Abstract
Purpose
To assess the effect of twice-daily nepafenac ophthalmic suspension 0.3% on postoperative cystoid-macular-edema (CME).
Patients and Methods
In this prospective, clinic-based, non-randomized case-series, 21 patients (21 eyes) were enrolled with either acute or chronic postoperative CME after cataract extraction. Patients were treated with twice-daily nepafenac 0.3% drops, and followed for at least a 4-month period. Best-corrected visual acuity (BCVA) and spectral-domain optical coherence tomography (SD-OCT)-derived central retinal thickness (CRT) were measured.
Results
From 21 patients, eight presented with acute postoperative CME and 13 with chronic CME. Mean follow-up was 4.82±1.24 months. No adverse events were reported during the study. Baseline BCVA was 0.49±0.36 logMAR and improved to 0.36±0.42 logMAR at the last follow-up visit (P<0.005). CRT decreased from 450.40±90.74 μm at baseline to 354.60±81.49 μm (P<0.05), following treatment.
Conclusion
Our outcomes strongly suggest that administrating nepafenac 0.3% drops on a twice-daily regimen could be a promising alternative for the management of postoperative CME. Additional studies are necessary to further validate our results.
Introduction
Cystoid macular edema (CME) is a common cause of poor visual outcome after intraocular surgery.Citation1,Citation2 CME is more frequently associated with uncomplicated cataract extraction with an incidence of 0.1–2.35%.Citation3 Clinically significant CME has an incidence of 1.17–4.04%, while subclinical CME is 4–10.9%.Citation4 However, it may be present secondary to other ocular surgeries such as complicated cataract extraction with rapture of posterior capsule, penetrating keratoplasty, trabeculectomy, and vitreoretinal surgery. It is also possible to occur after neodymium-doped yttrium aluminium garnet (Nd:YAG) laser capsulotomy and panretinal photocoagulation.Citation5 Other predisposing factors include diabetes mellitus, uveitis, and use of prostaglandin analogs.
CME develops by leakage of dilated perifoveal capillaries, with fluid accumulating in the outer plexiform layer of the macula, forming the typical petalloid appearance in fluorescein angiography.Citation6 The pathogenesis of this condition is associated with the blood–retinal barrier disruption induced by prostaglandins and other inflammatory mediators. Optical coherence tomography (OCT) is a reliable tool to diagnose CME by displaying the foveal cysts, and can detect the morphologic changes at an early stage. Intraretinal fluid is accumulated in the outer plexiform layer, and Muller fibers determine the arrangement of cystoid cavities leading to the characteristic OCT appearance and increasing the thickness of the macula.
In most cases, CME is self-limited but sometimes it may revert to a chronic condition with significant impairment in visual acuity. CME is characterized as acute when it appears within 4 months after surgery, late onset when it appears more than 4 months, and chronic when it lasts over 6 months.Citation7
Different types of nonsteroidal anti-inflammatory drugs (NSAIDs) have been proposed for prevention and management of CME, including bromfenac, nepafenac, diclofenac, flurbiprofen, and ketorolac tromethamine.Citation8 Nepafenac is approved in Europe to treat postoperative inflammation related to cataract surgery and reduce the risk of postoperative ME after cataract surgery in diabetic patients. Two forms of Nepafenac solution are available; 0.1% with dosage frequency 3-times daily and 0.3% once daily for better compliance. Studies show improvement in visual acuity in patients with 90 days use of nepafenac after cataract surgery instead of vehicle, proving the efficacy of NSAIDs in CME.Citation9,Citation10 In comparison with other NSAIDs, nepafenac is considered to have better corneal permeability to intraocular tissues after topical administration, reaching the posterior segment of the eye.Citation8,Citation11
In this study, we investigated the efficacy of 0.3% nepafenac twice daily in patients with established postoperative macula edema after cataract surgery.
Patients and Methods
This prospective, clinic-based, non-randomized study was conducted at the Ophthalmology Department of the University Hospital of Heraklion. The research followed the tenets of the declaration of Helsinki. The protocol for use of nepafenac 0.3% twice daily in the management of postoperative macular edema was reviewed and approved by the institutional review board of the University Hospital of Heraklion. Informed consent was obtained for all patients, in which all benefits and potential adverse events that could arise during the study were stated in detail.
