Abstract
Obinutuzumab is a novel glycoengineered type II anti-CD20 monoclonal antibody with a higher affinity for CD20 epitope, enhanced antibody-dependent cellular cytotoxicity and direct cell death, leading to superior cytotoxicity compared with rituximab. The approval of obinutuzumab by US Food and Drug Administration was based on a pivotal, phase III, randomized trial of chlorambucil monotherapy (n=118), chlorambucil plus obinutuzumab (n=333), or chlorambucil plus rituximab (n=330) in previously untreated patients with CLL. Obinutuzumab was administered intravenously as 1,000 mg on days 1, 8, and 15 of cycle 1 and day 1 of subsequent cycles. Obinutuzumab plus chlorambucil was associated with an overall response rate of 78% and a median progression-free survival of 26.7 months. Overall, obinutuzumab was fairly well tolerated in this pivotal study. The incidence of grade 3 or higher adverse events was infusion-related reactions (20%), neutropenia (33%), thrombocytopenia (10%), and infections (7%). Obinutuzumab in combination with chlorambucil is a safe and effective new treatment option for previously untreated elderly patients with CLL. It should become the new standard of care for these patients with significant co-morbidities who are not candidates for fludarabine-based therapy. Obinutuzumab combination therapy with several agents that inhibit kinases involved in the B-cell receptor signaling pathway, as well as many other agents utilized in the frontline and relapsed/refractory setting, is currently under investigation. As the results from these studies become available, the role of obinutuzumab is expected to expand to other settings.
Introduction
The American Cancer Society estimates 14,620 new cases of chronic lymphocytic leukemia (CLL) to be diagnosed in the USA in 2015 with approximately 4,650 deaths predicted to occur.Citation1 CLL, the most common lymphoproliferative disorder in the Western countries, is characterized by progressive accumulation of mature lymphocytes in the peripheral blood, bone marrow, and lymphoid tissues. CLL usually manifests as lymphocytosis with characteristic phenotype on B-cells (CD5+ and CD23+).Citation2 Two separate staging systems (Rai and Binet) have been created to establish prognostic implications for survival. While specific differences exist between these systems, they generally take into account the site of disease involvement, presence of anemia and thrombocytopenia.Citation3,Citation4 New prognostic factors have been recently identified with the advent of molecular profiling. Genomic features such as unmutated immunoglobulin heavy-chain variable (IGHV) status and cytogenetic abnormalities such as del (17p), TP53 mutation and expression of CD38, and zeta-chain-associated protein kinase (ZAP-70) are associated with poor prognosis, including shorter progression-free survival (PFS) and overall survival (OS). Recently, mutations in NOTCH1, SF3B1, and BIRC3 genes were also found to be associated with poor prognosis.Citation5 CLL is mainly a disease of the elderly population with a median age at diagnosis of 72 years. Clinical manifestation of CLL can vary from a long-term indolent disease to a rapidly progressive disease with OS ranging from months to decades; nonetheless, it remains an incurable disease with currently available therapies with the exception of hematopoietic stem cell transplantation.Citation6
Management of CLL is usually reserved for patients with stage III or IV disease or those with bulky lymphadenopathy, hepatosplenomegaly, constitutional symptoms (fatigue, night sweats, fever without infection, weight loss), threatened end-organ function, progressive anemia (hemoglobin [Hgb] <10 g/dL) or thrombocytopenia (platelet <100×109/L). The choice of treatment depends on various factors such as patient fitness, clinical stage of the disease, cytogenetic abnormalities, prior therapies, and response to previous agents.Citation5,Citation7 The combination of fludarabine, cyclophosphamide, and rituximab (FCR) is currently recommended as first-line