Abstract
Coronary and cerebrovascular atherothrombosis are the leading cause of mortality and morbidity worldwide. Novel antiplatelet agents have been established for the management of patients with clinically evident coronary atherothrombosis and are increasingly used in these patients. These agents, however, have shown limited efficacy in the prevention of cerebrovascular events and potential harm in patients with history of stroke or transient ischemic attack. Herein, the efficacy and safety of two established antiplatelet agents in patients with stroke – aspirin and clopidogrel – are reviewed with a focus on the use and challenges related to novel antiplatelet agents – prasugrel, ticagrelor, and vorapaxar – in patients at risk for and with a history of stroke or transient ischemic attack.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Stroke, a sudden loss of a neurological function due to ischemia or bleeding in the brain, is a leading cause of acquired disability and is second only to myocardial infarction as the most common cause of death in western countries.Citation1,Citation2 Ischemia, due to the embolic or thrombotic occlusion of an intracerebral artery, is the most common etiology of stroke (∼80%–90%), while hemorrhagic stroke and subarachnoid hemorrhages are less common.Citation3 A history of cerebrovascular accident (CVA; ie, stroke or transient ischemic attack [TIA]) in patients with concomitant coronary artery disease increases the risk of death, myocardial infarction, or recurrent stroke – both ischemic and hemorrhagic.Citation4–Citation9 In the Reduction of Atherothrombosis for Continued Health (REACH) registry, a CVA that occurred ≤1 year from enrollment was associated with a risk of recurrent stroke of any type that was greater compared with that of a more remote event.Citation4 Anticoagulants are established in the management of patients at risk for cardioembolic stroke (eg, atrial fibrillation). Antiplatelet agents, such as aspirin and clopidogrel, have reduced the incidence of ischemic stroke in patients with known symptomatic cerebrovascular disease and in those at high risk for atherothrombosis and are currently used both for the management of acute non-cardioembolic ischemic stroke and for secondary stroke prevention.Citation10 In the last several years, a number of novel antiplatelet agents have emerged in patients with acute and chronic coronary atherothrombosis.Citation11–Citation14 These agents, however, have shown limited efficacy and/or potential harm in cerebrovascular ischemic events, particularly in patients with previous CVA. Herein, the efficacy and safety of two established antiplatelet agents for the management of non-cardioembolic stroke – aspirin and clopidogrel – are reviewed with a focus on the use and challenges related to novel antiplatelet agents – prasugrel, ticagrelor and vorapaxar – in patients at risk for and with a history of CVA.
Aspirin and clopidogrel
Aspirin has been tested extensively in patients with stroke. An irreversible inhibitor of cyclooxygenase-1 that produces a permanent defect in thromboxane A2-mediated platelet activation, aspirin is the only agent that has proved beneficial in acute stroke. A large trial that tested aspirin (300 mg) started within 48 hours after symptom onset showed a nonsignificant reduction in mortality (9.0% versus 9.4%) and a significant reduction in recurrent ischemic strokes within 14 days (2.8% versus 3.9%; P<0.001), a benefit not offset by any significant excess in hemorrhagic stroke.Citation15 According to American guidelines for the early management of acute stroke, oral aspirin (initial dose 325 mg) should be started in most patients within 24–48 hours of symptom onset (Class I; Level of Evidence A).Citation16 Aspirin is also indicated for secondary stroke prevention. A meta-regression analysis of placebo-controlled trials of aspirin therapy for secondary stroke prevention estimated a 15% (95% confidence interval [CI] 6%–23%) relative risk reduction for stroke of any type (hemorrhagic or ischemic).Citation17 According to the American guidelines for the prevention of stroke, aspirin (50–325 mg/day) monotherapy (Class I; Level of Evidence A) is an acceptable option for patients with non-cardioembolic ischemic stroke or TIA.Citation18
Clopidogrel, a second-generation thienopyridine, is a prodrug that needs to be metabolized in the liver via cytochrome P450 to produce the active moiety, which prevents adenosine diphosphate-induced platelet activation and aggregation by irreversibly inhibiting the P2Y12 receptor.Citation19 Clopidogrel is rapidly absorbed after oral administration with peak plasma levels approximately 1 hour after dosing. The active metabolite (2-oxo-clopidogrel) is short lived, with a half-life of approximately 30 minutes.Citation20
