Abstract
Aim
To evaluate the efficacy of glycopyrrolate oral solution (1 mg/5 mL) in managing problem drooling associated with cerebral palsy and other neurologic conditions.
Method
Thirty-eight patients aged 3–23 years weighing at least 27 lb (12.2 kg) with severe drooling (clothing damp 5–7 days/week) were randomized to glycopyrrolate (n = 20), 0.02–0.1 mg/kg three times a day, or matching placebo (n = 18). Primary efficacy endpoint was responder rate, defined as percentage showing ≥3-point change on the modified Teacher’s Drooling Scale (mTDS).
Results
Responder rate was significantly higher for the glycopyrrolate (14/19; 73.7%) than for the placebo (3/17; 17.6%) group (P = 0.0011), with improvements starting 2 weeks after treatment initiation. Mean improvements in mTDS at week 8 were significantly greater in the glycopyrrolate than in the placebo group (3.94 ± 1.95 vs 0.71 ± 2.14 points; P < 0.0001). In addition, 84% of physicians and 100% of parents/caregivers regarded glycopyrrolate as worthwhile compared with 41% and 56%, respectively, for placebo (P ≤ 0.014). Most frequently reported treatment-emergent adverse events (glycopyrrolate vs placebo) were dry mouth, constipation, and vomiting.
Interpretation
Children aged 3–16 years with problem drooling due to neurologic conditions showed a significantly better response, as assessed by mTDS, to glycopyrrolate than to placebo.
ClinicalTrials.gov identifier
NCT00425087.
What this paper adds
In a randomized, placebo-controlled, Phase III clinical trial, glycopyrrolate oral solution (1 mg/5 mL) was found to be significantly superior to placebo in controlling problem drooling in children aged 3–16 years with problem drooling associated with neurologic conditions.
Significantly greater percentages of physicians and parents/caregivers of patients in the glycopyrrolate oral solution group regarded treatment as worthwhile.
Glycopyrrolate oral solution was generally well tolerated.
Introduction
Sialorrhea (drooling or excessive salivation) is an unintentional loss of saliva from the mouth. Although normal in infants, drooling usually stops when at 15–18 months of age and is considered pathologic if present after age 4 years. The most common cause of sialorrhea is neuromuscular dysfunction; other causes are hypersecretion and sensory or anatomic dysfunction, eg, failure of lip closure or infrequent swallowing.Citation1 In children with cerebral palsy and other neuromuscular conditions, drooling may be due to hypersalivation and/or oral motor dysfunction.Citation2,Citation3
The prevalence of moderate-to-severe sialorrhea in developmentally disabled individuals is estimated at 10%–37%.Citation4–Citation6 Among children with cerebral palsy who attended special schools, the incidence was 58% (93 of 160 children), 33% of whom drooled severely.Citation7 In a survey of parents of 1437 children with cerebral palsy, 34% reported that drooling was an occasional problem, and an additional 16% found it to be a frequent problem, requiring daily changes of clothing.Citation8
Complications of sialorrhea can be both physical and psychological and can have a negative impact on quality of life. Drooling can result in perioral chapping, irritation, and maceration, with secondary infection of the facial skin, dehydration due to chronic loss of fluids, and increased risk of silent saliva aspiration that can result in recurrent respiratory infections.Citation9 Moreover, drooling increases wetness, clothing odor, and social embarrassment; lowers self-esteem; and limits vocational opportunities.Citation9 In the school setting, children may be unable to share books or computer electronics,Citation1 and levels of care and dependency may increase.Citation10,Citation11
Treatment options explored to control drooling in children and adults include behavioral approaches, such as prompts to swallow or wipeCitation12 or preventing individuals from putting their fingers or objects into their mouths;Citation13 surgery to decrease salivary flow,Citation14 intraglandular injection of botulinum toxin type A,Citation15,Citation16 and anticholinergic agents such as benztropine, glycopyrrolate, trihexyphenidyl hydrochloride, and scopolamine patches.Citation11,Citation17