therapy for patients less than 70 years of age and without co-morbidities. Until recently, it was recommended that elderly patients (age ≥70 years) or those with significant co-morbidities should receive rituximab in combination with chlorambucil as frontline therapy.Citation5–Citation11 Although the addition of rituximab to chlorambucil has improved PFS and complete response (CR) rates compared with chlorambucil monotherapy, it did not result in survival benefit.Citation12 Other options include bendamustine, fludarabine or cyclophosphamide/prednisone ± rituximab, rituximab, cladribine, and chlorambucil. Elderly patients remain underrepresented in majority of the CLL studies, and available data have not shown superiority of one regimen over another until recently. Patients with del (17p) do not benefit from these regimens, and alemtuzumab-containing regimens as well as some novel targeted therapies are the only effective options for these patients.Citation5 Rituximab, the first monoclonal antibody against CD20 antigen expressed on the surface of the human B cells, was approved in 2010 for previously untreated CLL. Its discovery revolutionized the treatment of CD20+ lymphoproliferative disorders after its initial approval in 1997; however, majority of the patients with CLL will eventually relapse after rituximab-containing immunochemotherapy, which highlights the need for developing superior therapeutic options.Citation13,Citation14 Ofatumumab, a second-generation anti-CD20 monoclonal antibody, was approved in 2009 for refractory CLL and recently, in April 2014, for previously untreated CLL.Citation15 On November 1, 2013, obinutuzumab, a third-generation anti-CD20 monoclonal antibody, became the first treatment approved with US Food and Drug Administration’s (FDA) breakthrough designation for use in combination with chlorambucil as a first-line therapy for previously untreated CLL.Citation16
Pharmacology
CD20 is expressed on B cells from pre-B-cell stage until post-germinal cells differentiate to become plasma cells. Because CD20 is neither shed nor internalized in normal B cells, it serves as an ideal target for mature B-cell malignancies such as CLL.Citation6 Monoclonal antibodies generally have three possible mechanisms of action: 1) antibody-dependent cellular cytotoxicity (ADCC), 2) complement-dependent cytotoxicity (CDC), and 3) direct growth inhibition and apoptosis, also known as direct cell death ().Citation17 Anti-CD20 monoclonal antibodies are classified as type I or type II based on their mode of CD20 binding and primary mechanism for catalysis. Type I antibodies (rituximab and ofatumumab) cause translocation of CD20 into lipid rafts within the cell membrane, which results in efficient CDC, with ofatumumab exhibiting stronger CDC.Citation18–Citation20
Figure 1 Schematic representation of the putative mechanisms mediating rituximab’s anticancer activity in NHL cells.
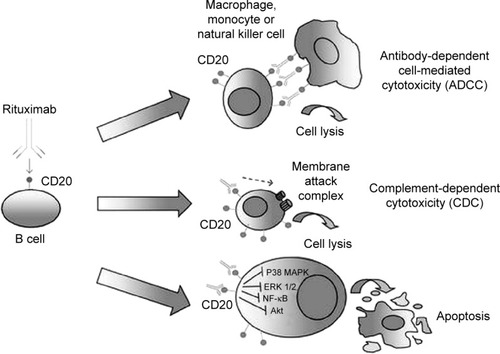
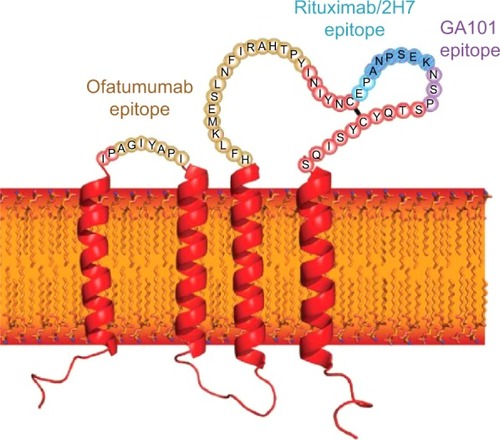
Type II antibodies (obinutuzumab), however, do not provoke localization of CD20 into lipid rafts; hence, it results in 10–1,000-fold less potent CDC compared with type I antibodies. ADCC is mediated by interaction between Fc region of the anti-CD20 antibody and FcγRIIIα (expressed on various immune effector cells). Obinutuzumab is a type II humanized CD20 IgG1 monoclonal antibody with glycoengineered Fc region, which leads to 100-fold greater ADCC compared with rituximab and ofatumumab. It also binds to CD20 epitope in a different space orientation and with a wider elbo-hinge angle compared with rituximab (). Obinutuzumab also mediates phagocytosis and superior induction of direct cell death compared with rituximab. Unlike type I monoclonal antibodies, the activity of obinutuzumab does not depend on classic apoptotic pathways, so it may have the ability to overcome typical apoptosis resistance mechanisms.Citation21,Citation22
Figure 2 The structure and topology of CD20 and the epitopes recognized by rituximab, ofatumumab, and obinutuzumab.