As monotherapy, clopidogrel has been tested for secondary stroke prevention in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial (against aspirin)Citation21 and the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial (against aspirin/ extended release dipyridamole).Citation22 The CAPRIE trial evaluated the efficacy of clopidogrel 75 mg as compared with aspirin 325 mg in 19,185 patients with prior myocardial infarction, symptomatic peripheral artery disease, or recent ischemic stroke (1 week to 6 months before randomization). The primary endpoint, a composite of vascular death, myocardial infarction, or ischemic stroke at 1 year, occurred in 5.3% of patients assigned to clopidogrel as compared with 5.8% of patients assigned to aspirin with a relative risk reduction (RRR) of 8.7% (95% CI 0.3–16.5; P=0.043). In the subgroup of patients who qualified for previous stroke, the effect of clopidogrel was directionally consistent (RRR 7.3%, 95% CI 5.7–18.7; P=0.26).Citation21 The PRoFESS trial compared clopidogrel 75 mg daily with the combination of very low-dose aspirin (25 mg) plus extended release dipyridamole (200 mg) in 20,333 patients with a history of ischemic stroke in the previous 90 days. The incidence of recurrent stroke of any type during an average follow-up of 2.5 years was similar between the two groups (hazard ratio [HR] 1.01, 95% CI 0.92–1.11); also, the incidence of ischemic stroke alone was similar (HR 0.97, 95% CI 0.88–1.07) but the incidence of hemorrhagic stroke was significantly higher in the aspirin plus dipyridamole arm (1.4% versus 1.0%, HR 1.42, 95% CI 1.11–1.83; P=0.006).Citation22
Following the demonstration of clear benefits provided by dual antiplatelet therapy (DAPT) with aspirin and clopidogrel in patients with acute coronary syndrome (ACS), the Management of Atherothrombosis with Clopidogrel in High-Risk Patients with Recent Transient Ischemic Attacks or Ischemic Stroke (MATCH)Citation23 and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA)Citation24 investigators evaluated this approach in patients with prior stroke and stable coronary artery disease. The MATCH trial randomized 7,599 patients with recent TIA or ischemic stroke (within 3 months) to DAPT with clopidogrel 75 mg plus aspirin (75 mg) or clopidogrel alone (75 mg) and followed them for 18 months. DAPT had no significant effect on the primary endpoint (a composite of ischemic stroke, myocardial infarction, vascular death, or rehospitalization for an acute ischemic event; RRR 6.4%, 95% CI −4.6–16.3; P=0.244); or the secondary endpoint of ischemic stroke alone (RRR 7.1%, 95% CI −8.5–20.4, P=0.35). However, the combination of aspirin and clopidogrel increased bleeding compared with clopidogrel alone with a 1.26% absolute increase in life-threatening bleeding (95% CI 0.64–1.88; P<0.0001) and a 0.40% absolute increase in symptomatic intracranial hemorrhage (95% CI −0.01–0.82). There was no mortality difference.Citation23 The CHARISMA trial randomized 15,603 patients with clinically evident cardiovascular disease or multiple risk factors for coronary artery disease to DAPT with aspirin and clopidogrel or aspirin monotherapy. Of the randomized patients, 35% (n=4,320) had a history of CVA at baseline. Like MATCH, CHARISMA did not show a benefit of DAPT as compared with aspirin alone in reducing the incidence of the primary endpoint of cardiovascular death, myocardial infarction, or stroke (6.8% versus 7.3%, RRR 0.93, 95% CI 0.83–1.05; P=0.22). The incidence of non-fatal ischemic stroke alone was not significantly reduced by DAPT (1.7% versus 2.1%, relative risk [RR] 0.81, 95% CI 0.64–1.02; P=0.07). Moderate but not severe bleeding according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) definition,Citation25 was increased in the combination therapy arm (2.1% versus 1.3%, RR 1.62, 95% CI 1.27–2.08; P<0.001).Citation24
The use of DAPT with aspirin plus clopidogrel has also been studied in a specific subset of ischemic stroke, including small artery disease. The Secondary Prevention of Small Subcortical Strokes (SPS3) trial randomized 3,020 patients with recent (within 6 months) symptomatic small subcortical brain infarcts identified by magnetic resonance imaging, also known as lacunar strokes. Patients were randomly assigned to receive 75 mg of clopidogrel plus aspirin (325 mg/day) or aspirin (325 mg/day) alone. The primary outcome was recurrent stroke of any type (ischemic or hemorrhagic). DAPT did not reduce the risk of the primary endpoint (125 strokes, 2.5% per year) as compared with aspirin alone (138 strokes, 2.7% per year) (HR 0.92, 95% CI 0.72–1.16; P=0.48). However, the risk of major hemorrhage (mostly systemic) was doubled by DAPT (HR 1.97, 95% CI 1.41–2.71; P<0.001). Importantly, DAPT was associated with an unexpected increase in all-cause mortality (HR 1.52, 95% CI 1.14–2.04; P=0.004) that led to an early termination of the study. These findings support the hypothesis that thrombosis may have a minimal role in precipitating occlusions of small, penetrating cerebral arteries,Citation26 which may also explain the low rate of recurrent events associated with lacunar strokes.Citation27 It is also possible that the use of a relatively high dose (325 mg) of aspirin may have contributed to the observed increase in bleeding complications.