Glycopyrrolate (glycopyrronium bromide) is a synthetic quaternary ammonium anticholinergic agentCitation11 with poor penetration of the blood–brain barrier, as shown by the direct measurement of radiolabeled glycopyrrolate in anesthetized dogs,Citation18 thus reducing the ability of glycopyrrolate to cause central nervous system effects.Citation19,Citation20 Glycopyrrolate was first approved for clinical use in 1961. Currently approved indications in the US include adjunctive treatment of peptic ulcer disease in adults and as a preoperative or intraoperative medication in adults and children 2 years of age and older. It is used preoperatively to inhibit salivation and excessive secretions of the respiratory tract. Additionally, glycopyrrolate has been used for children with tracheostomies who have difficulty managing secretions. Off-label use of commercially available oral glycopyrrolate tablets has been shown to decrease drooling in children with cerebral palsy.Citation8,Citation9,Citation21,Citation22 However, these tablets require compounding to dose pediatric patients, and the highly variable pharmacokinetics of glycopyrrolate resulted in wide-ranging doses and adverse events, leading to treatment discontinuation.Citation9 These findings have encouraged the development of a novel liquid formulation of glycopyrrolate, oral glycopyrrolate solution (1 mg/5 mL), providing more accurate pediatric dosing and titration. We have performed a randomized, double-blind, placebo-controlled Phase III trial to assess the efficacy and safety of this new formulation in managing problem drooling associated with cerebral palsy and other neurologic conditions in children. We also assessed the effectiveness of a training manual, designed to help educate patients and caregivers to identify adverse events (AEs) secondary to treatment with oral glycopyrrolate solution and to manage dosing regimens.
Material and methods
Participants
Male and female patients weighing at least 27 lb (12.2 kg) and previously diagnosed with cerebral palsy, mental retardation, or another neurologic condition associated with problem drooling were eligible for enrollment. Problem drooling was defined as drooling in the absence of treatment such that clothing became damp approximately 5–7 days/week. Patients with oral feeding problems or who used a tube for feeding were included. Female patients of childbearing potential were required to have a negative pregnancy test at the pretreatment visit and were counseled on the importance of not becoming pregnant.
Patients were excluded if their extent of drooling was wetness of the lips and chin but their clothes did not become damp on most days; if they had used glycopyrrolate liquid within approximately 24 hours of baseline; if they had used any anticholinergic or cholinergic medications prohibited by the protocol within three plasma half-lives of that medication prior to baseline; or if they had medical conditions contraindicating anticholinergic therapy or treatment with the study medication.
The study was conducted according to Good Clinical Practice guidelines and in full compliance with the World Assembly Declaration of Helsinki and its most recent amendments.
Interventions
The initial treatment dose was calculated based on body weight and assigned at the randomization visit. The initial dose was 0.02 mg/kg three times a day, and was titrated according to schedule over a 4-week period to optimal response, with a maximum dose of 0.1 mg/kg or 3 mg, three times a day, whichever was less. Since high-fat foods reduce the oral bioavailability of glycopyrrolate oral solution administered shortly after a meal, we advised that the test liquid be administered at least 1 hour before or 2 hours after meals at 7–8 am, 1–2 pm, and 7–8 pm. Parents/caregivers were instructed on how to measure dose levels using an oral syringe and a dosing cup; for patients with gastrostomy feeding tubes, Luer lock syringes were provided. The FDA-required training manual, which included the dose titration schedule, was provided to each parent/caregiver; it was also used to facilitate the recognition of AEs and the need for dose adjustments.
Outcomes
Primary outcome measures
The primary efficacy endpoint was responder rate, based on change in degree (severity and frequency) of drooling, as measured by parents/caregivers, using the modified 9-point Teacher’s Drooling Scale (mTDS), which was assessed at baseline and at weeks 2, 4, 6, and 8. The mTDS is scored from 1 (dry, never drools) to 9 (profuse: clothing, hands, tray, and objects become wet; frequently). At the request of the FDA, the primary endpoint was changed to “dichotomized mTDS,” which defined responders as those having an increase ≥3 units on the mTDS.