Pharmacokinetics
Based on the population pharmacokinetics, mean volume of distribution, terminal clearance, and half-life of obinutuzumab are approximately 3.8 L, 0.09 L/day, and 28 days, respectively. The metabolism of obinutuzumab has not been clearly studied; however, antibodies are mainly cleared via catabolism. The elimination of obinutuzumab follows a complex, compartment model and consists of a linear and time-dependent, saturable, nonlinear clearance pathway. The time-dependent, nonlinear clearance pathway is predominant during therapy initiation, but as treatment continues, linear clearance pathway predominates, suggesting target drug–mediated deposition.Citation18 The relationship between pharmacokinetics, clinical response and/or tumor burden has been inconclusive in Phase I/II trials, although Phase II studies reported worse response rates in patients with higher tumor burden, highlighting the importance of dosing schedule for optimal efficacy.Citation23 The volume of distribution and steady-state clearance are expected to increase with body weight, but this is associated with minimal change in overall exposure; hence, dose modification is not recommended. Obinutuzumab has not been tested in patients with severe renal impairment (CrCL ≤30 mL/min) and those with hepatic impairment; however, dose modifications are not expected since monoclonal antibodies are expected to be cleared via proteolytic enzymes.Citation16
Therapeutic potential of obinutuzumab
In vitro studies comparing rituximab and obinutuzumab have demonstrated variable responses to individual CLL sample, although CLL cell depletion from whole blood by obinutuzumab was consistently stronger than rituximab.Citation2,Citation18 Several Phase I/II studies have demonstrated clinical activity of obinutuzumab in CLL (). In a Phase I study of 22 heavily pretreated patients with relapsed non-Hodgkin’s lymphoma or CLL, obinutuzumab (200–2,000 mg) was administered as induction therapy weekly for 4 weeks. Patients who did not progress after induction therapy continued to receive obinutuzumab every 3 months for a maximum of eight doses. Five patients had CLL, all of whom were refractory to prior fludarabine therapy and received a median of four prior therapies. Separate results for patients with CLL are not available. At the end of induction, obinutuzumab therapy yielded a partial response (PR) of 23%.Citation24
Table 1 Efficacy of obinutuzumab in CLL
Table 3 Ongoing or planned studies of obinutuzumab in CLL
In the Phase Ib Galton study, a total of 41 previously untreated patients with CLL were randomized to receive obinutuzumab plus fludarabine and cyclophosphamide (n=21) or bendamustine (n=20) for six 28-day cycles. Obinutuzumab in combination with fludarabine and cyclophosphamide yielded an overall response rate (ORR) of 62% (CR =10%) compared with 90% (CR =20%) when combined with bendamustine. With the median follow-up of 23.5 months in one cohort and 20.7 months in the other cohort, none of the patients have relapsed or died. This study confirmed that obinutuzumab could be safely administered with standard chemotherapeutic regimens for the treatment of previously untreated patients with CLL.Citation25,Citation26
GAUGUIN was a Phase I/II study that evaluated the safety and efficacy of obinutuzumab monotherapy in heavily pretreated patients (median of three prior therapies) with relapsed/refractory (R/R) CLL. In the Phase I dose escalation study, obinutuzumab was administered to 13 patients as a flat dose ranging from 400 mg to 2,000 mg (days 1 and 8 of cycle 1; day 1 of cycles 2–8). Approximately 33% of the patients had high-risk cytogenetics (del [17p] or del [11q]) and approximately 70% of the patients had unmutated IGHV status. Obinutuzumab monotherapy yielded an ORR of 62%, all of which were PRs. The median duration of response (DOR) was 10.5 months at 38.7 months follow-up. Based on the preliminary efficacy data as well as modeling and simulation of pharmacokinetic data, which showed faster elimination of obinutuzumab in the first cycle than later cycles, a higher dose was selected for Phase II study. Obinutuzumab was administered as 1,000 mg intravenously (days 1, 8, and 15 of cycle 1; day 1 of cycle 2–8) for ten infusions to a total of 20 patients with R/R CLL. Of the 16 evaluable patients, the best ORR was 30% with one patient achieving a CR. At a median follow-up of 28.8 months, median PFS and median DOR in responders were 10.7 months and 8.9 