The only study that showed a possible benefit of DAPT in patients with stroke was the Clopidogrel in High-Risk Patients with Acute Non-Disabling Cerebrovascular Events (CHANCE) trial,Citation28–Citation30 conducted in 5,170 Chinese patients randomized within 24 hours of the onset of ischemic minor stroke or high-risk TIA to clopidogrel (loading dose of 300 mg followed by 75 mg/day for 90 days) plus aspirin (75–300 mg/day loading dose followed by 75 mg/day for the first 21 days) or placebo plus aspirin (75–300 mg loading dose followed by 75 mg/day for 90 days). The primary outcome was stroke of any type (ischemic or hemorrhagic) at 90 days. The study showed that DAPT, as compared to aspirin alone, importantly reduced the incidence of stroke (HR 0.68, 95% CI 0.57–0.81; P<0.001) and the composite of stroke, myocardial infarction, or vascular death (HR 0.69, 95% CI 0.58–0.82; P<0.001) (). The incidence of hemorrhagic stroke was similar between the two groups (HR 1.01, 95% CI 0.38–2.70; P=0.98).Citation30 Thus, it is possible that the combination of the selection of patients early after the index event (when recurrence is highest) with TIA or a small stroke (associated with a low risk for hemorrhagic conversion) and a short duration of therapy (21 days) might have hit the “sweet spot” by providing an anti-ischemic effect without an increase in bleeding. A similar trial, called Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT), is currently studying these therapies in patients with TIA and minor stroke with a narrower time window (treatment within 12 hours after symptoms onset).Citation31 A summary of all clopidogrel trials is provided in .
Figure 1 Cumulative incidence of survival free of stroke of any type in the clopidogrel plus aspirin (red lines) and aspirin (blue lines) groups.