Secondary outcome measures
Secondary outcome measures included daily mean parent/caregiver mTDS scores at weeks 2, 4, and 6; AUC analysis of all mTDS evaluations from screening to week 8; proportion of patients who discontinued treatment due to lack of efficacy; global assessments by the parent/caregiver, by patients deemed cognitively capable by the investigator, and by physicians, performed at week 8 or at the last visit, using a 5-point scale, ranging from 1 (strongly agree) to 5 (strongly disagree) in response to the statement “This is a worthwhile treatment”; assessments using a modified Behavioral and Medical Rating Scale (mBMRS);Citation23 and tabulation and description of all AEs.
The mBMRS is a scale that measures the frequency of 28 prespecified symptoms at each study visit using a scale of 1 through 4, where 1 represents not at all; 2, just a bit; 3, quite a bit; and 4, very much. This scale can be used by parents/caregivers to identify possible AE-related behaviors and physiologic effects in patients taking study drug. The mBMRS score at each visit was the average of nonmissing responses to the 28 symptoms; the score was calculated only if none of these symptoms was missing. The behavioral and symptom subscales of the mBMRS were the means of the first 12 and last 16 symptoms, respectively. A positive change reflected improvement, and a negative change reflected worsening.
We also assessed the effectiveness of the training manual, “Glycopyrrolate Liquid for the Treatment of Problem Drooling Associated with Cerebral Palsy or Other Neurologic Conditions in Children: For Parents and Caregivers of Patients,” to educate parents and caregivers about drooling. The most frequent AEs and the beneficial effects of glycopyrrolate oral solution in these patients were also identified.
Safety was evaluated by physical examination, 12-lead ECG, clinical laboratory test results, and urinalysis, assessed at baseline and at week 8 or study discontinuation.
Randomization
Prospective patients were screened within 3 weeks of dosing. Those receiving anti-sialogenic compounds or other medications with anticholinergic or cholinergic activity underwent a washout phase prior to baseline, beginning 8 days before randomization. Doses of study medication were titrated over a 4-week period to optimal response, after which patients remained on that dose for an additional 4 weeks.
Patients were randomized 1:1 to oral glycopyrrolate oral solution or matching placebo oral solution (similar in color and taste) three times a day. During the first 4 weeks, doses were titrated weekly to the optimal tolerated response for each study participant, but not exceeding 1.5–3.0 mg per dose based on weight, with the optimal tolerated dose reached by week 4. Five dose levels (0.02 mg/kg three times a day, 0.04 mg/kg three times a day, 0.06 mg/kg three times a day, 0.08 mg/kg three times a day, and 0.1 mg/kg three times a day) were evaluated. After the optimal dose level was reached, patients continued to receive the same medication and dose, for a total of 8 weeks.
Blinding
As patients receiving placebo would be expected to continue drooling chronically, caregivers of patients in this group were specifically encouraged to keep patients in the study until at least the end of the 4-week titration period.
Statistical methods
Data from all centers were combined. All percentages were based on the total number of patients in each group (two-sided P values). Patients who dropped out before the end of the study had the lowest rank carried forward. All statistical hypothesis tests used a type I (alpha) error of 0.05. All statistical calculations were performed using SAS® software (v 9.1.3; SAS Institute, Cary, NC).
According to the statistical analysis plan, all patients who received at least one dose of study drug were to be included in the safety population, and all randomized patients were to be included in the intent-to-treat (ITT) analysis of efficacy. In practice, two patients were randomized to treatment before the protocol was amended to set an upper age limit, and these patients no longer met the inclusion criteria. Thus, efficacy was assessed in a modified ITT (mITT) population, defined as all randomized patients who were within the age range of the final, amended protocol and received at least one dose of study medication. Consequently, these two patients were included in the analyses of safety but not of efficacy.
Results
This study was conducted between November 2002 and April 2007. The study duration, from first patient screened to last patient completed, was approximately 4.5 years. A temporary hold was placed on enrollment from November 2005 to September 2006 (10 months) pending receipt of orphan drug designation for glycopyrrolate liquid, which was granted on June 9, 2006, for the indication “treatment of pathologic (chronic moderate to severe) drooling in pediatric patients” by the US Food and Drug Administration Office of Orphan Products Development.