months, respectively. Less-impressive response rates in the Phase II of this study were thought to be related to higher tumor burden of enrolled patients, suggesting that doses higher than 1,000 mg may be necessary in this specific patient population. Similar to outcomes seen with ofatumumab, a trend for a dose–response relationship was seen in Phase I of this study. Based on all the available clinical data in B-cell malignancies in Phase I/II studies as well as modeling and simulation, a flat dose of 1,000 mg was chosen to be administered on days 1, 8, and 15 of the first cycle for Phase III studies to rapidly achieve and maintain adequate drug levels.Citation23,Citation27–Citation29
Since earlier Phase I/II studies suggested a dose–response relationship with obinutuzumab, GAGE study (Phase II) was designed to evaluate the safety and efficacy of two doses of obinutuzumab in 80 patients with previously untreated CLL. Of the enrolled patients, 54% had unmutated IGHV status and 10% had del (17p). Obinutuzumab was administered as 1,000 mg (100 mg day 1; 900 mg day 2; 1,000 mg days 8 and 15 of cycle 1; 1,000 mg day 1 of cycles 2–8) or 2,000 mg (100 mg day 1; 900 mg day 2; 1,000 mg day 3; 2,000 mg days 8 and 15 of cycle 1; 2,000 mg day 1 of cycles 2–8) every 21 days. Preliminary analysis at 11 months showed an ORR of 49% and 67% in patients receiving obinutuzumab 1,000 mg and 2,000 mg, respectively (P=0.08).Citation30
CLL11 was a Phase III, randomized, controlled, open-label study that enrolled 781 previously untreated patients with CLL with co-morbidities in a three-arm, two-stage trial. The study was conducted to assess whether the addition of either rituximab or obinutuzumab could enhance the efficacy of chlorambucil monotherapy in elderly, previously untreated patients with CLL. Notable eligibility criteria included a Cumulative Illness Rating Scale (CIRS) total score >6 or CrCl of 30–69 mL/min. Notable exclusion criteria included inadequate liver function, positive hepatitis (hepatitis B or C) serology, or immunization to live vaccine within 28 days prior to randomization. Patients were randomized to receive either obinutuzumab plus chlorambucil (G-Clb; stage 1, n=238; stage 2, n=333) or rituximab plus chlorambucil (R-Clb; stage 1, n=233; stage 2, n=330) or chlorambucil alone (stage 1, n=118) in six 28-day cycles. Chlorambucil was administered orally at a dose of 0.5 mg/kg on days 1 and 15 of each cycle. Obinutuzumab was administered intravenously as 1,000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of cycles 2–6. To ameliorate the infusion-related reactions (IRRs) seen in 89% of the first 53 patients receiving obinutuzumab in an earlier study, the protocol was amended to administer first obinutuzumab infusion over 2 days. The first dose was split between day 1 (100 mg) and day 2 (900 mg) and was administered to 42% of the patients. Rituximab was administered intravenously at 375 mg/m2 on day 1 of cycle 1 and 500 mg/m2 on day 1 of cycles 2–6. Dose modifications were not permitted. Enrolled patients represented a homogenous population that is representative of majority of the new CLL diagnoses. The median age of enrolled patients was 73 years. Of note, approximately 60% of the patients in each group had unmutated IGHV status.Citation31 The primary endpoint of the study was PFS, and key secondary endpoints included response rates, minimal residual disease, and OS. At 3 months after the end of treatment, compared with chlorambucil monotherapy, the median PFS was significantly prolonged for patients receiving obinutuzumab and chlorambucil (11.1 months vs 26.7 months, hazard ratio [HR] =0.18, P<0.001) and those receiving rituximab and chlorambucil (11.1 months vs 16.3 months, HR =0.44, P<0.001). Similar benefit was seen in all subgroups except in patients with del (17p). G-Clb resulted in significantly prolonged PFS compared with R-Clb (HR =0.39, P<0.001). The OS was also prolonged in patients receiving G-Clb compared with those receiving chlorambucil alone (rates of death: 20% vs 9%, HR =0.41, P=0.002). Survival benefit with G-Clb over R-Clb was not observed (HR =0.66, P=0.08); however, longer follow-up may be required to observe the mortality benefit. Median OS was not reached in either of the combination therapy arms. G-Clb yielded higher overall, complete, and molecular response rates compared with R-Clb ().Citation31 Results of this pivotal trial led to the approval of obinutuzumab by FDA in November 2013.