Patients who developed postoperative CME after cataract surgery were included in the study in a consecutive manner. CME was defined as central macular thickness over 30% of preoperative baseline in OCT;Citation9 this percentage is above the 10% coefficient of variation that is associated with the variability of repeated measurements of spectral domain OCT.Citation12 CME that appeared up to 4 months after operation and had a duration of less than 4 months was deemed as acute, while cases with a duration of more than 4 months were categorized as chronic.
All patients had undergone a standard preoperative ophthalmologic examination prior to their operation, including slit lamp biomicroscopy, intraocular pressure, fundoscopy, and optical coherence tomography (OCT, Heidelberg Engineering, Inc., Heidelberg, Germany). Prior to OCT imaging, pupils were always dilated using Tropicamide 0.5% (Topical, Demo) and Phenylephrine Hydrochloride 5% (Phenylephrine, Cooper) to achieve good quality results. If artifacts were detected the measurements were repeated. The CRT was measured using a macular thickness map analysis program; the central subfield thickness and not a point central thickness was used, as it has been shown to have greater reliability for macula thickness evaluation.Citation9,Citation13 All patients needed to have a normal preoperative OCT in order to be included in the study. All types of senile cataract such as nuclear, cortical, or posterior subcapsular were enrolled; however, cataract secondary to trauma or uveitis that could enhance the incidence of CME were excluded. All patients were operated on using phacoemulsification.
Patients with ocular history of retinal diseases such as epiretinal membrane, age-related macula degeneration, diabetic retinopathy, and retinal vein occlusion were excluded from the study. Furthermore, patients with diabetes, inflammatory conditions such as uveitis, and patients using prostaglandin analogs or carbonic anhydrase inhibitors due to glaucoma were also excluded. Patients with known allergies reaction or hypersensitivity to NSAIDs or any component of the product were also excluded. To enroll patients with postoperative ME, a medication free period was required as follows; for patients that had received dexamethasone implant, a minimum of 6 months since the last implant; for patients that had received intravitreal triamcinolone, a minimum of 3 months after the last injection and for patients on topical therapy, a minimum 2 weeks without any drop installation.
Best corrected visual acuity (BCVA) was recorded before drug initiation and at every visit until the last follow-up using Early Treatment Diabetic Retinopathy Study (ETDRS) charts at a distance of 4 meters as a logarithm of the minimum angle of resolution (logMAR).
All eyes were treated with topical nepafenac 0.3% (Nevanac 0.3%; Alcon Research, Ltd., Fort Worth, TX, USA) twice a day. Nepafenac was delivered as monotherapy without any other use of eye drops such as antibiotic, antiglaucoma, or steroid drugs. Slit lamp examination and fundoscopy were performed monthly to evaluate macular changes and to report any adverse event caused by drug usage during the study. More specifically, the examination included integrity of the cornea and corneal epithelium using fluorescence staining, evaluation of conjunctiva for hyperemia, intraocular pressure measurements, evaluation of anterior chamber for inflammatory cells or flare, and evaluation of the macula and optic nerve and peripheral retina. All patients received nepafenac 0.3% for at least 4 months to a maximum of 6 months.
Statistical Analysis
The efficacy variables included measurements of BCVA and CRT. Data were recorded in terms of means±standard deviation (SD). Statistical analysis was performed using SPSS software (statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 25. Normality of data distribution was checked and CRT and visual acuity measurements pre- and post-treatment were compared. A Shapiro–Wilk’s test (P>0.05) and a visual inspection of their histograms, normal Q-Q plots, and box plots showed that BCVA and CRT pre- and post-treatment were not normally distributed.Citation14 BCVA pre-treatment skewness was 2.472 [standard error (SE)=0.512] and Kurtosis 8.988 (SE=0.992) and BCVA post-treatment skewness was 2.915 (SE=0.512) and Kurtosis of 10.177 (SE=0.992). CRT pre-treatment skewness was 1.256 (SE-0.512) and Kurtosis 0.312 (SE=0.992) and CRT post-treatment skewness was 1.684 (SE=0.512) and Kurtosis 2.884 (SE=0.992).Citation15,Citation16 Non-parametric Wilcoxon signed-rank test was performed when comparing BCVA and CRT prior and after treatment with nepafenac solution. P-value<0.05 was accepted for statistical significance.