Forty-seven patients were screened at ten US clinical trial sites, and 38 patients aged 3–23 years were randomized to treatment with glycopyrrolate oral solution or placebo (). Of them, 36 patients were 3–16 years of age and two were older. The mITT population included 19 patients in the glycopyrrolate oral solution group and 17 in the placebo group. Their demographic and baseline clinical characteristics were similar (). Most patients had cerebral palsy, 16 (84.2%) in the glycopyrrolate oral solution group and 14 (82.4%) in the placebo group. In the glycopyrrolate oral solution group, 14 patients each (87.5%) had spastic cerebral palsy and were quadriplegic; in the placebo group, 13 (92.9%) each had spastic cerebral palsy and were quadriplegic. In the glycopyrrolate oral solution group, ten patients (52.6%) had oral feeding problems and seven (36.8%) used a tube for feeding; in the placebo group, eight (47.1%) had oral feeding problems and five (29.4%) used a tube for feeding. Eighteen patients (94.7%) in the glycopyrrolate oral solution and 15 (88.2%) in the placebo group were well-nourished.
Figure 1 CONSORT flow diagram.
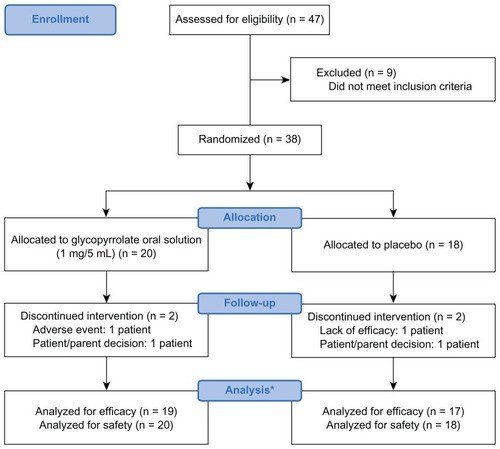
Table 1 Demographic and baseline clinical characteristics
Exposure to study drug
The mean daily dose of glycopyrrolate oral solution was 0.15 mg/kg, with 13 patients (68.4%) having mean daily doses ≥0.1 mg/kg to ≤0.2 mg/kg. The mean length of drug exposure in this group was 55.4 days (89.5% for >50 to ≤100 days); 17 of 19 (89.5%) completed the study, with ten reaching the highest dose level for baseline weight. Of the 17 who completed the 4-week maintenance period, 13 missed at least one dose of study drug (estimated compliance: 90%–100% for patients with up to 168 doses). Overall, the 19 patients in the glycopyrrolate oral solution group required 56 up-titrations and 11 down-titrations.
Within the placebo group, 15 of 17 patients (88.2%) completed the study, with 14 (82.3%) reaching the highest dose level for baseline weight. Of the 15 who completed the study, seven missed at least one dose of study drug (estimated compliance: 91%–100% for patients with up to 168 doses). Overall, the 17 patients in the placebo group required 67 up-titrations and three down-titrations.
Efficacy
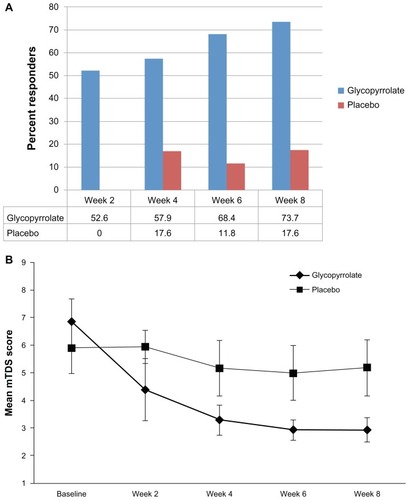
At week 8, 14 of 19 patients (73.7%) in the glycopyrrolate oral solution group and three of 17 (17.6%) in the placebo group exhibited at least a 3-point improvement in mTDS score (P = 0.0011, Fisher’s exact test; ). A beneficial effect of glycopyrrolate oral solution was observed as early as 2 weeks after treatment initiation (52.6%; P = 0.0007), with the proportion of responders increasing continuously through week 8 (). Mean improvements in mTDS score at week 8 were 3.94 points (SD: 1.95, 95% confidence interval [CI]: 2.97–4.91, median: 4.30 points) in the glycopyrrolate oral solution group and 0.71 points (SD: 2.14, 95% CI: −0.43–1.84, median: 0.25 points) in the placebo group (P < 0.0001).
