs.Citation15,Citation29,Citation30 Despite differences in patient ethnicity, similarities in hemostatic success between turoctocog alfa trials may be attributed to similarities in the treatment guidelines and evaluation instruments. Furthermore, hemostatic success with turoctocog alfa was similar to that observed in other hemophilia clinical trials of approved treatments in China, including Advate® (100%; Shire/Takeda) and Kogenate®FS (100%; Bayer), although in those studies the population size was slightly smaller and the majority of patients had mild/moderate hemophilia.Citation31,Citation32
Turoctocog alfa provided successful bleeding prevention in several major surgeries performed during guardian 7, including complex procedures such as removal of a pseudotumor, a corrective genitourinary surgery and fixation of a femoral fracture. Despite the presence of hemophilic arthropathy in many patients, no orthopedic procedures or joint replacements were performed, perhaps due to a relatively young study population and patients having to finance the procedures.
The safety of turoctocog alfa in guardian 7 was well tolerated in all age groups and comparable with other turoctocog alfa trials investigating PTPs.Citation15,Citation29,Citation30 No patient developed FVIII inhibitors, as expected for this population of PTPs.
PK results in pediatric patients (>12 years) and adults were consistent with previous observations in other turoctocog alfa clinical trials with PK assessments.Citation30,Citation33 Mean FVIII activity following administration of turoctocog alfa was lower in adolescent patients, which may partly be influenced by the small sample size (n=3). Furthermore, mean IR and AUC were lower, and mean CL was higher in adolescent patients compared with the overall PK population. For patients undergoing PK assessment, mean body mass index (BMI) was lower in adolescents than adults (15.3 kg/m2 versus 21.1 kg/m2), which could explain the difference in PK results, as BMI has previously been shown to be positively correlated with IR and AUC, and negatively correlated with CL.Citation34 There was no positive correlation with age for any of the PK parameters assessed. This differs from results observed in a global trial of turoctocog alfa, which suggested that IR, t½ and AUC increase with age whereas CL decreases.Citation33
During guardian 7, HAEM-QOL scores improved with time until the end of the trial for all age groups. Previous analysis of HAEM-QOL data has identified substantial impairments in the “physical health” domain for hemophilia patients, with HAEM-A-QOL scores strongly influenced by the presence of arthropathy and target joints.Citation35,Citation36 In this trial, patients of all ages reported substantial improvements in the “physical health” domain – which may in part be due to a reduction in joint bleeds and resolution of target joints – suggesting that long-term prophylaxis with turoctocog alfa offers the opportunity to improve QoL.
Conclusion
In conclusion, turoctocog alfa was safe and effective for prophylaxis and treatment of bleeding episodes in patients from China with severe hemophilia A across all ages. Furthermore, findings suggest that maintaining standard prophylaxis with FVIII aids the resolution of target joints in this population, providing the opportunity to improve QoL and reduce the long-term burden of care.
Data Sharing Statement
Data sets from Novo Nordisk-sponsored clinical research completed after 2001 for product indications approved in both the EU and US will be shared with bona fide researchers submitting a research proposal requesting access to data. The access request proposal form and the access criteria can be found at novonordisk-trials.com. Data will be available permanently after research completion and approval of product and product use in both the EU and US on a specialised Statistical Analysis System data platform. The analyses available for use will be those as approved by the Independent Review Board according to the IRB Charter (see novonordisk-trials.com). Individual participant data will be shared in data sets in a de-identified/-anonymised format. In addition, the study protocol and redacted Clinical Study Report will be available according to Novo Nordisk data sharing commitments.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The trial was sponsored by Novo Nordisk A/S (Bagsværd, Denmark). The authors thank all the participants, their families, investigators and trial staff who were involved in the trial. We also thank Lars Korsholm and Kerstin Pietzko (previously at Novo Nordisk) for their review and input to the manuscript, and James McCary, BSc (AXON Communications, London, UK) for medical writing and editorial support in preparation of the manuscript.
Disclosure
RW has received consulting fees from Novo Nordisk, is a steering committee member for Bayer, and is a member of the speakers’ bureaus for Novo Nordisk. IM is an employee of Novo Nordisk A/S. SZ is an employee of Novo Nordisk (China) Pharmaceuticals Co., Ltd. MP is a paid consultant to and reports personal fees from Novo Nordisk A/S. RY has received speaker/consultancy fees from Bayer, Novo Nordisk, Pfizer, Roche and Takeda, outside the submitted work. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47.