Table 2 Efficacy and safety outcomes from CLL11
Updated analysis of CLL11 with a data cutoff of April 2014 was recently published. As seen in the earlier analysis, the median PFS continued to remain significantly prolonged with G-Clb compared with R-Clb (29.2 vs 15.4 months, HR =0.40, P<0.001). Updated analysis, however, did not show any significant difference in OS between G-Clb and R-Clb (HR =0.70, P=0.06). This is likely secondary to the small number of death events in both arms (45 of 333 patients died in the G-Clb arm; 63 of 330 patients died in the R-Clb arm); hence, the data still may require further follow-up to show mortality benefit between the anti-CD20 monoclonal antibodies. Previously observed OS benefit of G-Clb over chlorambucil monotherapy was again confirmed (HR =0.47, P=0.0014); however, unlike previous analysis, updated analysis showed prolonged OS with R-Clb compared with chlorambucil monotherapy (HR =0.60, P=0.0242). Even though majority of the patients initially receiving chlorambucil monotherapy crossed over to either G-Clb or R-Clb arm as next-line agents, mortality benefit of G-Clb and R-Clb over chlorambucil monotherapy was still maintained. Time to next antileukemic treatment was longer with G-Clb compared with R-Clb (42.7 vs 32.7 months, HR =0.54, P<0.001).Citation32 A separate subgroup analysis of CLL11 was conducted to evaluate the efficacy and safety of obinutuzumab in the setting of R/R CLL. A total of 30 patients who initially received chlorambucil monotherapy developed progressive CLL within 6 months of chlorambucil monotherapy were treated with G-Clb (dosage and administration same as CLL11) for six cycles. At the end of the treatment, the ORR was 87% (CR =7%, incomplete CR =3%, and PR =77%), and the PFS was 17.2 months. The results of this subgroup analysis suggest the role of G-Clb for patients with CLL who have failed prior chlorambucil monotherapy.Citation33
Safety
Overall, obinutuzumab is fairly well tolerated with the most common adverse events (AEs) being IRRs and hematological toxicities. In one of the Phase I studies, the most common grade 3–4 AEs included neutropenia (48%–55%) and IRRs (20%).Citation26 In the Phase II part of the GANGUIN trial, obinutuzumab was well tolerated with no dose-limiting toxicities reported over the 400–2,000 mg dose range. The most common AEs included IRRs (any grade =95%; grade 3 =30%), all of which occurred during the first infusion. Grade 3–4 hematological AEs included neutropenia (25%) and thrombocytopenia (15%). Of note, in Phase I/II GANGUIN study, both early-and late-onset neutropenia were reported. Of the 13 patients with grade 3–4 neutropenia, six had a first onset within 30 days of treatment exposure. Infections were reported in 30% of the patients; however, only 15% of all infections were grade 3 (bacterial, herpes zoster, and testicular abscess). None of the infections were grade 4.Citation23
Higher dose of obinutuzumab has not been associated with any additional safety concerns. In the Phase I/II GAGE trial, obinutuzumab 2,000 mg dosing schema was associated with grade 3–4 IRR of 11% compared with 23% associated with the standard, FDA approved, 1,000 mg dosing schema. The reason why higher dose was associated with a lower incidence of IRR remains to be investigated. The incidence of grade 3–4 neutropenia and infections was similar between both dosing schemas.Citation30
