Abstract
Progressive airflow limitation is a hallmark feature of chronic obstructive pulmonary disease (COPD) that ultimately leads to breathlessness, impaired quality of life, and reduced exercise capacity. Pharmacotherapy is used in patients with COPD to prevent and control symptoms, reduce both the frequency and severity of exacerbations, improve health status, and increase exercise tolerance. These strategies are intended to address management issues which promote both current disease control and a reduction in the risk of disease deterioration in the future. At the present time, long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) are available for maintenance therapy in patients with persistent symptoms. Tiotropium was the first LAMA to be approved for management of COPD, and many studies have described its beneficial effects on multiple clinically relevant outcomes. Glycopyrronium bromide (NVA237), a new LAMA, has been developed and received regulatory approval for management of COPD in a number of countries around the world. Results from pivotal Phase III trials suggest that NVA237 is safe and well tolerated in patients with moderate to severe COPD, and provides rapid and sustained improvements in lung function. Further, these changes are associated with statistically and clinically meaningful improvements in dyspnea, health-related quality of life, and exercise tolerance. Treatment with NVA237 also results in a significant reduction in risk of exacerbations and the need for rescue medication, and has been comparable with tiotropium with respect to safety and efficacy outcomes. Finally, emerging data indicate that NVA237 is efficacious both as monotherapy and in combination with indacaterol.
Introduction
Progressive limitation in airflow is a hallmark feature of chronic obstructive pulmonary disease (COPD) that ultimately leads to breathlessness, impaired quality of life, and reduced exercise capacity.Citation1,Citation2 Unlike other common chronic conditions, COPD continues to be a leading cause of morbidity and mortality worldwide.Citation1,Citation2
Pharmacotherapy is used in patients with COPD to prevent and control symptoms, reduce the frequency and severity of exacerbations, improve health status, and increase exercise tolerance.Citation1 These strategies are intended to address management issues which promote both current disease control and a reduction in risk of deterioration of the disease in the future.Citation1 Given that increased airway resistance contributes to the symptoms of COPD, treatment to a large extent relies on use of bronchodilators which influence the activity of beta2 and muscarinic receptors in the airway.Citation1 At the present time, long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) are available for maintenance therapy in patients with persistent symptoms. Tiotropium was the first LAMA to be approved for the management of COPD, and many studies have described its beneficial effects on many clinically relevant outcomes.Citation1 More recently, the Seebri® Breezhaler® (glycopyrronium bromide) a new, once daily, LAMA developed as NVA237 by Novartis Pharma AG, Basel, Switzerland, has received regulatory approval for management of COPD in a number of countries around the world.Citation3 Aclidinium bromide is a twice-daily LAMA that has also been approved recently in the European Union and in the US for the treatment of COPD.Citation4 This review focuses on the role of the novel LAMA, NVA237, in the management of COPD. The clinical evidence supporting the benefits of NVA237 focus on data from pivotal Phase III studies. The clinical data are presented in keeping with the Global Initiative for Chronic Obstructive Lung Disease 2011 recommendations,Citation1 with a view to providing pragmatic strategies that can be adopted in both primary and secondary care environments. The emerging utility of NVA237 in management of COPD, including its role in combination with the LABA indacaterol, is also reviewed to provide an evolving perspective on how it may be incorporated into future guidelines on COPD management.
Mechanism and onset of action
Anticholinergic bronchodilators such as ipratropium and oxitropium were originally introduced as short-acting preparations and more recently as a longer-acting formulation (tiotropium).Citation5 These older compounds have played an important role in the management of COPD for almost 25 years. The effectiveness of these therapies stems in part from the fact that vagally mediated bronchoconstriction represents a significant reversible component of airflow obstruction in patients with COPD. The competition with acetylcholine for binding to the muscarinic (M3) receptor on bronchial smooth muscle cells, thereby abolishing bronchomotor tone, is a central function of antimuscarinic agents.Citation5 Newer bronchodilator therapies, with a longer duration of action, appear to be more efficacious than shorteracting agents for improving relevant clinical outcomes in patients with COPD of varying disease severity, in large part because prolonged stenting of the airways allows for more complete lung emptying over time.Citation1