- Melchiorre D, Manetti M, Matucci-Cerinic M. Pathophysiology of hemophilic arthropathy. J Clin Med. 2017;6(7):63. doi:10.3390/jcm6070063
- Hartmann J, Croteau SE. 2017 Clinical trials update: innovations in hemophilia therapy. Am J Hematol. 2016;91(12):1252–1260. doi:10.1002/ajh.24543
- Bertamino M, Riccardi F, Banov L, Svahn J, Molinari AC. Hemophilia care in the pediatric age. J Clin Med. 2017;6(5):54. doi:10.3390/jcm6050054
- Arruda VR, Doshi BS, Samelson-Jones BJ. Emerging therapies for hemophilia: controversies and unanswered questions. F1000Res. 2018;7:F1000Faculty Rev–1489. doi:10.12688/f1000research.12491.1
- Poon MC, Luke KH. Haemophilia care in China: achievements of a decade of World Federation of Hemophilia treatment centre twinning activities. Haemophilia. 2008;14(5):879–888. doi:10.1111/j.1365-2516.2008.01821.x
- Zhao H, Yang L, Long C, et al. Hemophilia care in China: review of care for 417 hemophilia patients from 11 treatment centers in Shanxi Province. Expert Rev Hematol. 2015;8(4):543–550. doi:10.1586/17474086.2015.1043263
- Li C, Zhang X, Zhao Y, et al. Status and trend analysis of prophylactic usage of recombinant factor VIII in Chinese pediatric patients with hemophilia A: reCare - a retrospective, Phase IV, non-interventional study. Curr Med Res Opin. 2017;33(9):1571–1578. doi:10.1080/03007995.2017.1333489
- Wu R, Luke KH. The benefit of low dose prophylaxis in the treatment of hemophilia: a focus on China. Expert Rev Hematol. 2017;10(11):995–1004. doi:10.1080/17474086.2017.1386096
- Zhang L, Li H, Zhao H, Zhang X, Ji L, Yang R. Retrospective analysis of 1312 patients with haemophilia and related disorders in a single Chinese institute. Haemophilia. 2003;9(6):696–702. doi:10.1046/j.1351-8216.2003.00826.x
- ClinicalTrials.gov. Interventional Studies of rFVIII for Haemophilia A in China (Clinicaltrials.gov search), 2019. Available from: https://clinicaltrials.gov/ct2/results?term=recombinant+FVIII&cond=Hemophilia+A&cntry=CN&age_v=&gndr=&type=Intr&rslt=&Search=Apply. Accessed September 2019.
- Thim L, Vandahl B, Karlsson J, et al. Purification and characterization of a new recombinant factor VIII (N8). Haemophilia. 2010;16(2):349–359. doi:10.1111/j.1365-2516.2009.02135.x
- Ahmadian H, Hansen EB, Faber JH, et al. Molecular design and downstream processing of turoctocog alfa (NovoEight), a B-domain truncated factor VIII molecule. Blood Coagul Fibrinolysis. 2016;27(5):568–575. doi:10.1097/MBC.0000000000000477
- Novo Nordisk. NovoEight (turoctocog alfa): summary of Product Characteristics, 2018. Available from: https://www.ema.europa.eu/en/documents/product-information/novoeight-epar-product-information_en.pdf. Accessed September 2019.
- Lentz SR, Janic D, Kavakli K, et al. Long-term safety and efficacy of turoctocog alfa in prophylaxis and treatment of bleeding episodes in severe haemophilia A: final results from the guardian 2 extension trial. Haemophilia. 2018;24(6):. doi:10.1111/hae.13617
- Yaish H, Matsushita T, Belhani M, et al. Safety and efficacy of turoctocog alfa in the prevention and treatment of bleeds in previously untreated paediatric patients with severe haemophilia A: results from the guardian 4 multinational clinical trial. Haemophilia. 2020;26(1):64–72. doi:10.1111/hae.13883
- Napolitano M, Nøhr AM. The Effect of Fluctuating Temperature on the Stability of Turoctocog Alfa for Hemophilia A. Drugs R D. 2019;19(4):381–390. doi:10.1007/s40268-019-00290-3
- Tischer B, Marino R, Napolitano M. Patient preferences in the treatment of hemophilia A: impact of storage conditions on product choice. Patient Prefer Adherence. 2018;12:431–441. doi:10.2147/PPA.S151812
- European Medicines Agency. Guideline on the clinical investigation of recombinant and human plasma-derived factor VIII products, 2018. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-recombinant-human-plasma-derived-factor-viii-products-revision-2_en.pdf. Accessed September 2019.