In the CLL11 trial, the most frequent grade 3 or higher AEs in the G-Clb arm included IRRs (20%), infections (12%), and hematological AEs such as neutropenia (33%), thrombocytopenia (10%), anemia (4%), and leukopenia (4%) (). The incidence of these AEs was similar with R-Clb except for lower rates of thrombocytopenia (3%) and IRRs (4%). With regards to grade 3–4 infections, no significant differences were found between three treatment arms despite a higher incidence of neutropenia with G-Clb. All grade 3–4 IRRs occurred during the first infusion of obinutuzumab, which led to discontinuation of therapy in 8% of the patients. The incidence of IRRs decreased significantly with subsequent therapy, 3% with second dose and <1% with subsequent doses. No grade 3–4 IRRs were reported beyond first dose. Of note, no significant difference in the IRRs was seen between G-Clb and R-Clb arms in the stage II of CLL11 trial; however, the incidence was higher in G-Clb group compared with R-Clb group (20% vs 4%, respectively). First administration of obinutuzumab has been shown to trigger immediate and strong release of cytokines (IL6, IL8, TNFα, IFNγ, and IL10) associated with a rapid destruction of circulating B cells, hence demonstrating a close temporal relationship between the release of cytokines and development of IRRs. When compared with rituximab, obinutuzumab results in more potent cytotoxicity and rapid B-cell depletion.Citation23,Citation34 In an exploratory analysis of CLL11 evaluating risk factors associated with IRR, type of treatment received, tumor burden, target antigen expression, and FCγR gene polymorphism were identified as potential risk factors. Patients with high tumor burden and/or high circulating lymphocyte count (>25×109/L) are at risk for tumor lysis syndrome and should receive appropriate pharmacologic prophylaxis.Citation35 In the pivotal trial, the incidence of tumor lysis syndrome was higher with obinutuzumab (4%) compared with rituximab (<1%). Other serious AEs included newly diagnosed neoplasms; however, the rates were comparable with both monoclonal antibodies. Updated analysis in April 2014 did not reveal any new safety concerns. A total of 4% of the patients died in the G-Clb arm due to AEs compared with 6% in the R-Clb arm.Citation31,Citation32
Obinutuzumab labeling includes a black box warning for hepatitis B reactivation and progressive multifocal leukoencephalopathy. Anti-CD20 monoclonal antibodies are associated with the highest risk of hepatitis B reactivation; hence, patients should be screened for risk of hepatitis B reactivation, and if at risk, antiviral therapy should be initiated to prevent this potentially fatal complication.Citation16,Citation36
Dosage and administration
Obinutuzumab is administered as an intravenous infusion for six 28-day cycles. The total dose is 1,000 mg with the exception of the first dose for cycle 1, which should be administered as 100 mg on day 1 and 900 mg on day 2. The first dose of the first cycle should be administered at 25 mg/h. Similar to rituximab, the second dose of cycle one can be titrated up to 50 mg/h every 30 minutes to a maximum rate of 400 mg/h. Subsequent infusions can be started at 100 mg/h and increased by 100 mg/h every 30 minutes up to a maximum rate of 400 mg/h. All patients should be premedicated with acetaminophen (650 – 1000 mg), intravenous glucocorticoid (dexamethasone 20 mg or methylprednisolone 80 mg), and an antihistamine (diphenhydramine 50 mg) prior to the first two infusions. For subsequent infusions, premedication with an antihistamine, for history of grade ≥1 IRR, and intravenous glucocorticoid, for history of grade ≥3 IRR, or a lymphocyte count >25 × 109/L, is recommended.Citation16
Formulary considerations
Obinutuzumab is supplied as a 1,000-mg single-use vial, which is recommended to be diluted in adequate amount of 0.9% sodium chloride to yield a final concentration of 0.4–4 mg/mL. Diluted solution for infusion should be used immediately; however, it can be stored up to 24 hours at 2°C–8°C followed by 48 hours at room temperature.Citation16 The current average wholesale prices for 1,000 mg of obinutuzumab (single vial) and rituximab (two 500-mg vials) are US$6,192 and US$8,233, respectively.Citation37 For a person with a body surface area of 2 m2, the cost of obinutuzumab for the first cycle will be approximately 56% higher than that of rituximab; however, the overall cost is fairly similar for six cycles of treatment duration.