Results
Twenty-one patients (21 eyes) were enrolled in the study, 13 males and eight females. From them eight patients were diagnosed with acute macular edema and 13 were diagnosed with chronic macular edema after cataract extraction. Three patients from the chronic CME group had posterior capsular rapture during cataract surgery. and summarize our results, presenting the type of CME (acute or chronic), visual acuity, and CRT of all patients prior and after treatment with nepafenac 0.3%. During the study no patient presented any adverse event that might be attributed to nepafenac dosage, including conjunctival hyperemia, toxic keratitis, and corneal melting. BCVA after nepafenac administration increased in most of the cases. Similarly, most patients were documented with decreased central macular thickness at the end of the follow-up. In patients with acute CME, mean BCVA increased from 0.58±0.60 to 0.46±0.56 after treatment without a statistically significant difference (Wilcoxon signed-rank test, P=0.144). In the same group mean CRT decreased from 449.86±95.09 to 329.14±34.73 with statistically significant difference (Wilcoxon signed-rank test, P=0.018). In patients with chronic CME, mean BCVA increased from 0.44±0.15 to 0.30±0.23 after treatment with statistically significant difference (Wilcoxon signed-rank test, P=0.017). In the same group mean CRT decreased from 450.69±92.28 to 368.31±96.59 with significant difference (Wilcoxon signed-rank test, P=0.050). and show the changes in BCVA and CRT in acute and chronic CME prior to and after treatment of patients, respectively.
Table 1 Visual Acuity and Central Macular Thickness Prior to Nepafenac Administration and in the Last Follow-Up in Patients with Acute and Chronic Macular Edema
Table 2 Mean±Standard Deviations (SD) of Best Corrected Visual Acuity (BCVA) and Central Macular Thickness (CRT) Prior to Nepafenac Administration and in the Last Follow-Up in Patients with Acute and Chronic Macular Edema. Statistical Significance: P<0.05.
Figure 1 Changes in best corrected visual acuity (BCVA) in acute and chronic cystoid macular edema (CME) prior to and after treatment of patients. Statistical significance, P<0.05.
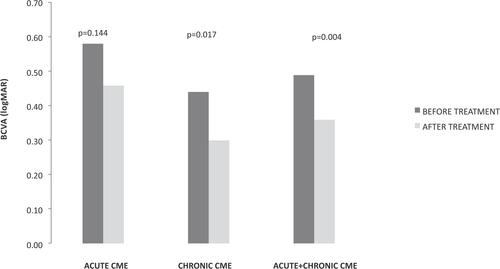
Figure 2 Changes in central retinal thickness (CRT) in acute and chronic cystoid macular edema (CME) prior to and after treatment of patients. Statistical significance, P<0.05.
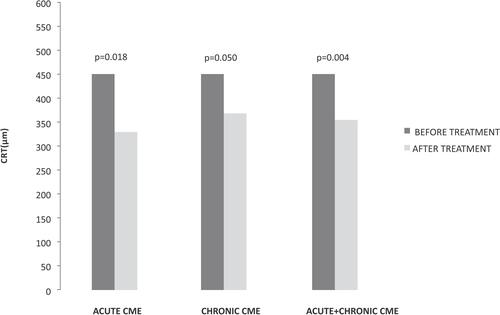
The anatomic restoration in OCT images and CRT measurements was combined with visual acuity improvement in most of the cases. In the group of acute CME, there were patients (three out of eight) with no visual acuity changes, even if CRT was decreased; however one of them already had visual acuity of 0.00 logMAR before treatment and so there was no expectation of visual improvement. In another case, although a significant decrease in CRT (188 μm) was documented, a minor decrease in visual acuity after therapy was attributed to the irregular corneal surface, probably related to cornea pathology. depicts an example of acute postoperative CME after cataract extraction. Nepafenac treatment lasted 4 months and showed a significant decrease of intraretinal fluid in the macula area with a simultaneous increase in visual acuity. In the group of chronic CME there were also two patients with a decrease in CRT, but no change in visual acuity, and two patients who had no improvement after therapy. shows an OCT of a patient with chronic CME before and after 4 months of nepafenac administration. This patient underwent cataract extraction 2 years before enrolment and was diagnosed with postoperative ME in his first-month follow up examination. The patient had received intravitreal steroids with good transient response; however, he proved to be a steroid responder with poor medical IOP control and the steroid therapy was terminated. He was enrolled in the study following the inclusion criteria described above and received nepafenac drops. In a period of 4 months, the patient appeared with minimal fluid in the macular area combined with increased visual acuity. The IOP remained normal without the need of any anti-glaucoma medication.