NVA237 (glycopyrronium bromide) is a new LAMA that acts as a competitive antagonist by binding to muscarinic receptors in bronchial smooth muscle, resulting in inhibition of acetylcholine-induced bronchoconstriction. It appears that NVA237 can to bind to all five subtypes of the muscarinic receptor (M1–M5).Citation6 It is the M3 receptor located on airway smooth muscle that is primarily involved in smooth muscle contraction. It is important to note that blockade of M2 receptors is associated with attenuation of the feedback inhibition of acetylcholine production, an activity that may reduce the bronchodilating effects of an antimuscarinic agent. Further, if cardiac M2 receptors are blocked, this may result in an increase in heart rate.Citation6 Therefore, the ideal antimuscarinic agent for use in COPD should have features that promote high affinity for M1 and M3 receptors and low affinity for M2 receptors. Tiotropium bromide and NVA237 share similar features, which include a higher selectivity for M3 receptors than for M2 receptors.Citation6–Citation8 Further, like tiotropium bromide and aclidinium bromide, NVA237 dissociates more slowly from the M3 receptor than from the M2 receptor.Citation6–Citation8
Clinical considerations
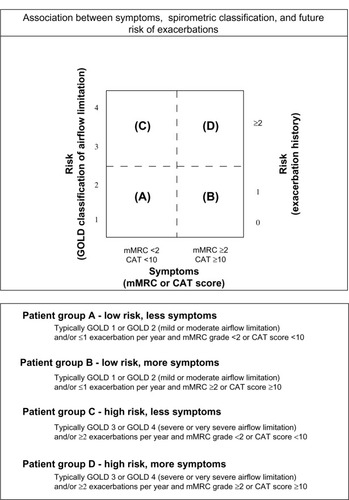
LAMAs and LABAs are recognized in the Global Initiative for Chronic Obstructive Lung Disease and the National Institute for Health and Clinical Excellence guidelinesCitation9,Citation10 as first-line maintenance therapies for patients with moderate-to-severe COPD (). Numerous studies have reported that both tiotropium and LABAs are efficacious for improving lung function and quality of life, and for reducing acute exacerbations and hospitalizations in patients with moderate-to-severe COPD.Citation10,Citation11–Citation16 At present, it is not entirely clear as to whether patients should be initiated on monotherapy with either a LAMA or a LABA or a combination of both. This uncertainty relates in part to the absence of long-term studies comparing these different approaches in patients with varying clinical features and levels of pulmonary impairment.
Figure 1 Global strategy for diagnosis, management and prevention of COPD: combined assessment of COPD.
Abbreviations: GOLD, Global initiative for Obstructive Lung Disease; COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council; CAT, COPD Assessment Test.
Until recently, tiotropium was the only LAMA approved for the management of COPD. Numerous studies have established that it is a safe and effective therapy and that it improves a range of important clinical outcomes. For example, compared with placebo, tiotropium is associated with superior bronchodilation,Citation17 increased inspiratory capacity,Citation18,Citation19 increased exercise capacity,Citation18,Citation20,Citation21 reduced dyspnea,Citation17,Citation22 improved health status,Citation17,Citation22 and reduced exacerbations.Citation17,Citation23 In patients with moderate-to-severe COPD, the newer once-daily agent, NVA237, has been shown to provide sustained 24-hour bronchodilation, to have a rapid onset of action, and to be safe and well tolerated.Citation6–Citation8 All doses of NVA237 evaluated (up to 200 μg) appear to be well tolerated, with doses of 50 μg or 100 μg once daily having greater efficacy than lower doses.Citation24–Citation26 The recent approval of NVA237 for use in the management of COPD offers clinicians another therapeutic option, and warrants a thorough review of the available literature outlining its clinical usefulness and its anticipated positioning in current guidelines.
Pivotal phase III trials
GLOW I
Study design and methods
In GLOW 1 (the Glycopyrronium bromide in COPD airways clinical study 1), patients had to complete a seven-day prescreening period followed by a 14-day run-in period in a double-blind, placebo-controlled manner.Citation27 Subjects who met the eligibility criteria were randomized in a 2:1 ratio to 26 weeks of treatment with either NVA237 50 μg or placebo once daily. Medication was administered via a single-dose dry-powder inhaler with low internal resistance (the Breezhaler device). Study outcomes included the efficacy, safety, and tolerability of once-daily NVA237 50 μg compared with placebo in patients with moderate to severe COPD. In total, 822 patients were randomized to NVA237 (n = 552) or placebo (n = 270).
Trough forced expiratory volume in one second (FEV1) at week 12 was the primary outcome. Enrolled patients included men and women >40 years of age, with a smoking history of ≥10 pack-years, a diagnosis of moderate to severe stable COPD,Citation9 a post-bronchodilator FEV1 ≥30% and <80% of the predicted normal, and post-bronchodilator FEV1/forced vital capacity <0.70.
Secondary outcome measures included assessment of breathlessness using the transition dyspnea index and health-related quality of life data captured using the St George’s Respiratory Questionnaire at week 26. Mean time to first moderate to severe COPD exacerbation and mean daily use of rescue medication over 26 weeks were also examined. Other outcome measures included trough FEV1 at the end of day 1 and at week 26. Finally, measurements of serial spirometry at various time points, including inspiratory capacity, were also evaluated.
Inhaled corticosteroids (ICS), intranasal corticosteroids, and H1 antagonists were allowed during the study period only if patients had been stabilized on a recommended and constant dose prior to study entry. The washout period for long-acting bronchodilator therapy prior to the run-in period was 48 hours for LABAs-ICS combinations and seven days for tiotropium. Patients were encouraged to use rescue medication on an “as-needed” basis. Patients using fixed-dose LABAs-ICS combination products were switched to an equivalent dose of ICS during the run-in phase and throughout the study period. During the screening period, the dose of ICS had to remain stable. Patients using ICS prior to screening continued on their prestudy regimen for the duration of the study. Salbutamol inhalers were provided to all patients for use as rescue medication during the study.