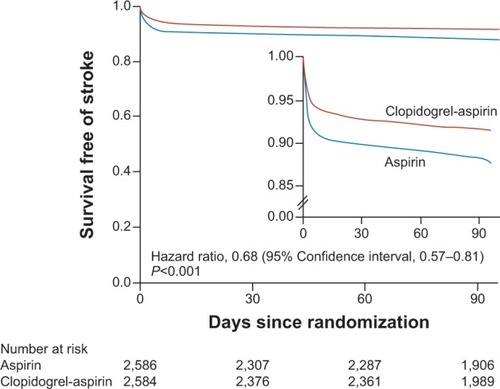
Table 1 Summary of all clopidogrel trials
Prasugrel
Prasugrel is a third-generation thienopyridine that is more efficiently metabolized compared with clopidogrel, thus producing a higher and more predictable level of P2Y12 inhibition.Citation32,Citation33 Prasugrel was tested against clopidogrel in aspirin-treated patients with ACS and known coronary anatomy in the pivotal Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). The TRITON-TIMI 38 trial compared prasugrel (60 mg loading dose and 10 mg daily maintenance dose) versus clopidogrel (300 mg loading dose and 75 mg daily maintenance dose) in 13,608 patients with moderate-to-high risk ACS presumed to receive percutaneous coronary intervention after diagnostic angiography. The primary endpoint was death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke. The key safety endpoint was major bleeding. At 15 months the primary endpoint was significantly reduced by prasugrel (HR 0.81, 95% CI 0.73–0.90; P<0.001), with an effect that was mostly evident on myocardial infarction and with no effect on stroke (HR 1.02, 95% CI 0.71–1.45; P=0.93). However, major bleeding was increased by prasugrel (2.4% versus 1.8%, HR 1.32, 95% CI 1.03–1.68; P=0.03) as well as life-threatening bleeding (1.4% versus 0.9%, HR 1.52, 95% CI 1.08–2.13; P=0.01) and fatal bleeding (0.4% versus 0.1%, HR 4.19, 95% CI 1.58–11.11; P=0.002). No clinical benefit and possibly net harm was found with prasugrel in patients who qualified for prior stroke or TIA at baseline, patients >75 years, and those weighing <60 kg. In particular, patients with prior CVA had significant worse clinical outcomes and more frequent bleeding (including intracranial bleeding) (HR 1.54, 95% CI 1.02–2.32; P=0.04) than those without such a history (HR 0.84, 95% CI 0.76–0.93; P<0.001).Citation11 Notably, the TRITON-TIMI 38 study excluded patients with a history of hemorrhagic stroke or an ischemic stroke within 3 months of enrollment, a population where the harm could have been even higher.Citation34 Prasugrel was also tested against clopidogrel in ACS patients managed medically in the A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY) trial, a trial that showed a neutral effect for prasugrelCitation35 and that excluded patients with prior history of TIA or stroke of any type. On the basis of these data, prasugrel is contraindicated in patients with a history of stroke of any type or TIA.Citation36
Ticagrelor
Ticagrelor is a first-in-class member of a novel class of oral P2Y12 inhibitors: the cyclopentyl-triazolo-pyrimidines. Unlike thienopyridines, ticagrelor is direct acting (ie, not a prodrug) and also reversible with a half-life of 6–12 hours.Citation37,Citation38
In the Platelet Inhibition and Patient Outcomes (PLATO)Citation12 trial 18,624 patients with ACS were randomized to ticagrelor (180 mg loading dose followed by 90 mg twice daily) or clopidogrel (300–600 mg loading dose followed by clopidogrel 75 mg once daily) for 12 months. All patients received aspirin 75–100 mg daily. The primary endpoint, a composite of death from vascular causes, myocardial infarction, or stroke at 12 months, occurred in 9.8% of patients receiving ticagrelor as compared with 11.7% of those receiving clopidogrel (HR 0.84, 95% CI 0.77–0. 