Abstract
Purpose
Unconjugated bilirubin is one of the most endogenous antioxidant substances. Mildly elevated total bilirubin concentrations may protect against cardiovascular disease and total death. However, most studies only focused on the association between serum total bilirubin and the risk of cardiovascular disease and total death. This study aimed to investigate the relationship between serum indirect bilirubin (IBIL) and the cardiovascular events in maintenance hemodialysis patients.
Patients and Methods:
This retrospective cohort study included 284 maintenance hemodialysis patients. Patients were divided into two groups according to the median IBIL level: high IBIL group (IBIL ≥3.0 μmol/L) and low IBIL group (IBIL <3.0 μmol/L). All demographic and laboratory data were recorded at baseline. The endpoint was cardiovascular events and all-cause mortality.
Results
During the median follow-up time of 62 months, 96 patients developed cardiovascular disease. There were 134 deaths. In Kaplan–Meier analysis curves, the risk of cardiovascular events in the low IBIL group was significantly higher than high IBIL group (P < 0.001). In multivariate Cox regression analysis, the risk of cardiovascular events in high IBIL group was 0.484 times (95% CI 0.278–0.844, P = 0.010) the risk in low IBIL group. However, there was no significant association between serum IBIL level and all-cause mortality (P = 0.269).
Conclusion
Our findings suggest that lower circulating IBIL levels were associated with the increased risk of cardiovascular events in maintenance hemodialysis patients.
Introduction
Between 40% and 50% of deaths among patients with end-stage renal disease (ESRD) are due to cardiovascular causes, and the risk of cardiovascular mortality in patients with ESRD is 8.8-fold higher than in the general population.Citation1,Citation2 Therefore, it is an important clinical priority to find novel and innovative biomarker and potential therapeutic target to reduce cardiovascular event and improve clinical outcomes in ESRD.
Unconjugated bilirubin possesses potent antioxidant,Citation3,Citation4 anti-inflammatory,Citation5 and possibly lipid-lowering properties.Citation6 Recent clinical studies show mildly elevated total bilirubin is associated with protection from kidney damage and dysfunction in chronic kidney disease patients,Citation7,Citation8 and low serum total bilirubin levels are also associated with the loss of residual kidney function in peritoneal dialysis patients.Citation9 Furthermore, unconjugated bilirubin functions intracellularly, as a potent inhibitor of NADPH oxidase complexes and albumin-bound bilirubin contributes significantly to the oxidant scavenging activity of plasma and may also potentially reduce endothelial dysfunction, which is strictly related to cardiovascular disease and total death.Citation3,Citation4,Citation10 Similar inverse associations have now been shown between serum total bilirubin concentrations and coronary artery disease,Citation11 coronary heart disease,Citation12 peripheral vascular disease,Citation13 and stroke.Citation14 Moreover, mildly elevated total bilirubin (TBIL) concentrations may protect against cardiovascular and total death.Citation15,Citation16 However, most previous studies only focused on TBIL without distinguishing between direct bilirubin (DBIL) and indirect bilirubin (IBIL). Few studies investigated the relationship between serum IBIL and all-cause mortality in dialysis patients.Citation17,Citation18 However, the relationship between serum IBIL and the cardiovascular events in hemodialysis patients remains unclear.
Therefore, in the present study, we conducted a retrospective cohort study to evaluate the relationship between serum IBIL and cardiovascular events and all-cause death in maintenance hemodialysis (MHD) patients.
Methods
Study Design and Population
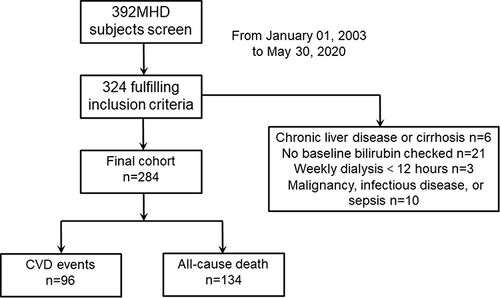
We conducted a retrospective single-center cohort study. The study population consists of 284 patients aged over 18 years with ESRD receiving maintenance hemodialysis for at least 3 months from Shanghai Fifth People’s Hospital. Study subjects were recruited between January 01, 2003, and May 30, 2020. Up to January 2022, the median follow-up time was 62 months. All of the patients were subjected to a standard bicarbonate dialysis session. Hemodialysis was performed 3 times weekly using single-use dialyzers with a membrane surface area of 1.5 to 1.7 m2. Exclusion criteria were (1) weekly dialysis for less than 12 hours; (2) baseline conditions of malignancy, infectious disease, or sepsis; (3) patients with chronic liver disease or cirrhosis; (4) patients with missing bilirubin levels ().