Efficacy data
Trough FEV1 was significantly higher at week 12 in patients receiving NVA237 (least squares mean ± standard error, 1.408 ± 0.0105 L), versus placebo (1.301 ± 0.0137 L, treatment difference 108 ± 14.8 mL, P < 0.001, ). Differences in trough FEV1 were observed as early as the end of day 1 and were maintained through week 26. Treatment with NVA237 was associated with significant improvements in FEV1 throughout the 24-hour periods on day 1 and at weeks 12 and 26 compared with placebo. The superiority of NVA237 in improving FEV1 compared with placebo was observed at all other visits and study time points. In a subpopulation of patients who underwent measurements of serial spirometry throughout the 24-hour periods on day 1 and at weeks 12 and 26, treatment with NVA237 resulted in significant improvement in FEV1 compared with placebo. Measurements of inspiratory capacity were obtained to evaluate the influence of NVA237 on lung hyperinflation at rest. In patients receiving NVA237, inspiratory capacity was significantly increased at all time points on day 1 and at weeks 12 and 26 (all P < 0.001, ) compared with placebo. Mean differences in inspiratory capacity for NVA237 versus placebo were 104 mL, 97 mL, and 113 mL (all P < 0.001) by the end of day 1, week 12, and week 26, respectively.
Figure 2 Trough FEV1 on Day 1 and at weeks 12 and 26.
Abbreviations: FEV1, forced expiratory volume in one second; LSM, least square mean.
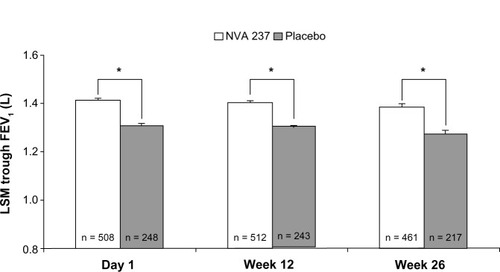
Table 1 Inspiratory capacity on day 1 and at weeks 12 and 26 in the FAS population
Dyspnea and quality of life data
Significant treatment differences in focal scores for the transition dyspnea index (1.04, P < 0.001) and total scores on the St George’s Respiratory Questionnaire (−2.81, P = 0.004) in favor of NVA237 were observed at week 26. A statistically significant reduction in the risk of first moderate to severe COPD exacerbation of 31% (P = 0.023) was observed in the patients receiving NVA237 despite a relatively low baseline exacerbation burden in the overall population at study entry. Administration of NVA237 was also associated with a reduction in use of rescue medication by 0.46 puffs per day (P = 0.005) versus placebo.
Safety data
The incidence of adverse events among patients receiving NVA237 was 57.5% compared with 65.2% in the placebo group. The higher frequency of adverse events in the placebo group was attributed to worsening of COPD. Other adverse events, including gastrointestinal disturbances, urination difficulty, urinary retention, and dry mouth occurred at low frequencies in both the NVA237 and placebo groups. Serious adverse events and discontinuations due to adverse events tended to be less frequent in patients receiving NVA237 than in those receiving placebo. Excluding serious worsening of COPD, which was less frequent in the NVA237 group, a similar percentage of serious adverse events were observed in the NVA237 and placebo groups.
Summary
The results of GLOW 1 suggest that NVA237 is safe and well tolerated in patients with moderate to severe COPD, providing rapid improvements in lung function that are sustained over time. Further, these latter changes were also associated with statistically significant and clinically meaningful improvements in dyspnea and health-related quality of life. Finally, treatment with NVA237 resulted in a significant reduction in risk of exacerbations and need for rescue medication.
GLOW 2
Study design and methods
In the multicenter, placebo-controlled, double-blind, parallel-group GLOW 2 (The GLycopyrronium bromide in COPD airWays clinical study 2), patients who fulfilled the inclusion criteria were randomized in a 2:1:1 ratio to receive NVA237 50 mg, placebo, or open-label tiotropium 18 mg for 52 weeks.Citation28 NVA237 50 mg and placebo were delivered using a single-dose dry-powder inhaler with low internal resistance (the Breezhaler device). Open-label tiotropium 18 mg was delivered via the HandiHaler® device (Boehringer Ingelheim, Ingelheim, Germany). All study treatments were administered in the morning between 8 am and 11 am for the duration of the 52-week study period following appropriate washout and run-in phases. Use of concomitant medications was similar to that described in the GLOW 1 study.Citation27
Trough FEV1 at week 12 was the primary outcome. Enrolled patients included males and females over 40 years of age, with a smoking history of ≥10 pack-years, a diagnosis of moderate to severe stable COPD,Citation9 a post-bronchodilator FEV1 ≥30% and <80% of the predicted normal, and post-bronchodilator FEV1/forced vital capacity <0.70.