92; P<0.001) with no significant difference in the incidence of stroke in the two groups (1.5% versus 1.3%, HR 1.17, 95% CI 0.91–1.52; P=0.22). The rate of death from any cause was also reduced with ticagrelor (4.5% versus 5.9% with clopidogrel, HR 0.78, 95% CI 0.69–0.89; P<0.001). No significant difference in the rates of major bleeding was found between ticagrelor and clopidogrel (11.6% versus 11.2%, HR 1.04, 95% CI 0.95–1.13; P=0.43). However, ticagrelor was associated with a higher rate of major bleeding not related to coronary artery bypass grafting (4.5% versus 3.8%, HR 1.19, 95% CI 1.02–1.38; P=0.03), including more instances of intracranial bleeding (HR 1.87, 95% CI 0.98–3.58; P=0.06).Citation12
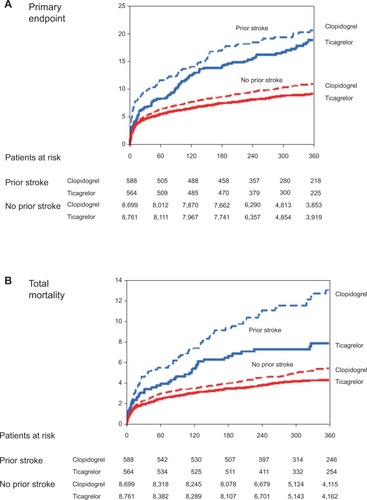
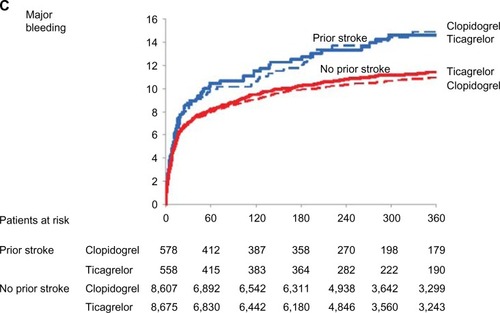
The effect of ticagrelor in patients with prior CVA was evaluated in a post hoc analysis of 1,152 ACS patients in PLATO of whom 69 patients had both prior TIA and prior stroke, 653 had prior ischemic stroke only, and 430 had a prior TIA only. Higher rates of myocardial infarction (HR 1.92, 95% CI 1.59–2.33; P<0.0001), death (HR 2.18, 95% CI 1.79–2.6; P<0.0001), stroke (HR 2.90, 95% CI 2.03–4.14; P<0.0001), and intracranial bleeding (HR 3.95, 95% CI 1.82–8.55; P<0.0005) were observed in patients with prior stroke or TIA compared to patients with non-CVA history, underscoring the high risk of this population. In this subset, ticagrelor, as compared with clopidogrel, had an effect that was directionally consistent with the overall PLATO trial with no significant interaction with the presence of stroke or TIA at baseline. Ticagrelor was associated with a nonsignificant reduction in the primary endpoint (19.0% versus 20.8%, HR 0.87, 95% CI 0.66–1.13; Pint=0.84) () with a numerical reduction in mortality (7.9% versus 13.0%, HR 0.62, 95% CI 0.42–0.91; Pint=0.19) () at 1 year and no significant increase in overall major bleeding complications (14.6% versus 14.9%, HR 0.99, 95% CI 0.71–1.37; Pint=0.77) (). Neither overall stroke rate (HR 1.13, 95% CI 0.59–2.17; Pint=0.89) nor hemorrhagic stroke rate (HR 0.67, 95% CI 0.11–4.03; Pint=0.25) showed a significant treatment-by-subgroup interaction. This analysis therefore indicated that ticagrelor had an effect consistent with the overall PLATO results.Citation39 These results, however, should be balanced with the small albeit significant increase in the risk of intracranial bleeding of the drugCitation12 and with a comparator arm (aspirin plus clopidogrel) that didn’t provide any ischemic benefit but caused increased bleeding compared to either clopidogrel or aspirin alone. Therefore, ticagrelor indication needs to be more extensively explored in adequately powered clinical trials evaluating patients with prior CVA.
Figure 2 Cumulative incidence of (A) the primary composite of cardiovascular death, myocardial infarction, and stroke; (B) mortality; and (C) major bleeding in the ticagrelor (solid lines) and clopidogrel (dotted lines) groups in patients with a history of prior stroke or transient ischemic attack (blue lines) and no previous stroke or transient ischemic attack (red lines) at baseline.