The endpoint was cardiovascular event and all-cause death. The cardiovascular event was defined as ischemic heart disease, cerebrovascular disease, peripheral vascular disease, heart failure, cardiac arrhythmia, and cardiac arrest. All-cause death was defined as any death. The study was performed according to the principles of the Declaration of Helsinki of 1975, revised in 2013, and the protocol of this retrospective single-center cohort study was approved by the Ethics Committee of Shanghai Fifth People’s Hospital of Fudan University (approval number 2018–145).
Data Collection
Baseline demographic data, clinical data and biochemical parameters were collected at the initiation of hemodialysis therapy. The baseline blood samples of each patient were collected at the initiation of hemodialysis therapy. These data were complemented by clinical assessment of blood pressure (BP), and fasting blood glucose. Diabetes was diagnosed on the basis of the World Health Organization criteria. Hypertension was defined as BP >140/90 mmHg and/or the use of antihypertensive medication.
Statistical Analysis
The Kolmogorov–Smirnov test was used to check the normality of the data distribution. Data are expressed as means ± SD for continuous parametric data, medians and interquartile ranges for continuous nonparametric data, and frequencies for categorical data. Potential differences among the two groups were assessed with t-test for normally distributed data, Mann–Whitney U for nonnormally distributed data. Cumulative survival curves for the cardiovascular disease and all-cause mortality were compared with the Log rank test, and the survival rate was calculated by the Kaplan–Meier method. The Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of cardiovascular disease, initially without adjustment and subsequently adjusting for clinical parameters. For all tests, P values of <0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, USA) and IBM SPSS software (SPSS Inc., Chicago, USA).
Results
Baseline Characteristics
The study population consists of 284 MHD patients. In order to ensure that the two groups have roughly the same number of people and get enough evidence, the patients were divided into the following two groups according to the median IBIL value (3.0 μmol/L): I1 (low IBIL group; <3.0 μmol/L) and I2 (high IBIL group; ≥3.0 μmol/L). There were 139 patients in the I1 group and 145 patients in the I2 group. The baseline characteristics and clinical outcomes of the study population are displayed in . In the I2 group, serum TBIL, DBIL, IBIL and serum albumin and calcium values were higher; MHD duration was longer, compared with those in the I1 group.
Table 1 Baseline Characteristics of Chronic Hemodialysis Patients Classified by the Serum Indirect Bilirubin Median Value
Survival Analysis
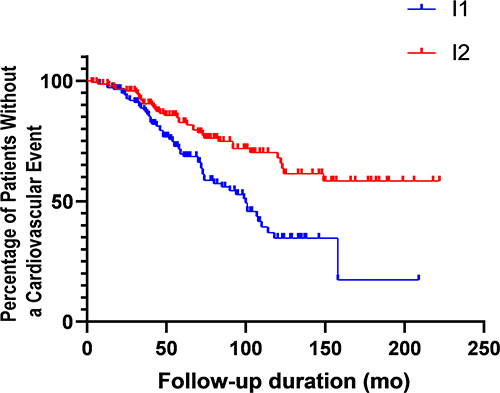
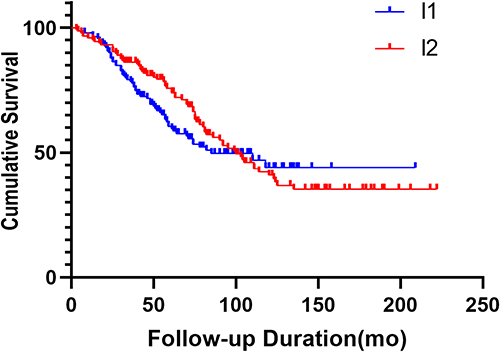
During the 62 months observation period, the all-cause mortality rate was 47.2% and 96 cardiovascular events occurred (). In the I1 group, 59 of the 139 patients developed cardiovascular events, while in the I2 group, 37 of the 145 patients developed cardiovascular disease. In Kaplan–Meier analysis curves for the cardiovascular events among 284 MHD patients, the risk of cardiovascular events in the I1 group was significantly higher than those in the I2 group (Log Rank χ2 = 12.97, P < 0.001, ). However, there was no significant difference in the risk of all-cause mortality between the two groups (Log Rank χ2 = 2.997, P = 0.428, ).