Other key secondary assessments included evaluation of dyspnea using the transition dyspnea index at week 26 and health status measurements derived from the total score on the St George’s Respiratory Questionnaire at week 52. Additional evaluations included comparisons between open-label tiotropium 18 mg, placebo, and NVA237 in relation to all the study endpoints. GLOW 2 was not adequately powered to demonstrate statistical superiority of NVA237 versus tiotropium.
Efficacy data
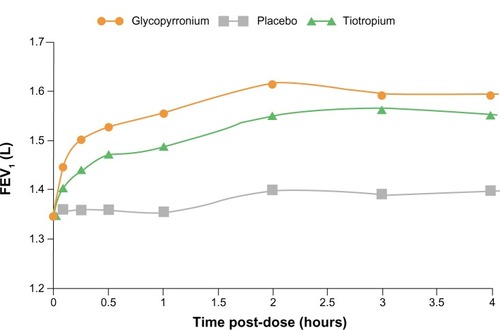
Of the 1066 patients randomized, 810 completed the study according to protocol requirements. Treatment with NVA237 resulted in a significant improvement in trough FEV1, which showed a mean increase of 97 mL (95% confidence interval [CI] 64.6–130.2; P , 0.001) at week 12. The trough FEV1 at week 12 in the tiotropium group showed a mean improvement of 83 mL (95% CI 45.6–121.4; P < 0.001, ). As early as the first dose on day 1, patients receiving NVA237 showed rapid improvement in FEV1 compared with placebo. During the time interval from five minutes to four hours post-dosing, NVA237 was associated with significantly greater improvements in FEV1 compared with both placebo (P < 0.001) and tiotropium (P < 0.01, ). With the exception of one predose measurement at −20 minutes at week 52 (P < 0.053), improvements in inspiratory capacity were significantly greater (P < 0.001) in patients receiving NVA237 and similar to improvements observed in the tiotropium group at almost all evaluated time points on day 1 and weeks 12 and 52.Citation28 Among patients receiving NVA237 and tiotropium, trough forced vital capacity was significantly greater compared with placebo at day 1 and at weeks 12, 26, and 52 (P < 0.001). Both NVA237 and tiotropium were associated with comparable numeric differences compared with placebo for all outcomes.
Figure 3 Trough forced expiratory volume in 1 s (FEV1) at day 1 and weeks 12, 26 and 52.
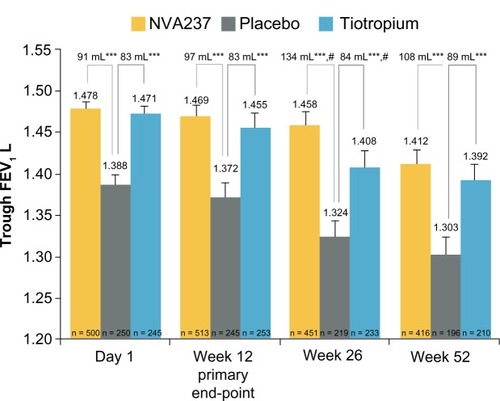
Figure 4 FEV1 at each time point up to 4 hours post-dose on day 1 in GLOw2.
Abbreviations: FEV1, forced expiratory volume in one second; GLOW, Glycopyrronium bromide in COPD airways; LSM, least squares means.
Dyspnea and quality of life data
At week 26, the focal score for the transition dyspnea index improved with NVA237 compared with placebo (2.13 versus 1.32, with a least squares mean treatment difference of 0.88 [95% CI 0.299–1.320]; P < 0.002). This change was comparable with the improvement observed in the tiotropium group versus the placebo group (with a least squares mean treatment difference of 0.95 [95% CI 0.356–1.521]; P = 0.002, ). The St George’s Respiratory Questionnaire total score improved significantly at 52 weeks with NVA237 and tiotropium compared with placebo (with a least squares mean treatment difference versus placebo of −3.32 [95% CI −5.287 to −1.346]; P < 0.001) and −2.84 [95% CI −5.105 to −0.571]; P = 0.014, respectively, .
Figure 5 Improvements in (A and B) dyspnea and (C and D) health-related quality of life with glycopyrronium versus placebo in GLOW2.
Abbreviations: HRQoL, health-related quality of life; GLOW, GLycopyrronium bromide in COPD airways; LSM, Least squares means; CI, Confidence interval; TDI, Transition Dyspnea index; od, once daily; OR, odds ratio; SGRQ, St George’s Respiratory Questionnaire.
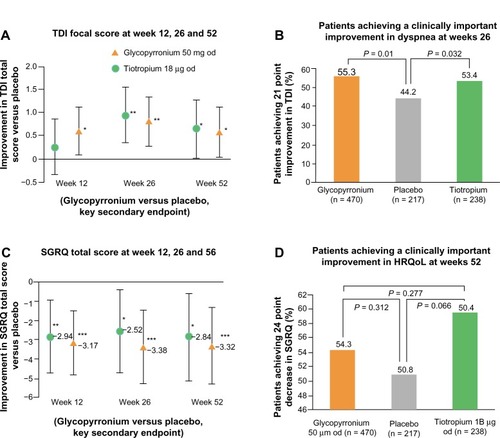
Exacerbation data
The risk of moderate to severe COPD exacerbations was reduced by 34% during treatment with NVA237 (P < 0.001) compared with placebo. Patients receiving treatment with NVA237 also required less rescue medication (P < 0.039 versus placebo, ).