Figure 3 Patient with acute pseudophakic ME before nepafenac administration and after 4 months therapy. Visual acuity elevated from 0.44 logMAR to 0.30 logMAR, and central macular thickness decreased from 601 μm to 379 μm. Green line represents the same area of the fovea prior to and after therapy in infrared picture, which corresponds to CME improvement.
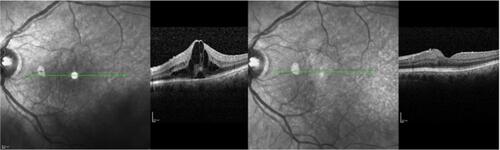
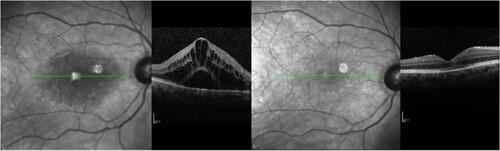
Figure 4 Patient with chronic CME. CME before nepafenac administration and after 4 months therapy. Visual acuity changed from 0.50 logMAR to 0.30 logMAR, and central macular thickness decreased from 499 μm to 255 μm. Green line represents the same area of the fovea prior to and after therapy in infrared picture, which corresponds to CME improvement.
Discussion
Our results strongly indicate that instillation of nepafenac 0.3% on a twice-daily basis could be an alternative for the management of established postoperative CME. Nepafenac 0.1% ophthalmic suspension, with a dosage of 3-times daily, has received approval from FDA to reduce the risk of postoperative CME in patients with diabetes mellitus, based on two randomized, controlled, Phase 3 studies.Citation17,Citation18 Studies demonstrated that nepafenac 0.3% given once daily before surgery and continued for 90 days is superior to vehicle in terms of improving visual acuity and reducing the risk of CME.Citation9
In our study we treat patients with established CME applying nepafenac 0.3% twice daily. In a recent preclinical study it was reported that administration of nepafenac 0.3% once daily could reach a concentration of active analog (amfenac) in the retina tissue at the level of 51% compared to instillation of nepafenac 0.1% three times per day.Citation19 Although this dosage has been found to be effective as prophylaxis for CME in diabetic patients who have undergone cataract surgery, it might be low for established CME.Citation9 For this reason, we decided to increase the dose of the drug to twice daily, in order to succeed and get stable therapeutic levels capable to treat established postoperative CME. Experiments conducted in rabbit eyes confirmed that intravitreal application of nepafenac up to a dose of 1.5 mg is not toxic.Citation20 Moreover, the bioavailability of a drug administered as eye drops is reported in the literature to be in the order of 5–10%.Citation12,Citation21 This means that the dose of nepafenac used in this study did not exceed the drug’s safety profile. In addition, the drug’s SPC states that no adverse effects are expected if the suggested topical dosage is exceeded.Citation13
In our study, the intraretinal fluid decreased in the majority of our patients after treatment with nepafenac and in parallel visual acuity improved both in acute and chronic CME cases. In both subgroups, there were cases that ended up with completely dry macula after a 3-month period of therapy. In chronic CME patients, CRT decreased up to 368 μm, and therapy, in some cases of edema lasting for over 8 months, resulted in total resolution of macular fluid in OCT. In the chronic CME group, only two out of 16 patients did not respond in the treatment, while the majority of patients ended up with a good anatomical and functional outcome. Given the self-limited nature of Irvine Gass syndrome, the efficacy of the treatment, especially in the chronic CME subgroup, strongly supports the therapeutic potential of the drug in the dosage used in this study.Citation3 None of our patients reported any adverse effect during the study.