Effect of IBIL Level on Cardiovascular Events
Univariate Cox regression survival analysis is summarized in . Briefly, age, serum IBIL, alanine aminotransferase and aspartate aminotransferase were the risk factors for cardiovascular events (P < 0.05). shows unadjusted and multivariable-adjusted HRs with 95% CIs according to baseline serum IBIL concentration as a continuous variable. In the multivariate Cox regression analysis, after adjustment for age, serum uric acid, hemoglobin, serum albumin, single-pool Kt/V (SpKt/V), hypersensitive C reactive protein (HsCRP) and the other traditional and dialysis-related risk factors, serum IBIL was also the independent risk factors for cardiovascular events (HRs = 0.773, 95% CI 0.660–0.906, P = 0.001). In the same multivariable-adjusted model, age (HRs = 1.038, 95% CI 1.016–1.061, P = 0.001), previous coronary artery disease (HRs = 3.839, 95% CI 1.832–8.044, P < 0.001) and previous congestive heart failure (HRs = 1.941, 95% CI 1.021–3.692, P = 0.043) were the independent risk factors for cardiovascular events. Consistently, there was no significant association between serum uric acid, SpKt/V, serum albumin, hemoglobin, HsCRP and cardiovascular events (). However, there was no significant association between serum IBIL and all-cause mortality (HRs = 0.818, 95% CI 0.574–1.167, P = 0.269).
Table 2 Risk Factors for Cardiovascular Events by the Cox Proportional Hazards Analysis in Maintenance Hemodialysis Patients
We converted serum IBIL into binary and tertile variables when performing the Cox regression analysis (). In the multivariate Cox regression analysis, compared with the I1 group, the fully adjusted HRs in the I2 group for cardiovascular events was 0.484 (95% CI 0.278–0.844, P = 0.010), after adjustment for age, serum uric acid, hemoglobin, serum albumin, SpKt/V, HsCRP and the other traditional and dialysis-related risk factors (). After adjustment for traditional and dialysis-related risk factors, individuals with IBIL in the upper tertile had an adjusted hazard ratio of 0.578 (95% CI 0.410–0.815, P = 0.002) for cardiovascular disease compared with those in the lower tertile.
Table 3 The Relationship Between Cardiovascular Events and the Baseline Serum Indirect Bilirubin by Multiple Cox Analysis
Discussion
Our study demonstrated that the occurrence of cardiovascular events was negatively correlated with increasing serum IBIL binary and tertile and actual IBIL values among MHD patients followed for 62 months. This relationship also remained significant even after adjusting for potential confounding factors, including age, serum uric acid, hemoglobin, serum albumin, spKt/V, HsCRP, and serum phosphorus. This is the first report on the association between cardiovascular events and serum IBIL levels in maintenance hemodialysis patients.