Figure 6 Kaplan–Meier plot of the time to first moderate or severe chronic obstructive pulmonary disease exacerbation.
Safety data
There were no apparent differences in safety profiles across the treatment groups. Pooled data related to adverse events in GLOW 1 and GLOW 2 are shown in .
Table 2 Most frequent adverse events (≥5% in any treatment group); SAEs occurring in ≥5 patients in any treatment group, deaths, discontinuations due to adverse events and electrocardiographic abnormalities; pooled data from GLOW1 and GLOW2
Summary
GLOW 2 demonstrated that NVA237 50 mg once daily providedsignificant improvements in lung function, dyspnea, health status, exacerbations, and use of rescue medication in comparison with placebo, and was comparable with tiotropium. The results of GLOW 2 suggest that NVA237 has features related to both efficacy and safety that will allow clinicians to consider it as an alternative LAMA for management of COPD.
GLOW 3
Study design and methods
In the multicenter GLOW 3 (GLycopyrronium bromide in COPD airWays clinical study 3), patients were randomized into two treatment arms using a crossover design, with placebo followed by NVA237 50 μg once daily or NVA237 50 μg once daily followed by placebo for three weeks, with a 14-day washout interval.Citation29 Men and women with moderate to severe COPD were eligible for enrollment in GLOW 3 if they were aged ≥40 years, had a smoking history of ≥10 pack-years, an FEV1 <80% and ≥40% of predicted normal, and FEV1/forced vital capacity <70%. The definition of diagnostic criteria for COPD was similar to that described in GLOW 1Citation27 and GLOW 2.Citation28
The primary outcome measure was the effect of NVA237 50 μg on exercise tolerance compared with placebo after three weeks of treatment on day 21. One hour after drug administration on days 1 and 21, exercise tolerance was measured by exercise endurance time during a submaximal cycle ergometry test. Submaximal exercise testing was performed according to recognized standards. Exercise endurance was defined as the time from commencement of loaded pedaling to cessation of exercise.Citation30 An important key secondary measurement included inspiratory capacity, derived spirometrically, at isotime, ie, the last time point in the submaximal constant-load cycle ergometry test at which the patient had a valid test result for both treatment periods.
Efficacy data
Ninety-five (88%) of 108 randomized patients completed the study. More than 96% of the patients completed periods 1 and 2. Their mean age was 60.5 years (58% male), with the majority being Caucasian (96%). During the incremental work test, the mean Wmax at screening was 87.8 ± 28.8 W, while the submaximal workload averaged 70.2 ± 23.1 W.
Both at rest and at peak exercise, inspiratory capacity was consistently higher in the NVA237 group compared with the placebo group on days 1 and 21 (P < 0.05, ). NVA237 also produced a statistically significant treatment difference in inspiratory capacity at isotime on day 1 versus placebo (P < 0.001) and on day 21 versus placebo (P < 0.001).
Table 3 IC at rest (prior to exercise) and peak exercise on Days 1 and 21
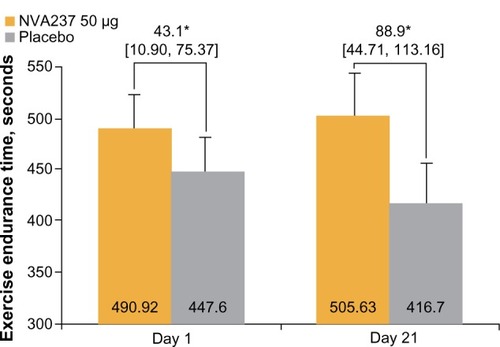
After three weeks of treatment with NVA237, exercise endurance time was significantly superior compared with placebo. The least squares mean treatment difference on day 21 between the NVA237 and placebo groups was 88.9 seconds, a change that corresponded to an approximately 21% between-group difference (P < 0.001, ). Data obtained on day 1 revealed a least squares mean treatment difference of 43.1 seconds between the groups, a change that corresponded to an approximately 10% between-group difference (P < 0.001).
Figure 7 Exercise endurance time on Days 1 and 21.
Abbreviation: LSM, least square mean.
Safety data
The overall proportion of patients who experienced at least one adverse event was comparable between those receiving NVA237 and those receiving placebo. Most adverse events were considered mild or moderate in severity and were not considered to be related to the study medication. The number of patients who discontinued study participation due to adverse events was low, whether treated with NVA237 or placebo. No deaths were reported during the study. The only serious study-related adverse event reported was a herniated disc diagnosed in a patient receiving NVA237; this was not considered to be drug-related. There were no unexpected changes in vital signs, electrocardiograms, and hematology or biochemistry parameters during the study.