Several modalities have been described for the management of postoperative CME. Steroids and NSAIDs are used alone or in combination as eyedrops to resolve macular edema after cataract extraction. Steroids have also tried in clinical practice by peribulbar or intravitreal injections. However, steroids are known to increase the intraocular pressure in a percentage of patients.Citation22 Oral carbonic anhydrase inhibitors are considered as a second-line alternative or complimentary to other drugs.Citation23 Finally, in some cases surgical management can be considered including vitrectomy and repositioning of intraocular lens.Citation7 In a recent review study about the treatment of postoperative macular edema, it is referred to the lack of evidence to provide the optimum therapy for macular edema after cataract surgery.Citation24 Non-steroidal anti-inflammatory drugs, however, represent the most commonly adopted drug category, which has a benefit but still with no adequate evidence compared to other treatments.Citation24 NSAIDs block the enzymes of cyclooxygenase (COX), reducing the production of prostaglandins that play a key role in CME development due to anterior segment inflammation after cataract extraction.Citation8,Citation9,Citation25 These drugs represent useful alternative of steroids because they do not induce adverse events such as an increase of intraocular pressure. Nepafenac is deemed to have a better penetration of the cornea compared to other NSAIDs, resulting in a better bioavailability in the anterior chamber; this in combination with the improved dosage facilitated with the 0.3% formulation may lead in a more effective way of handling intraocular inflammation.Citation25 In our study, we used the drug in a twice daily dose in an effort to reach therapeutic levels adequate to treat established CME in patients operated on for cataract extraction. Our results strongly indicate that using nepafenac 0.3% twice daily in patients with acute or chronic CME can improve their anatomic and functional course with a dosage that permits good patient’s compliance. In the majority of previous studies, NSAIDs are used as eyedrops four times per day for the management of macular edema for a long time period, something that could have an impact on patient’s compliance.Citation26,Citation27 Moreover, the lack of steroid related complications as well as the improved safety profile of topical application as compared to intravitreal injections represent additional advantages of topical nepafenac treatment.
In this study there are limitations that should be mentioned. This was a non-controlled study and as a result our findings were not compared to the natural course of the disease. This is of obvious importance especially for the acute CME cases, given their trend to be self-limited. Fluorescein angiography was not performed to diagnose Irvine Gass syndrome. However, OCT is considered a valid tool for establishing the diagnosis of postsurgical CME as it provides characteristic morphological changes in the macula, including well-defined cysts with intraretinal fluid (IRF) accumulation in the outer plexiform layer and subretinal fluid (SRF) associated with macular thickening. Finally, the number of patients enrolled was small, necessitating bigger studies in order to extract safer conclusions.
Conclusion
In conclusion nepafenac 0.3% twice daily was used successfully to treat patients who developed acute or chronic postoperative CME after cataract extraction. Additional research with a controlled study that will include a larger number of patients would help to further confirm our results.
Acknowledgements
The abstract of this paper was presented at the 9th annual congress on Controversies in Ophthalmology:Europe (COPHy EU) 2018 as a poster presentation with interim findings. The poster’s abstract was published in the “Congress program” and in the “Abstract book”. Athanassios K Giarmoukakis and Styliani V Blazaki are co-first authors in this study.
Disclosure
Miltiadis K Tsilimbaris reports grants, personal fees from Novartis Hellas, personal fees from Bayer Hellas, grants, personal fees from Allergan Hellas, grants from Alcon Hellas, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
- Gass JD, Norton EW. Fluorescein studies of patients with macular edema and papilledema following cataract extraction. Trans Am Ophthalmol Soc. 1966;64:232–249.
- Irvine AR. Cystoid maculopathy. Surv Ophthalmol. 1976;21:1–17. doi:10.1016/0039-6257(76)90045-X
- Loewenstein A, Zur D. Postsurgical cystoid macular edema. Dev Ophthalmol. 2010;47:148–159.
- Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC; United Kingdom Pseudophakic Macular Edema Study Group. Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology. 2016;123(2):316–323. doi:10.1016/j.ophtha.2015.10.001
- Shimura M, Yasuda K, Nakazawa T, et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1617–1624.
- Rossetti L, Autelitano A. Cystoid macular edema following cataract surgery. Curr Opin Ophthalmol. 2000;11:65–72. doi:10.1097/00055735-200002000-00010
- Zur D, Loewenstein A. Postsurgical cystoid macular edema. Dev Ophthalmol. 2017;58:178–190.
- Walters T, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007;33:1539–1545.
- Singh RP, Lehmann R, Martel J, et al. Nepafenac 0.3% after cataract surgery in patients with diabetic retinopathy: results of 2 randomized phase 3 studies. Ophthalmology. 2017;124(6):776–785. doi:10.1016/j.ophtha.2017.01.036
- Kusbeci T, Eryigit L, Yavas G, Inan UU. Evaluation of cystoid macular edema using optical coherence tomography and fundus fluorescein angiography after uncomplicated phacoemulsification surgery. Curr Eye Res. 2012;37(4):327–333. doi:10.3109/02713683.2011.635402
- Hariprasad SM, Callanan D, Gainey S, et al. Cystoid and diabetic macular edema treated with nepafenac 0.1%. J Ocul Pharmacol Ther. 2007;23:585–590. doi:10.1089/jop.2007.0062
- Prausnitz MR, Noonan JS. Permeability of cornea, sclera and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–1488.
- Available from: https://www.ema.europa.eu/en/documents/product-information/nevanac-epar-product-information_en.pdf. Accessed October 13, 2020.
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52(3/4):591–611.
- Razali NM, Wah YB. Comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Liliefors and Anderson-Darling test. J Sat Model Anal. 2011;2(1):21–33.
- Doane DP, Seward LE. Measuring skewness. J Stat Educ. 2011;19(2):1–18. doi:10.1080/10691898.2011.11889611
- Singh R, Alpern L, Jaffe GJ, et al. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clin Ophthalmol. 2012;6:1259–1269. doi:10.2147/OPTH.S31902
- Pollack A, Staurenghi G, Sager D, et al. Prospective randomized clinical trial to evaluate the safety and efficacy of nepafenac treatment for the prevention of macular edema associated with cataract surgery in patients with diabetic retinopathy. Br J Ophthalmol. 2016.
- Chastain JE, Sanders ME, Curtis MA, et al. Distribution of topical ocular nepafenac and its active metabolite amfenac to the posterior segment of the eye. Exp Eye Res. 2016;145:58–67. doi:10.1016/j.exer.2015.10.009
- Afrashi F, Hashas AS, Shahbazov C, et al. Reliability of intravitreal nepafenac in rabbits. J Ocul Pharmacol Ther. 2015;31(1):43–50. doi:10.1089/jop.2014.0053
- Scruggs J, Wallace T, Hanna C. Route of absorption of drug and ointment after application to the eye. Ann Ophthalmol. 1978;10(3):267–271.
- Khurana RN, Palmer JD, Porco TC, Wieland MR. Dexamethasone intravitreal implant for pseudophakic cystoid macular edema in patients with diabetes. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):56–61. doi:10.3928/23258160-20150101-09
- Ismail RA, Sallam A, Zambarakji HJ. Pseudophakic macular edema and oral acetazolamide: an optical coherence tomography measurable, dose-related response. Eur J Ophthalmol. 2008;18(6):1011–1013. doi:10.1177/112067210801800626
- Wielders LH, Schouten JS, Aberle MR, et al. Treatment of cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2017;43(2):276–284. doi:10.1016/j.jcrs.2016.06.041
- Yüksel B, Karti Ö, Kusbeci T. Topical nepafenac for prevention of post-cataract surgery macular edema in diabetic patients: patient selection and perspectives. Clin Ophthalmol. 2017;11(11):2183–2190. doi:10.2147/OPTH.S132810
- Rho DS. Treatment of acute pseudophakic cystoid macular edema: diclofenac versus ketorolac. J Cataract Refract Surg. 2003;29(12):2378–2384.
- Warren KA, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina. 2010;30(2):260–266. doi:10.1097/IAE.0b013e3181b8628e