Data on the association between serum bilirubin and the risk of cardiovascular events in dialysis patients are limited and inconclusive. A cohort study followed for 50 months comprised 1080 chronic hemodialysis patients demonstrated that individuals with serum total bilirubin in the upper tertile had an adjusted hazard ratio of 0.40 for cardiovascular event, compared with those in the lower tertile.Citation19 However, this research did not have unconjugated bilirubin data. In 1,419 patients with angina pectoris undergoing percutaneous coronary intervention, those with higher total bilirubin levels experienced significantly fewer MACEs (cardiac death, myocardial infarction, target-vessel revascularization, or unstable angina pectoris/heart failure, over 2.4-yr follow-up) than patients with lower total bilirubin concentrations.Citation20 Moreover, investigation of 3316 Ludwigshafen Risk and Cardiovascular Health Study participants revealed that increased total bilirubin predicted lower mortality over a period of 10.4 yr.Citation21 Furthermore, a study included 2936 subjects followed for 5.4 years showed that higher serum total bilirubin was associated with a decreased risk of developing cardiovascular death in asymptomatic diabetic patients.Citation16 However, few studies drew opposite conclusions. A retrospective cohort study included 740 peritoneal dialysis subjects followed for 28 months demonstrated all-cause mortality was higher in the patients in the higher TBIL group than in the lower TBIL group and in patients in the higher IBIL group than in the lower IBIL group.Citation17 And a nationwide retrospective cohort study investigating 47,650 hemodialysis patients followed for 27.6 ± 12 months indicated that high serum total bilirubin level is associated with high mortality in patients undergoing long-term hemodialysis.Citation22 A large retrospective study investigating 1111 patients indicated that patients with ST-segment elevation myocardial infarction and higher total bilirubin undergoing percutaneous coronary intervention and stent placement had increased MACE and rate of cardiac death during their hospital admission.Citation23 The discrepancies in ethnicity and inclusion criteria may be confounding factors that caused these different results. However, most research did not have unconjugated bilirubin data, which had strong anti-oxidant effects.
Recently, two studies investigated the relationship between serum IBIL and all-cause mortality in dialysis patients. A multicenter retrospective cohort study followed for 24 months comprised 885 maintenance hemodialysis patients demonstrated that patients with IBIL ≤ 3.3 μmol/L had hazard ratio of 1.661 for all-cause death, compared with those with baseline IBIL 3.4–4.8 μmol/L (P = 0.013). The Kaplan–Meier curves showed higher all-cause mortality in patients with IBIL ≤ 3.3 μmol/L (P = 0.015).Citation18 Zhan et alCitation17 found that there was a 56% higher risk of 5-year mortality in the higher IBIL group than in the lower IBIL group. However, our study showed that there was no significant association between serum IBIL level and all-cause mortality (P = 0.269). The discrepancies in sample size and the follow-up time may cause different results.
The high risk of cardiovascular morbidity and mortality in MHD individuals is associated with a high prevalence of traditional risk factors for cardiovascular disease (hypertension, diabetes, and dyslipidemia). Apart from these risk factors, a series of nontraditional risk factors including calcium and phosphate abnormalities, oxidative stress, and inflammation may render ESRD patients more prone to develop excess risks of cardiovascular disease.Citation24 Up to now, many researchers have tried to assess several risk factors to improve life expectancy in ESRD patients. Unconjugated bilirubin affects atherosclerosis by several inhibiting mechanisms, including vascular smooth muscle cell proliferation, low-density lipoprotein oxidation, and endothelial dysfunction.Citation25 Additionally, elevated total bilirubin levels are associated with decreased oxidative stress status and augmented endothelium-dependent vasodilation in male gilbert syndrome subjects who have mild unconjugated hyperbilirubinemia.Citation26 Moreover, in spontaneously hypertensive rats, the administration of hemin for 3 months elevated unconjugated bilirubin levels and total antioxidant capacity and reduced left ventricular hypertrophy, hypertension, ventricular phospholipase C activity, circulating aldosterone, and urinary excretion of oxidized lipid.Citation27 Furthermore, lipid soluble unconjugated bilirubin prevents the oxidation of cardiolipin and decreases the infarct size in the heart during ischemia.Citation28 At last, unconjugated bilirubin, which is not hydrophobic substances and plasma albumin-bound, could not be removed by hemodialysis and can keep its strong anti-oxidant effects in MHD patients. Thus, in a special group undergoing hemodialysis, high IBIL could keep protective role. In our study, in Cox regression analysis, serum IBIL levels were a risk factor for cardiovascular events (P < 0.05). However, there was no significant association between serum DBIL, TBIL and cardiovascular disease (P > 0.05).