Figure 2 (A) Percent responders in each group, defined as >3-point change on the mTDS; (B) mean mTDS scores (+2 SEMs) over time for the mITT population.
Statistically significant group differences were observed for both the investigator and parent/caregiver global assessments of study medication. For patients in the glycopyrrolate oral solution group, 84.2% of investigators and 100% of parent/caregivers agreed that the treatment was worthwhile, compared with 41.2% of investigators (P = 0.0140; Fisher’s exact test) and 56.3% of parent/caregivers (P = 0.0017; Fisher’s exact test) of patients in the placebo group.
Safety
All 20 patients treated with glycopyrrolate oral solution and 15 of 18 (83.3%) who received placebo had at least one treatment-emergent AE (TEAE), including 15 (75%) and seven (39%), respectively, who had TEAEs considered by the investigator to be related to treatment. Four patients (20%) in the glycopyrrolate oral solution group, but none in the placebo group, had at least one severe TEAE. One patient (5.0%) in the glycopyrrolate oral solution group experienced a serious AE, generalized tonic-clonic seizure activity followed by generalized convulsions, 8 days after the last dose of study drug, which was not considered related to study drug; no placebo patient had a serious AE. One patient in the glycopyrrolate oral solution group (5.0%) and one in the placebo group (5.6%) discontinued treatment because of a TEAE. The most common adverse reactions were dry mouth, vomiting, constipation, and nasal congestion ().
Table 2 Treatment-emergent adverse reactions occurring in ≥15% of patients treated with glycopyrrolate oral solution (1 mg/5 mL) and a greater frequency than placebo
The mBMRS analysis was based on available scores from only five patients in the glycopyrrolate oral solution group and four in the placebo group. Their mean increases from baseline were 0.20 and 0.13, respectively, and their mean increases in behavioral subscale score were 0.44 and 0.23, respectively. Of all AEs reported in the study, 39.9% were identified by the parent/caregiver, and 4.5% by the investigator using the mBMRS.
Discussion
We have shown here that the responder rate to glycopyrrolate oral solution was significantly greater than the responder rate to placebo in the management of severe to moderate drooling associated with cerebral palsy and other neurologic conditions in children aged 3–16 years, and that glycopyrrolate oral solution was well-tolerated in these children. More than 85% of patients in this study had cerebral palsy, classified as spastic and quadriplegic; were developmentally disabled; had impaired speech; resided at home with a parent or foster parent/guardian; and had no history of oral glycopyrrolate use. In addition, both investigators and parents/caregivers assessed glycopyrrolate oral solution as superior to placebo in controlling drooling.
In this trial, the maximum recommended dosage of glycopyrrolate oral solution was 0.1 mg/kg or 3 mg TID, whichever was lower, according to the dose titration schedule. The liquid formulation provides a premixed uniform solution for accurate, individualized pediatric dosing and titration based on patient response to treatment and permits more precise weight-based dosing than is possible with oral tablets.Citation24 Once an initial dose has been selected, clinical signs can be used to titrate the dose for each child over several weeks until control of drooling is deemed satisfactory. Caregiver use of the training manual and continued discussion with the child’s physician can assist in facilitating dose adjustments.
The mBMRS, which measures the frequency of prespecified symptoms at each study visit,Citation23 was used by parents/caregivers to identify possible AE-related behaviors and physiologic effects in patients taking glycopyrrolate oral solution. A positive change reflected improvement, and a negative change reflected worsening. The most common AEs observed with glycopyrrolate oral solution are related to its mechanism of action as an anticholinergic agent. Glycopyrrolate oral solution is contraindicated in patients with conditions that preclude anticholinergic therapy, including glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon, complications of ulcerative colitis, and myasthenia gravis; in patients taking solid oral potassium chloride; and in those with constipation or intestinal pseudo-obstruction.