Conclusion
Obinutuzumab is approved by US FDA for use in combination with chlorambucil in previously untreated patients based on the results of CLL11. Obinutuzumab in combination with chlorambucil should be considered the new standard of care for previously untreated patients with CLL with co-morbidities that are not eligible for fludarabine-based therapies as it is the first study to show survival benefit over another regimen in this patient population. The response rates, however, remain grim for patients with del (17p); hence, ibrutinib, a Bruton’s Tyrosine Kinase inhibitor, remains an important treatment option as frontline therapy for patients with del (17p). However, it is associated with an increased risk of atrial fibrillation and bleeding complications.Citation5
Combination chemoimmunotherapy with FCR is associated with an ORR of 90% (CR =44%) and median PFS of 4.8 years; hence, it remains the standard of care for previously untreated, younger, fit patients.Citation5–Citation11 Whether the substitution of obinutuzumab for rituximab in the FCR regimen results in better outcomes is currently under investigation (NCT01300247). Obinutuzumab-based combination therapies are being studied as frontline and salvage therapy for R/R CLL () as well as other subtypes of non-Hodgkin’s lymphomas. Compared with chlorambucil monotherapy, bendamustine and alemtuzumab in combination with rituximab have yielded superior response rates and PFS in previously untreated elderly patients with CLL. Alemtuzumab monotherapy has also shown superior outcomes in patients with del (17p) compared with chlorambucil monotherapy. Several combinations of obinutuzumab and bendamustine are currently under evaluation in various stages of clinical trial; however, there are currently no trials evaluating efficacy and safety of alemtuzumab plus obinutuzumab, an important area of investigation for future.Citation8,Citation38–Citation41
Increasing evidence shows that antigen-dependent and antigen-independent B-cell receptor (BCR) signaling and proteins in the B-cell CLL/lymphoma 2 (Bcl-2) family play a central role in the pathogenesis of CLL. Understanding of this pathophysiology has recently led to the development and approval of two targeted kinase therapies, ibrutinib and idelalisib.Citation38 Obinutuzumab combination therapy is under investigation with several agents that inhibit kinases involved in the BCR signaling pathway ().
Ofatumumab, a different anti-CD20 monoclonal antibody, was recently approved for use in combination with chlorambucil for previously untreated patients with CLL based on the results of a Phase III trial demonstrating an ORR of 82% and median PFS of 22.4 months. Ofatumumab plus chlorambucil (O-Clb) failed to show a survival benefit over chlorambucil monotherapy.Citation42 Ladyzynski and colleagues recently published a Bayesian network meta-analysis of randomized clinical trials involving treatment-naive, symptomatic patients with CLL. Of all the treatment options evaluated in the network analysis (chlorambucil, fludarabine, O-Clb, R-Clb, and G-Clb), G-Clb was found to be the most effective therapy with respect to projected mean PFS and was associated with the highest potential of prolonging OS of patients.Citation43 Reyes and colleagues recently presented results of a study comparing the cost-effectiveness of G-Clb versus O-Clb using a Markov model. G-Clb use was associated with an increase of 0.83 life years and 0.79 quality-adjusted life year as well as US$4,500 gain in incremental cost per quality-adjusted life years relative to O-Clb. Treatment with O-Clb was higher by US$3,600 per patient relative to G-Clb. This analysis suggested that treatment with G-Clb is highly cost-effective compared with O-Clb.Citation44
As the place in therapy for obinutuzumab expands, head-to-head comparison with ofatumumab in addition to other agents commonly used as frontline therapy and in relapsed/refractory setting (bendamustine plus rituximab) will become critical. Except for CLL11, no other well-designed, randomized, Phase III trial to date has shown that targeting CD20 antigen in patients with CLL and coexisting conditions results in improved survival. Survival benefit was not seen when G-Clb and R-Clb were compared; however, G-Clb was superior in achieving better minimal residual disease negative, which has been predicted to be associated with longer OS in patients receiving fludarabine-based chemoimmunotherapy. With longer patient follow-up, this difference may become apparent. Results of ongoing studies will be critical in determining whether obinutuzumab in combination with targeted kinase inhibitors such as ibrutinib and idelalisib will further enhance efficacy without significant impact on AEs.