Hypoalbuminemia is also a risk factor for cardiovascular disease.Citation24,Citation29 The present study demonstrated that serum albumin value in patients with IBIL ≥ 3.0 μmol/L was higher than that in patients with IBIL <3.0 μmol/L, which may have potentially increased the protective effect of IBIL which is albumin-bound. However, in multivariate Cox regression analysis, low IBIL was still an independent risk factor for cardiovascular events in MHD patients even after adjusting for serum albumin and other potential confounding factors.
The present study has some limitations. First, this was a retrospective, relatively small sample size, single-center study that might have selection bias and reduce the statistical power. Second, we only had all-cause mortality, but we did not know the exact cause of death of the uremia patients. Third, the potential mechanism behind the relationship between serum IBIL and cardiovascular disease in MHD individuals was not clarified. Fourth, this was a retrospective study, which can only reveal associations but not causality.
Conclusion
The current study demonstrated that lower serum IBIL concentrations were associated with the increased risk of cardiovascular events in uremia patients undergoing hemodialysis independent of established cardiovascular disease risk factors. If our findings are further confirmed by future studies, routine measurements of serum IBIL could help identify those patients at high risk of cardiovascular disease.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethical Approval
Since this study was a retrospective one using clinical data, and it did not involve further invasive intervention, treatment, or costs to patients, the study received a consent exemption and it was approved by the ethics committee of Shanghai Fifth People’s Hospital of Fudan University (approval number 2018-145).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have no conflicts of interest for this work.
Additional information
Funding
References
- Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl_3):iii28–iii34. doi:10.1093/ndt/gfy174
- de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789. doi:10.1001/jama.2009.1488
- Stec DE, Storm MV, Pruett BE, Gousset MU. Antihypertensive actions of moderate hyperbilirubinemia: role of superoxide inhibition. Am J Hypertens. 2013;26(7):918–923. doi:10.1093/ajh/hpt038
- Maruhashi T, Kihara Y, Higashi Y. Bilirubin and endothelial function. J Theroscler Thromb. 2019;26(8):688–696. doi:10.5551/jat.RV17035
- Tran DT, Jeong YY, Kim JM, Bae HB, Son SK, Kwak SH. The anti-inflammatory role of bilirubin on “two-hit” sepsis animal model. Int J Mol Sci. 2020;21(22):8650. doi:10.3390/ijms21228650
- Bulmer AC, Verkade HJ, Wagner KH. Bilirubin and beyond: a review of lipid status in Gilbert’s syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res. 2013;52(2):193–205. doi:10.1016/j.plipres.2012.11.001
- Li J, Liu D, Liu Z. Serum total bilirubin and progression of chronic kidney disease and mortality: a systematic review and meta-analysis. Front Med. 2021;25(7):549. doi:10.3389/fmed.2020.00549
- Tanaka S, Ninomiya T, Masutani K, et al. Prognostic impact of serum bilirubin level on long-term renal survival in IgA nephropathy. Clin Exp Nephrol. 2015;19(6):1062–1070. doi:10.1007/s10157-015-1096-0
- Tsujikawa H, Tanaka S, Hara M, et al. Association of lower serum bilirubin with loss of residual kidney function in peritoneal dialysis patients. Ther Apher Dial. 2020;24(2):202–207. doi:10.1111/1744-9987.12865
- Salvatore T, Galiero R, Caturano A, et al. Coronary microvascular dysfunction in diabetes mellitus: pathogenetic mechanisms and potential therapeutic options. Biomedicines. 2022;10(9):2274. doi:10.3390/biomedicines10092274
- Jain V, Ghosh RK, Bandyopadhyay D, et al. Serum bilirubin and coronary artery disease: intricate relationship, pathophysiology, and recent evidence. Curr Probl Cardiol. 2021;46(3):100431. doi:10.1016/j.cpcardiol.2019.06.003