Four prior studies have assessed glycopyrrolate for the treatment of sialorrhea in children with cerebral palsy and other neurologic conditions; two were open-label,Citation21,Citation22 one a retrospective review,Citation8 and one a prospective, randomized, double-blind, dose-ranging trial.Citation9 In the randomized trial, 39 children aged ≥4 years were assigned to one of two dosage regimens based on weight. Children <30 kg were started at 0.6 mg, with weekly increases to 1.2 mg, 1.8 mg, and 2.4 mg, whereas children >30 kg were started at 1.2 mg, with weekly increases to 1.8 mg, 2.4 mg, and 3.0 mg. In that trial, commercially available glycopyrrolate tablets were ground up and placed into gelatin capsules. If the child could not swallow the capsule, the parent was allowed to take the capsule apart and place its powdered contents into the child’s food. In that study, almost all children demonstrated a marked improvement in drooling. AEs also increasd as the dose increased, with 20% of the children stopping glycopyrrolate due to behavioral problems, constipation, excessive oral dryness, or urinary retention.
In children with cerebral palsy, neurodevelopmental delays may disturb lip closure, intraoral tongue suction, and swallowing, resulting in sialorrhea due to disturbed coordination of tongue mobility, not to hypersalivation.Citation24 A recent meta-analysis of surgical management of drooling found most evidence to be of low quality and heterogeneous, with varying levels of success.Citation14 Use of botulinum toxin is not likely to supplant surgical treatment due to its temporary effects, but it may be useful in empirically selected candidates for durable surgical treatment of the major salivary glands.Citation14
Although the number of patients in this trial was small, most clinical studies of orphan drugs do not include large numbers of subjects.Citation25
Conclusion
Treatment with glycopyrrolate oral solution significantly improves problem drooling in children aged 3–16 years with cerebral palsy and other neurologic conditions. Using the mTDS as the primary outcome measure, children treated with individually optimized doses of glycopyrrolate oral solution showed a significantly better clinical response rate than did children who received placebo.
Acknowledgment
The authors thank BelMed Professional Resources, New Rochelle, NY, for editorial support, with funding provided by Shionogi Inc.
Disclosure
Robert S Zeller, MD, has served as an advisor to Shionogi Inc. Hak-Myung Lee, PhD, Paul Cavanaugh, PhD, and Jennifer Davidson, DO, are employees of Shionogi Inc. The study was sponsored by Shionogi Inc, and ResearchPoint, a Shionogi company.
References
- HocksteinNGSamadiDSGendronKHandlerSDSialorrhea: a management challengeAm Fam Physician2004692628263415202698
- HarrisSRPurdyAHDrooling and its management in cerebral palsyDev Med Chil Neurol198729807811
- HusseinIKershawAETahmassebiJFFayleSAThe management of drooling in children and patients with mental and physical disabilities: a literature reviewInt J Paediatr Dent199883119558540
- EkedahlCSurgical treatment of droolingActa Otolaryngol1974772152204819034
- Van De HeyningPHMarquetJFCretenWLDrooling in children with cerebral palsyActa Otorhinolaryngol Belg1980346917057223419
- SyedaFAhsanFNunezDAQuality of life outcome analysis in patients undergoing submandibular duct repositioning surgery for sialorrhoeaJ Laryngol Otol200712155555817078897
- TahmassebiJFCurzonMEPrevalence of drooling in children with cerebral palsy attending special schoolsDev Med Child Neurol20034561361712948328
- BachrachSJWalterRSTrzcinskiKUse of glycopyrrolate and other anticholinergic medications for sialorrhea in children with cerebral palsyClin Pediatr (Phila)1998374854909729704