Summary of GLOW 1, 2, and 3
Based on the data highlighted in the Phase III studies described above, NVA237 should become an important addition to the armamentarium for COPD management. Improvements in trough FEV1 with NVA237 compared with both placebo and tiotropium were clinically relevant and appeared to be sustained over time. Changes in inspiratory capacity were significantly greater than in the placebo group and comparable with tiotropium, suggesting that NVA237 will have a very positive impact on minimizing the deleterious influence of both static and dynamic hyperinflation on COPD-related outcomes. It is also evident that NVA237 has a beneficial influence on dyspnea, quality of life, and exercise tolerance, and that it is associated with a significant reduction in COPD-related exacerbations among patients with moderate to severe disease.
Evolving role of NVA237 in COPD
Combination therapy with long-acting bronchodilators
There is a growing focus on promoting a strategy of maximal bronchodilation in patients with COPD, particularly among those individuals with persistent symptoms and disability.Citation1 Depending on the stage of disease, a single bronchodilator may not be adequate for many individuals. At the present time, there is a lack of clarity around when to initiate therapy with a combination of drugs from different classes. Combining bronchodilators of different pharmacologic classes has been shown to be superior to the individual components and associated with a reduction in the risk of side effects compared with increasing the dose of a single bronchodilator.Citation1 Evidence describing combinations of long-acting bronchodilators (LAMAs/LABAs) suggest that these preparations may be effective in the management of COPD, given their positive influence on optimizing symptom control, their potential to improve patient compliance, and their ability to simplify disease management.
Addition of a second long-acting bronchodilator to therapy for patients in groups B–D is recommended in the revised Global Initiative for Chronic Obstructive Lung Disease 2011 guidelineCitation1 as a strategy to optimize symptom control (). Addition of ICS to LABAs/LAMAs combination therapy is recommended for patients at risk of experiencing COPD exacerbations in groups C or D ().Citation1 Interestingly, despite reports suggesting that ICS have limited efficacy in terms of modifying the long-term decline of FEV1 in COPD,Citation31,Citation33 these medications continue to be widely used in patients with COPD.Citation1
LABAs and LAMAs appear to have a separate but complementary influence on bronchodilation.Citation34 The primary effect of LABAs appears to be mediated by stimulation of β2-adrenergic receptors located in airway smooth muscle. The antagonistic influence of LAMAs at the level of the muscarinic receptors in airway smooth muscle serves to block the signaling cascade that ultimately results in smooth muscle contraction.Citation35
There is growing evidence that LAMA/LABA combinations are efficacious in terms of improving trough FEV1, dyspnea, quality of life, and use of rescue medication compared with these agents used alone.Citation34,Citation36–Citation44 At the present time, there is a number of new LABA/LAMA combinations being studied in the management of COPD.Citation45 Much of the evidence describing the benefits of this strategy centers around the free combination of the LAMA tiotropium with the LABAs, formoterol, salmeterol, and indacaterol. Several once-daily fixed-dose LABALAMA combinations are currently in development, and include QVA149 (indacaterol-glycopyrronium), vilanterol-umeclidinium, and olodaterol-tiotropium.Citation45 The fixed-dose LABA/LAMA combination furthest in development is indacaterol-glycopyrronium (QVA149).
QVA149
In a double-blind, four-period, crossover Phase II study, 154 patients with moderate to severe COPD were randomized to receive QVA149 (indacaterol 300 μg + NVA237 50 μg), indacaterol 300 μg, indacaterol 600 μg, or placebo for seven days followed by a seven-day washout period between each treatment.Citation46 Compared with placebo and indacaterol, QVA149 significantly increased trough FEV1 on days 1 and 7 (primary endpoint). QVA149 also had a rapid onset of action at five minutes post-dose on day 1, and was associated with sustained bronchodilation over a 24-hour period. Further, improvement in FEV1 at all the post-baseline time points on day 1 were significantly greater with QVA149 than with indacaterol or placebo (P < 0.05). Compared with placebo and indacaterol, trough forced vital capacity and peak FEV1 on days 1 and 7 were also significantly greater for QVA149 (P < 0.05). Overall, all treatments were well tolerated. Recent results from Phase III studiesCitation47,Citation48 show that QVA149 provides significant and sustained improvement in lung function compared with placebo, tiotropium, salmeterol-fluticasone, and the separate components, indacaterol and NVA237 (ILLUMINATE and SHINE studies, respectively), over 26 weeks, with significant symptomatic benefits also reported. It is relevant to note that the SHINE study enrolled more than 2100 patients and met the primary endpoint by demonstrating the superiority in trough FEV1 (P < 0.001) achieved by once-daily QVA149 compared with once-daily indacaterol or once-daily NVA237 in patients with moderate to severe COPD.Citation47 Taken together, the emerging data indicate that NVA237 is efficacious both as monotherapy and in combination with indacaterol. The choice between monotherapy versus a LABA/LAMA combination as initial maintenance therapy will be driven by the expectation of achieving maximal bronchodilation and superior clinical outcomes compared with single use of either a LABA or LAMA in patients with persistent and disabling symptoms. As more data on LABA/LAMA combination therapy become available, it is very likely that this approach will be the most appropriate one for achieving optimal outcomes in patients with established COPD.