- Li C, Wu W, Song Y, Xu S, Wu X. The nonlinear relationship between total bilirubin and coronary heart disease: a dose-response meta-analysis. Front Cardiovasc Med. 2022;8:761520. doi:10.3389/fcvm.2021.761520
- Lan Y, Liu H, Liu J, Zhao H, Wang H. The relationship between serum bilirubin levels and peripheral arterial disease and gender difference in patients with hypertension: BEST study. Angiology. 2020;71(4):340–348. doi:10.1177/0003319719900734
- Choi Y, Lee SJ, Spiller W, et al. Causal associations between serum bilirubin levels and decreased stroke risk: a two-sample Mendelian randomization study. Arterioscler Thromb Vasc Biol. 2020;40(2):437–445. doi:10.1161/ATVBAHA.119.313055
- Vitek L, Hubacek JA, Pajak A, et al. Association between plasma bilirubin and mortality. Ann Hepatol. 2019;18(2):379–385. doi:10.1016/j.aohep.2019.02.001
- Chen SC, Lin CP, Hsu HC, et al. Serum bilirubin improves the risk predictions of cardiovascular and total death in diabetic patients. Clin Chim Acta. 2019;488:1–6. doi:10.1016/j.cca.2018.10.028
- Zhan X, Yang M, Chen Y, et al. Relationship between serum bilirubin levels and mortality in patients on peritoneal dialysis. Ren Fail. 2019;41(1):532–539. doi:10.1080/0886022X.2019.1628062
- Tian ML, Song WL, Li Q, et al. Association of low serum indirect bilirubin level with all-cause mortality in maintenance hemodialysis patients. Zhonghua Yi Xue Za Zhi. 2019;99(28):2203–2207. doi:10.3760/cma.j.issn.0376-2491.2019.28.010
- Ho Y, Chen TW, Huang TP, Chen YH, Tarng DC. Bilirubin links HO-1 and UGT1A1*28 gene polymorphisms to predict cardiovascular outcome in patients receiving maintenance hemodialysis. Antioxidants. 2021;10(9):1403. doi:10.3390/antiox10091403
- Yao HM, Shen DL, Zhao XY, et al. Prognostic value of total bilirubin in patients with angina pectoris undergoing percutaneous coronary intervention. Int J Clin Exp Med. 2015;8(9):15930–15939.
- Zulus B, Grünbacher G, Kleber ME, März W, Renner W. The UGT1A1*28 gene variant predicts long-term mortality in patients undergoing coronary angiography. Clin Chem Lab Med. 2018;56(4):560–564. doi:10.1515/cclm-2017-0692
- Su H, Kao C, Lin Y, et al. Relationship between serum total bilirubin levels and mortality in uremia patients undergoing long-term hemodialysis. Atherosclerosis. 2017;265:155–161. doi:10.1016/j.atherosclerosis.2017.09.001
- Chung SR, Yang TH, Shin HC, et al. Initial total bilirubin and clinical outcome in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention with drug-eluting stents. Circ J. 2016;80(6):1437–1444. doi:10.1253/circj.CJ-15-1397
- Stenvinkel P, Heimbürger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–1911. doi:10.1046/j.1523-1755.1999.00422.x
- Bulmer AC, Bakrania B, Du Toit EF, et al. Bilirubin acts as a multipotent guardian of cardiovascular integrity: more than just a radical idea. Am J Physiol Heart Circ Physiol. 2018;315(3):H429–H447. doi:10.1152/ajpheart.00417.2017
- Maruhashi T, Soga J, Fujimura N, et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation. 2012;126(5):598–603. doi:10.1161/CIRCULATIONAHA.112
- Ndisang JF, Jadhav A. Upregulating the heme oxygenase system suppresses left ventricular hypertrophy in adult spontaneously hypertensive rats for 3 months. J Card Fail. 2009;15(7):616–628. doi:10.1016/j.cardfail.2009.02.003
- Ben-Amotz R, Bonagura J, Velayutham M, Hamlin R, Burns P, Adin C. Intraperitoneal bilirubin administration decreases infarct area in a rat coronary ischemia/reperfusion model. Front Physiol. 2014;5:53. doi:10.3389/fphys.2014.00053
- Querido S, Quadros Branco P, Silva Sousa H, et al. Hypervolemia, hypoalbuminemia and mitral calcification as markers of cardiovascular risk in peritoneal dialysis patients. Rev Port Cardiol. 2017;36(9):599–604. doi:10.1016/j.repc.2016.12.014