Vorapaxar
Vorapaxar is a selective, orally active, potent, and competitive protease-activated receptor 1 antagonist that potently inhibits thrombin-induced platelet aggregation. Vorapaxar is rapidly absorbed via the oral route and has peculiar pharmacokinetics with a half-life greater than 7 days (159–311 hours). It is metabolized and eliminated primarily by biliary and gastrointestinal routes.Citation40 Its Phase III program included two large trials in patients with acute and chronic coronary atherothrombosis, namely Thrombin-Receptor Antagonist Vorapaxar in Acute Coronary Syndromes (TRACER)Citation13 and Trial to Assess the Effects of SCH 530348 in Preventing Heart Attack and Stroke in Patients with Arteriosclerosis (TRA 2P-TIMI 50).Citation14
The TRACER trial randomized 12,944 patients with ACS without ST-segment elevation to vorapaxar (40 mg loading dose followed by 2.5 mg/day) or placebo on top of standard of care. The primary endpoint was a composite of death from cardiovascular causes, myocardial infarction, stroke, recurrent ischemia with rehospitalization, or urgent coronary revascularization. The trial was terminated early after a safety review due to a significant increase in the risk of major bleeding complication in the vorapaxar arm (HR 1.35, 95% CI 1.16–1.58; P<0.001), including intracranial hemorrhage (HR 3.39, 95% CI 1.78–6.45; P<0.001). In addition, vorapaxar did not significantly reduce the primary efficacy endpoint (HR 0.92, 95% CI 0.85–1.01; P=0.07) despite a promising effect on myocardial infarction (HR 0.88, 95% CI 0.79–0.98; P=0.02).Citation13
In the TRA 2P-TIMI 50 trial, vorapaxar (2.5 mg daily) was compared to placebo for a median of 30 months in 26,449 patients who had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease. Vorapaxar significantly reduced the primary efficacy endpoint (composite of cardiovascular death, myocardial infarction, stroke, or urgent coronary revascularization) compared with placebo (HR 0.87, 95% CI 0.80–0.94; P<0.001) and increased the risk of moderate to severe bleeding (HR 1.66, 95% CI 1.43–1.93; P<0.001), including intracranial hemorrhage (HR 1.94, 95% CI 1.39–2.70; P<0.001). After 2 years, the data and safety monitoring board recommended discontinuation of the study treatment in patients with a history of stroke owing to the risk of intracranial hemorrhage.Citation14 The efficacy and safety of vorapaxar in patients with prior ischemic stroke was specifically assessed in a subgroup analysis of the TRA 2P-TIMI 50 which showed that in patients with prior ischemic stroke who receive standard antiplatelet therapy, adding vorapaxar increased the risk of intracranial hemorrhage without an improvement in major vascular events, including ischemic stroke.Citation41
How to use old and new antiplateletagents in patients presenting with ACS and with prior stroke?
The development of novel platelet inhibitors for the treatment of patients with ACS that showed not only limited efficacy but also potential harm in patients with prior stroke represents a common clinical dilemma for the management of patients with both conditions. Optimal management of these patients should quantify and individualize the ischemic and bleeding risks. In particular, the risk related to the type of stroke (minor stroke or TIA, lacunar stroke, cardioembolic stroke) and its timing compared to the index ACSCitation4 and the risk associated with the ACS event (eg, Global Registry of Acute Coronary Events [GRACE] and Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association guidelines [CRUSADE] risk scores).Citation9,Citation42 Herein are presented some general principles to help individualize the selection of antiplatelet agent(s), the duration of antiplatelet therapy, and also the type of revascularization in this population according to the type of presenting ACS, ie, with or without persistent ST elevation.
ST elevation ACS
ACS with persistent ST elevation represents a clinical setting where the time needed for an adequate patient evaluation and therapeutic decision often has to struggle against the need to “save muscle”. In this setting, the time available to collect all the information related to the previous cerebrovascular event might not be adequate. In this setting it may be useful to privilege an approach that allows a short duration of DAPT (such as the use of bare-metal stents or second-generation drug-eluting stents).Citation43–Citation45 In general, clopidogrel should be favored as the adenosine diphosphate-receptor blocker in these patients.
Non-ST elevation ACS
In patients with non-ST elevation ACS (ie, non-ST-segment myocardial infarction or unstable angina) physicians have typically more time to clarify the history of CVA (TIA, cardioembolic stroke, lacunar or presumed atherothrombotic stroke) and quantify the expected risks and the benefits of revascularization (eg, GRACE risk score).Citation42 For example, in patients admitted for non-ST-segment myocardial infarction with a known history of recent lacunar stroke, long-term DAPT therapy post stent implantation would likely increase the risk of bleeding and mortality.Citation27 If the expected benefits of coronary revascularization (GRACE risk score >140, large ischemic area of myocardium) are relevant, DAPT duration should be minimized. On the other hand, patients with unstable angina at low risk might be managed conservatively.
Patients with a history of primary hemorrhagic stroke represent a formidable challenge, since most of the above-mentioned trials excluded these patients. An accurate evaluation of the benefits of revascularization should be balanced against the high hemorrhagic risks. Short-duration DAPT or monotherapy with clopidogrel may be appropriate according to the coronary revascularization strategy (ie, conservative or invasive) ().Citation46,Citation47
Conclusion
The lack of benefit observed with novel antiplatelet agents for the prevention of a first or recurrent cerebrovascular event, such as prasugrel or vorapaxar (with the possible exception of ticagrelor), that proved beneficial for the treatment of coronary atherothrombosis underscores the importance of carefully weighing the benefit and risk of antiplatelet therapy in different clinical settings.