Delivery of NVA237via Breezhaler device
The Breezhaler has been shown to be a user-friendly device, and is a single-dose dry-powder inhaler suitable for use by patients with COPD of all ages with a wide range of disease severity.Citation49,Citation50 Irrespective of the degree of pulmonary impairment and patient age, studies with the Breezhaler have shown that it delivers a consistent dose of drug, with no reported device failures. Further, the Breezhaler has lower internal resistance properties compared with the HandiHaler used to deliver tiotropium (specific airflow resistances of 2.2 and 5.1 × 10−2 kPa1/2 L−1 minute, respectively).Citation49 The specific airflow resistance properties of the Diskus® and Turbuhaler® are 2.1 × 10−2 kPa1/2 L−1 minute and 3.2 × 10−2 kPa1/2 L−1 minute, respectively.Citation51 An inhaler’s internal resistance will determine the effort patients have to make to achieve adequate inspiratory flows for effective and reproducible dose delivery.Citation52,Citation53 Therefore, low-resistance devices would tend to require less patient effort in order to generate adequate inspiratory flows compared with high-resistance devices, making them more suitable for older patients and for those with severe COPD, who may experience difficulties in generating adequate flows.
Conclusion
Bronchodilator therapy is a central strategy in the management of symptomatic COPD, and the agents that are currently available have been shown to have very positive effects on many important clinical outcomes. NVA237 (glycopyrronium bromide), the newest LAMA to receive approval for management of COPD, appears to have a clinically and statistically meaningful impact on many relevant management parameters described in the pivotal studies, ie, GLOW 1,Citation27 GLOW 2,Citation28 and GLOW 3.Citation29 The inhaled route will continue to be the preferred route of administration in order to minimize systemic effects. Not uncommonly, the complex procedures involved in use of the inhaler devices to deliver medication and frequent dosing are the main barriers to compliance among patients with COPD.
A number of recent advances in the treatment of COPD have the potential to make management of the disease more convenient for patients, particularly for those who need LABA/LAMA combination therapy, and provide long-term, day-to-day control of their symptoms. Research aimed at developing LAMAs with efficacy and safety equivalent to tiotropium, such as NVA237, provides alternatives for patients with COPD. The development of LABAs with a 24-hour duration of action has also permitted the development of LABA/LAMA combinations for once-daily administration in a single inhaler. Many of these combinations have been shown to be efficacious in terms of significantly improving lung function and other outcomes compared with monotherapy, with either equivalent or fewer side effects. Further research should focus on examining the long-term efficacy and safety of different bronchodilator combinations, especially their ability to improve patientrelevant outcomes, and their impact on the natural history of COPD when introduced early in the disease process.
Disclosure
The author has received research, consulting, and lecturing fees from GlaxoSmithKline, Sepracor, Schering Plough, Altana, Methapharma, AstraZeneca, ONO Pharma, Merck Canada, Forest Laboratories, Novartis Canada/ US, Boehringer Ingelheim (Canada) Ltd, Pfizer Canada, SkyePharma, and KOS Pharmaceuticals.
References
- Global Initiative for Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease2011 Available from: http://www.goldcopd.org/guidelines-gold-summary-2011.html
- ViegiGPistelliFSherrillDLMaioSBaldacciSCarrozziLDefinition, epidemiology and natural history of COPDEur Respir J200730993101317978157
- UK Medicines Information New Drugs OnlineGlycopyrrolate2012 Available from: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4793Accessed April 12, 2013
- UK Medicines Information New Drugs OnlineAclidinium bromide2012 Available from: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4515Accessed April 12, 2012
- TashkinDPLong-acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safetyCurr Opin Pulm Med2010169710520019615
- SykesDADowlingMRCharltonSJExploring the mechanism of agonist efficacy: a relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptorMol Pharmacol20097654355119498041
- VogelmeierCBanerjiDNVA237, a long-acting muscarinic antagonist, as an emerging therapy for chronic obstructive pulmonary diseaseTher Adv Respir Dis2011516317321511677
- SechaudRRanardDZhang-AubersonLde la MotteSDrollmannAKaiserGPharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD)Int J Clin Pharmacol Ther20125011812822257577
- global Initiative for Chronic Obstructive Lung Disease (GOLD 2008)Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease Available from: http://www.ncbi.nlm.nih.gov/pubmed/17507545
- National Institute for Health and Clinical ExcellenceChronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary careLondon, UKNational Clinical Guideline Centre2010 Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/EnglishAccessed April 13, 2013
- BuhlRBanjeriDProfile of glycopyrronium for once-daily treatment of moderate-to-severe COPDInt J Chron Obstruct Pulmon Dis2012772974123118536
- VogelmeierCHedererBGlaabTTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med20113641093110321428765
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med200735677578917314337