- World Medical Association. Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Last amended by the 59th WMA General Assembly, Seoul, Republic of Korea, 2008. Available from: https://www.wma.net/wp-content/uploads/2018/07/DoH-Oct2008.pdf. Accessed September 2019.
- International Conference on Harmonisation. ICH Harmonised Tripartite Guideline. Good Clinical Practice, 01-May-1996, Geneva, Switzerland; 1996. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed September 2019.
- Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. doi:10.1111/jth.12672
- Li C, Zhang X, Zhao Y, et al. Long-term efficacy and safety of prophylaxis with recombinant factor VIII in Chinese pediatric patients with hemophilia A: a multi-center, retrospective, non-interventional, phase IV (ReCARE) study. Curr Med Res Opin. 2017;33(7):1223–1230. doi:10.1080/03007995.2017.1310720
- Yao W, Xiao J, Cheng X, et al. The efficacy of recombinant FVIII low-dose prophylaxis in Chinese pediatric patients with severe hemophilia A: a retrospective analysis from the ReCARE study. Clin Appl Thromb Hemost. 2017;23(7):851–858. doi:10.1177/1076029616679507
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi:10.1056/NEJMoa067659
- Manco-Johnson MJ, Soucie JM, Gill JC. Prophylaxis usage, bleeding rates, and joint outcomes of hemophilia, 1999 to 2010: a surveillance project. Blood. 2017;129(17):2368–2374. doi:10.1182/blood-2016-02-683169
- Zhao Y, Xiao J, Yang R, et al. Efficacy of standard prophylaxis versus on-demand treatment with Bayer's sucrose-formulated recombinant FVIII (rFVIII-FS) in Chinese children with severe hemophilia A. Pediatr Hematol Oncol. 2017;34(3):138–148. doi:10.1080/08880018.2017.1313921
- Thrombosis and Hemostasis Group, Hematology Society of Chinese Medical Association, Hemophilia Treatment Center Collaborative Network of China. Consensus of Chinese expert on the diagnosis and treatment of hemophilia (version 2017). Zhonghua Xue Ye Xue Za Zhi. 2017;38(5):364–370. doi:10.3760/cma.j.issn.0253-2727.2017.05.002
- Lentz SR, Misgav M, Ozelo M, et al. Results from a large multinational clinical trial (guardian™1) using prophylactic treatment with turoctocog alfa in adolescent and adult patients with severe haemophilia A: safety and efficacy. Haemophilia. 2013;19(5):691–697. doi:10.1111/hae.12159
- Kulkarni R, Karim FA, Glamocanin S, et al. Results from a large multinational clinical trial (guardian™3) using prophylactic treatment with turoctocog alfa in paediatric patients with severe haemophilia A: safety, efficacy and pharmacokinetics. Haemophilia. 2013;19(5):698–705. doi:10.1111/hae.12165
- Shi J, Zhao Y, Wu J, Sun J, Wang L, Yang R. Safety and efficacy of a sucrose-formulated recombinant factor VIII product for the treatment of previously treated patients with haemophilia A in China. Haemophilia. 2007;13(4):351–356. doi:10.1111/j.1365-2516.2007.01472.x
- Zhang L, Zhao Y, Sun J, Wang X, Yu M, Yang R. Clinical observation on safety and efficacy of a plasma- and albumin-free recombinant factor VIII for on-demand treatment of Chinese patients with haemophilia A. Haemophilia. 2011;17(2):191–195. doi:10.1111/j.1365-2516.2010.02395.x
- Jiménez-Yuste V, Lejniece S, Klamroth R, et al. The pharmacokinetics of a B-domain truncated recombinant factor VIII, turoctocog alfa (NovoEight(R)), in patients with hemophilia A. J Thromb Haemost. 2015;13(3):370–379. doi:10.1111/jth.12816
- Tiede A, Goldmann G, Miljic P, et al. Body mass index was found to be the best predictor for the pharmacokinetics of recombinant factor VIII. Haemophilia. 2018;24:215–216.
- Wyrwich KW, Krishnan S, Poon JL, et al. Interpreting important health-related quality of life change using the Haem-A-QoL. Haemophilia. 2015;21(5):578–584. doi:10.1111/hae.12642
- Varaklioti A, Kontodimopoulos N, Niakas D, Kouramba A, Katsarou O. Health-related quality of life and association with arthropathy in Greek patients with hemophilia. Clin Appl Thromb Hemost. 2018;24(5):815–821. doi:10.1177/1076029617733041