While most trials with combinations of antiplatelet agents in patients with prior stroke showed negative results, the CHANCE trial indicated that it is still possible to find a “sweet spot” where DAPT may be useful.Citation30 However, the CHANCE findings may not be necessarily generalizable to non-Chinese patients and confirmatory trials in other populations will be necessary.
Future research is needed to understand the potential role of established and novel antiplatelet agents (especially ticagrelor), alone or in combinations, and the optimal duration of therapy in the management of patients with prior CVA.
Disclosure
The authors report no conflicts of interest in this work.
References
- HeuschmannPUDi CarloABejotYEuropean Registers of Stroke (EROS) InvestigatorsIncidence of stroke in Europe at the beginning of the 21st centuryStroke20094051557156319325154
- NicholsMTownsendNLuengo-FernandezREuropean Cardiovascular Disease Statistics 2012BrusselsEuropean Heart Network and European Society of Cardiology2012 Available from: http://www.escardio.org/about/documents/eu-cardiovascular-disease-statistics-2012.pdfAccessed February 22, 2014
- RogerVLGoASLloyd-JonesDMHeart disease and stroke statistics – 2012 update: a report from the American Heart AssociationCirculation20121251e2e22022179539
- DucroqGAmarencoPLabreucheJA history of stroke/ transient ischemic attack indicates high risks of cardiovascular event and hemorrhagic stroke in patients with coronary artery diseaseStroke20131276730738
- MukherjeeDEagleKAKline-RogersEGRACE InvestigatorsImpact of prior peripheral arterial disease and stroke on outcomes of acute coronary syndromes and effect of evidence-based therapies (from the Global Registry of Acute Coronary Events)Am J Cardiol200710011617599431
- BhattDLPetersonEDHarringtonRACRUSADE InvestigatorsPrior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromesEur Heart J200930101195120219339264
- BrilakisESHernandezAFDaiDQuality of care for acute coronary syndrome patients with known atherosclerotic disease: results from the Get With the Guidelines ProgramCirculation2009120756056719652090
- JensenJKMedinaHMNorgaardBLAssociation of ischemic stroke to coronary artery disease using computed tomography coronary angiographyInt J Cardiol2012160317117421543126
- SubherwalSBachRGChenAYBaseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) bleeding scoreCirculation2009119141873188219332461
- LiaoJKSecondary prevention of stroke and transient ischemic attack: is more platelet inhibition the answer?Circulation2007115121615162117389280
- WiviottSDBraunwaldEMcCabeCHPrasugrel versus clopidogrel in patients with acute coronary syndromesN Eng J Med20073572020012015
- WallentinLBeckerRCBudajATicagrelor versus clopidogrel in patients with acute coronary syndromesN Engl J Med2009361111045105719717846
- TricociPHuangZHeldCThrombin-receptor antagonist vorapaxar in acute coronary syndromesN Engl J Med20123661203322077816
- MorrowDABraunwaldEBonacaMPVorapaxar in the secondary prevention of atherothrombotic eventsN Engl J Med2012366151404141322443427
- International Stroke Trial Collaborative GroupThe International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischaemic strokeLancet19973499065156915819174558
- JauchECSaverJLAdamsHPJrGuidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/ American Stroke AssociationStroke201344387094723370205
- JohnsonESLanesSFWentworthCE3rdSatterfieldMHAbebeBLDickerLWA meta-regression analysis of the dose–response effect of aspirin on strokeArch Intern Med1999159111248125310371234
- FurieKLKasnerSEAdamsRJGuidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/ American Stroke AssociationStroke201142122727620966421
- HerbertJMSaviPP2Y12, a new platelet ADP receptor, target of clopidogrelSemin Vasc Med20033211312215199474
- SaviPPereilloJMUzabiagaMFIdentification and biological activity of the active metabolite of clopidogrelThromb Haemost200084589189611127873
- CAPRIE Steering CommitteeA randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE)Lancet19963489038132913398918275