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- WedzichaJACalverleyPMSeemungalTAThe prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/ fluticasone propionate or tiotropium bromideAm J Respir Crit Care Med2008177192617916806
- AaronSDVandehmheenKLFergussonDTiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary diseaseAnn Intern Med200714654555517310045
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- O’DonnellDEFlugeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J20042383284015218994
- CelliBZuwallackRWangSKestenSImprovement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumesChest20031241743174814605043
- CasaburiRFactors determining constant work rate exercise tolerance in COPD and their role in dictating the minimal clinically important difference in response to interventionsCOPD2005213113617136973
- MaltaisFHamiltonAMarciniukDImprovements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest20051281168117816162703
- DonohueJFvan NoordJABatemanEDA 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest2002122475512114338
- NiewoehnerDERiceKCoteCPrevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med200514331732616144890
- FogartyCHattersleyHDi ScalaLDrollmannABronchodilatory effects of NVA237, a once daily long-acting muscarinic antagonist, in COPD patientsRespir Med201110533734221144724
- VerkindreCFukuchiYFlemaleASustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsRespir Med20101041482148920541381
- VogelmeierCVerkindreCCheungDSafety and tolerability of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsPulm Pharmacol Ther20102343844420416390
- D’UrzoAFergusonGTvan NoordJAEfficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trialRespir Res20111215622151296
- KerwinEHebertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J2012401106111423060624
- BeehMKSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J Chron Obstruct Pulmon Dis2012750351322973092
- European Respiratory SocietyClinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise TestingEur Respir J199710266226899426113
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Updated2010 Available from: http://www.goldcopd.org/Guidelines/guideline-2010-gold-report.html
- TashkinDCelliBDecramerMPrescribing patterns according to COPD treatment guidelines in patients enrolled in a global clinical trialProc Am Thorac Soc20063A111
- KarnerCCatesCJThe effect of adding inhaled corticosteroids to tiotropium and long-acting beta(2)-agonists for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20119CD00903921901729
- CazzolaMMolimardMThe scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPDPulm Pharmacol Ther20102325726720381630
- RouxEMolimardMSavineauJPMarthanRMuscarinic stimulation of airway smooth muscle cellsGen Pharmacol1998313493569703200
- van NoordJAAumannJLJanssensEComparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPDEur Respir J20052621422216055868
- van NoordJAAumannJLJanssensEEffects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPDChest200612950951716537846
- van NoordJAAumannJLJanssensECombining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptomsRespir Med2010104995100420303247
- TashkinDPLittnerMAndrewsCPTomlinsonLRinehartMDenis-MizeKConcomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trialRespir Med200810247948718258423
- TashkinDPDonohueJFMahlerDAEffect of arformoterol twice daily, tiotropium once daily, and their combination in patients with COPDRespir Med200910351652419208459
- MaltaisFBeckEWebsterDFour weeks once daily treatment with tiotropium+olodaterol (BI 1744) fixed dose combination compared with tiotropium in COPD patientsEur Respir J201036S54P5557
- CazzolaMDi MarcoFSantusPThe pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPDPulm Pharmacol Ther200417353914643169
- VogelmeierCKardosPHarariSGansSJStengleinSThirlwellJFormoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month studyRespir Med20081021511152018804362
- MahlerDAD’UrzoABatemanEDConcurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, doubleblind comparisonThorax20126778178822544891
- D’UrzoAVogelmeierCFuture of chronic obstructive disease managementExpert Rev Respir Med2012628529922788943
- van NoordJABuhlRLaforceCQVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary diseaseThorax2010651086109120978028
- BatemanEFergusonGTBarnesNBenefits of dual bronchodilation with QVA149 once daily versus placebo, indacaterol, NVA237 and tiotropium in patients with COPD: the SHINE studyAbstract presented at the European Respiratory Society Congress 2012Vienna, AustriaSeptember 1–5, 2012
- VogelmeierCBatemanEPallanteJOnce-daily QVA149 significantly improves lung function and symptoms compared to twice-daily fluticasone/salmeterol in COPD patients: the ILLUMINATE studyAbstract presented at the European Respiratory Society CongressVienna, AustriaSeptember 1–5, 2012
- ChapmanKRFogartyCMPeckittCDelivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPDInt J Chron Obstruct Pulmon Dis2011635336321760722
- PavkovRMuellerSFiebichKCharacteristics of a capsule based dry powder inhaler for the delivery of indacaterolCurr Med Res Opin2010262527253320843166
- BroedersMEMolemaJVermueNAFolgeringHTPeak inspiratory flow rate and the slope of the inhalation profiles in dry powder inhalersEur Respir J20011878078311757627
- WieshammerSDreyhauptJDry powder inhalers: which factors determine the frequency of handling errors?Respiration200875182517911976
- TerzanoCDry powder inhalers and the risk of errorRespiration200875141518185025