- MierRJBachrachSJLakinRCBarkerTChildsJMoranMTreatment of sialorrhea with glycopyrrolate: a double-blind, dose-ranging studyArch Pediatr Adolesc Med20001541214121811115305
- Van der BurgJJJongeriusPHVan HulstKVan Limbeek RotteveelJJDrooling in children with cerebral palsy: effect of salivary flow reduction on daily life and careDev Med Child Neurol20064810310716417664
- ReddihoughDSReidSMPloverCEvaluation of glycopyrrolate in the treatment of chronic droolingDegen Neurol Neuromusc Dis2011137
- Van der BurgJJDiddenRJongeriusPHRotteveelJJA descriptive analysis of studies on behavioural treatment of drooling 1970–2005Dev Med Child Neurol20074939039417489816
- FairhurstCBCockerillHManagement of drooling in childrenArch Dis Child Educ Pract Ed201196253020675519
- ReedJMansCKBrietzkeSESurgical management of drooling: a meta-analysisArch Otolaryngol Head Neck Surg200913592493119770427
- LimMMaceANouraeiSASandhuGBotulinum toxin in the management of sialorrhoea: a systematic reviewClin Otolaryngol20063126727216911641
- ReidSMJohnstoneBRWestburyCRawickiBReddihoughDSRandomized trial of botulinum toxin injections into the salivary glands to reduce drooling in children with neurological disordersDev Med Child Neurol20085012312818201301
- JongeriusPHvan TielPvan LimbeekJGabreelsFJRotteveelJJA systematic review for evidence of efficacy of anticholinergic drugs to treat droolingArch Dis Child20038891191414500313
- ProakisAGHarrisGBComparative penetration of glycopyrrolate and atropine across the blood-brain and placental barriers in anesthetized dogsAnesthesiology197848339344646152
- OlsenAKSjøgrenPOral glycopyrrolate alleviates drooling in a patient with tongue cancerJ Pain Symptom Manage19991830030210534970
- RautakorpiPMannerTAli-MelkkilaTKailaTOlkkolaKKantoJPharmacokinetics and oral bioavailability of glycopyrrolate in childrenPharmacol Toxicol1998831321349783332
- BlascoPAStansburyJCGlycopyrrolate treatment of chronic droolingArch Pediatr Adolesc Med19961509329358790123
- SternLMPreliminary study of glycopyrrolate in the management of droolingJ Paediatr Child Health19973352549069045
- Camp-BrunoJAWinsbergBGGreen-ParsonsARAbramsJPEfficacy of benztropine therapy for droolingDev Med Child Neurol1989313093192666205
- VillarrealMAOrphan Drug Act: Background and Proposed Legislation in the 107th CongressCRS Report for Congress: Library of Congress (US)2001 Order Code RS209716
- ErasmusCEVan HulstKRotteveelLJDrooling in cerebral palsy: hypersalivation or dysfunctional oral motor controlDev Med Child Neurol20095145445919207297
Appendix
List of all participants and sites at which this study was performed:
Delaware: Stephen J Bachrach, MD, and James Jeffrey Malatack, MD, Alfred I DuPont Hospital for Children, Wilmington, DE; Kentucky: Lynn R Campbell, MD, Shriners Hospital for Children, Lexington, KY; Louisiana: Phillip E Gates, MD, Hospital for Children, Shreveport, LA; New York: Vijaya Atluru, MD, Clinical Trials Center, Mineola, NY; Stephen B Sulkes, MD, University of Rochester Medical Center, Rochester, NY; Ohio: Phillip Watson, MD, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Tennessee: Shiva Shankar Natarajan, MD, Mid-South Physicians Group, P.L.L.C., Germantown, TN; Texas: Wilfred Castro, MD, Alamo City Clinical Research, San Antonio, TX; Nancy Noble Dodge, MD, Texas Scottish Rite Hospital for Children, Dallas, TX; Warren A Marks, MD, Cook Children’s Health Care System, Fort Worth, TX; Robert S Zeller, MD, Texas Children’s Hospital, Houston, TX; Virginia: Richard Stevenson, MD, University of Virginia Kluge Children’s Rehabilitation Center, Charlottesville, VA.