- DienerHCSaccoRLYusufSEffects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active, and placebo-controlled studyLancet Neurol200871087588418757238
- DienerHCBogousslavskyJBrassLMAspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trialLancet2004364943133133715276392
- BhattDLFoxKAHackeWClopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic eventsN Engl J Med2006354161706171716531616
- GUSTO InvestigatorsAn international randomized trial comparing four thrombolytic strategies for acute myocardial infarctionN Engl J Med1993329106736828204123
- WardlawJMWhat causes lacunar stroke?J Neurol Neurosurg Psychiatry200576561761915834013
- BenaventeORWhiteCLPearceLThe Secondary Prevention of Small Subcortical Strokes (SPS3) studyInt J Stroke20116216417521371282
- KennedyJHillMDRyckborstKJEliasziwMDemchukAMBuchanAMFASTER InvestigatorsFast assessment of stroke and transient ischemic attack to prevent early recurrence (FASTER): a randomized controlled pilot trialLancet Neurol200761196196917931979
- HankeyGJJohnstonSCEatonJDEffect of clopidogrel plus ASA vs ASA early after TIA and ischaemic stroke: a substudy of the CHARISMA trialInt J Stroke2011613921205234
- WangYWangYZhaoXClopidogrel with aspirin in acute minor stroke or transient ischemic attackN Engl J Med20133691111923803136
- JohnstonSCEastonJDFarrantMPlatelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial: rationale and designInt J Stroke20138647948323879752
- JakubowskiJAWintersKJNaganumaHWallentinLPrasugrel: a novel thienopyridine antiplatelet agent: a review of preclinical and clinical studies and the mechanicistic basis for its distinct antiplatelet profileCardiovasc Drug Rev200725435737418078435
- AngiolilloDJCapranzanoPPharmacology of emerging novel platelet inhibitorsAm Heart Journal2008156Suppl 2S10S1518657681
- WiviottSDAntmanEMGibsonCMEvaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet inhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38)Am Heart J2006152462763516996826
- RoeMTArmstrongPWFoxKAPrasugrel versus clopidogrel for acute coronary syndromes without revascularizationN Engl J Med2012367141297130922920930
- US Food and Drug AdministrationCardiovascular and Renal Drug Advisory Committee Briefing Document: Prasugrel for ACSSilver Spring, MDFood and Drug Administration2009 Available from: http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4412b1-01-FDA.pdfAccessed December 11, 2013
- HustedSvan GiezenJTicagrelor: the first reversibly binding oral P2Y12 receptor antagonistCardiovasc Ther200927425927419604248
- TengRButlerKPharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, in healthy subjectsEur J Clin Pharmacol201066548749620091161
- JamesSKStoreyRFKhurmiNSTicagrelor versus clopidogrel in patients with acute coronary syndromes and a history of stroke or transient ischemic attackCirculation2012125232914292122572911
- AngiolilloDJCapodannoDGotoSPlatelet thrombin receptor antagonism and atherothrombosisEur Heart J2010311172819948715
- MorrowDAAlbertsMJMohrJPEfficacy and safety of vorapaxar in patients with prior ischemic strokeStroke201344369169823396280
- LimMJEagleKAGoreJMTreating patients with acute coronary syndromes with aggressive antiplatelet therapy (from the Global Registry of Acute Coronary Events)Am J Cardiol200596791792116188516
- PalmeriniTBiondi-ZoccaiGDella RivaDStent thrombosis with drug eluting stents: is the paradigm shifting?J Am Coll Cardiol201362211915192124036025
- KolandaiveluKSwaminathanRGibsonWJStent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer–drug coatingsCirculation2011123131400140921422389
- PalmeriniTBiondi-ZoccaiGDella RivaDStent thrombosis with drug-eluting stent and bare-metal stents: evidence from a comprehensive network meta-analysisLancet201237998241393140222445239
- FlynnRWMacDonaldTMMurrayGDMacWalterRSDoneyASPrescribing antiplatelet medicine and subsequent events after intracerebral hemorrhageStroke201041112606261120947854
- ViswanathanARakichSMEngelCAntiplatelet use after intracerebral hemorrhageNeurology200666220620916434655