Disclosure
The author reports no conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts and Figures 2015Atlanta, GAAmerican Cancer Society2015
- DesaiAEl-BakkarHAbdul-HayMNovel agents in the treatment of chronic lymphocytic leukemia: a review about the futureClin Lymphoma Myeloma Leuk201515631432225445466
- BinetJLAuquierADighieroGA new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysisCancer1981481982067237385
- RaiKRSawitskyACronkiteEPChananaADLevyRNPasternackBSClinical staging of chronic lymphocytic leukemiaBlood1975462192341139039
- National Comprehensive Cancer NetworkNon-Hodgkin’s Lymphoma. Version 2. 2015. NCCN Clinical Practice Guidelines in Oncology Available from: http://www.nccn.orgAccessed March 1, 2015
- JaglowskiSMAlinariLLapalombellaRMuthusamyNByrdJCThe clinical application of monoclonal antibodies in chronic lymphocytic leukemiaBlood2010116193705371420610811
- HallekMChronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatmentAm J Hematol201388804816
- ChesonBDMonoclonal antibody therapy of chronic lymphocytic leukemiaBest Pract Res Clin Hematol201023133143
- HallekMFingerle-RowsonGFinkA-MFirst-line treatment with fludarabine, cyclophosphamide, and rituximab improves overall survival in previously untreated patients with advanced chronic lymphocytic leukemia: results of a randomized phase III trial on behalf of an International Group of Investigators and the German CLL Study GroupBlood200911422535 ASH Annual Meeting Abstracts19451549
- EichhorstBDreylingMRobakTMonteserratEHallekMChronic lymphocytic leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow upAnn Oncol201122suppl 6vi50vi5421908504
- FischerKJBahloAMFinkRExtended follow up of the CLL8 protocol, a randomized phase III trial of the German CLL Study Group comparing fludarabine and cyclophosphamide (FC) to FC plus rituximab (FCR) for previously untreated patients with CLL: results on survival, progression free survival, delayed neutropenia and secondary malignancies confirm superiority of FCR regimenBlood2012120435 ASH Annual Meeting Abstracts
- HillmenPGribbenJGFollowsGARituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: final analysis of an open-label phase II studyJ Clin Oncol201432121236124124638012
- Roche Pharmaceutical IncProduct Information. Rituximab (Rituxan)South San Francisco, CARoche Pharmaceutical Inc1997
- CartronGTrappeRUSolal-CelignyPHallekMInterindividual variability of response to rituximab: from biological origins to individualized therapiesClin Cancer Res2011171193021208903
- Glaxo Group LimitedProduct Information. Ofatumumab (Arzerra)Brentford, UKGlaxo Group Limited2009
- Genentech IncProduct Information Obinutuzumab (Gazyva)South San FranciscoGenentech Inc2013
- SmolejLTargeted treatment of chronic lymphocytic leukemia: clinical potential of obinutuzumabPharmgenomics Pers Med201481725691812
- CerquozziSOwenCClinical role of obinutuzumab in the treatment of naïve patients with chronic lymphocytic leukemiaBiologics20159132225733804
- HoySObinutuzumab: a review of its use in patients with chronic lymphocytic leukemiaDrugs20157528529625586272
- KleinCLammensASchäferWEpitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional propertiesMABs201351223123211638
- GolayJDa RoitFBolognaLGlycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximabBlood20131222024822491
- RafiqSButcharJPCheneyCComparative assessment of clinically utilized CD-20 directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage propertiesJ Immunol20131902702271123418626
- CartronGde GuibertSDilhuydyMSObinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase I/II GAUGUIN studyBlood2014124142196220225143487
- SehnLHAssoulineSESteartDA phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignanciesBlood2012119225118512522438256
- BrownJRO’BrienSKingsleyCDSafety and efficacy of obinutuzumab with fludarabine/cyclophosphadmide or bendamustine in the initial therapy of patients with chronic lymphocytic leukemia: results from phase 1b Galton trial (GA04779g) abstract 52355th American Society of Hematology Annual Meeting and ExpositionSan Francisco2013
- BrownJRO’BrienSKingsleyCDObinutuzumab (G) plus fludarabine/cyclophosphamide (F-GC) or bendamustine (G-B) in the initial therapy of CLL patients: the phase 1b GALTON trialBlood20151251822792785
- CartronGde GuibertSDilhuydyMSPhase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemiaBlood200911422884 ASH Annual Meeting Abstracts
- CartronGMorschhauserFThieblemontCResults from a phase II study of obinutuzumab monotherapy in relapsed/refractory CLL [abstract]Haematologica201196suppl 239 Abstract 0101
- MorschhauserFSallesGCartronGDose selection for phase III studies of the monoclonal anti-CD20 antibody obinutuzumab (GA101) – a rational approachHaematologica201196suppl 2390
- FlynnJMByrdJCKippsTJObinutuzumab (GA101) 1,000 mg versus 2,000 mg in patients with chronic lymphocytic leukemia (CLL): results of the phase II GAGE (GA04768g) trialJ Clin Oncol201432suppl 5 abstract 7083
- GoedeVFischerKBuschRObinutuzumab plus chlorambucil in patients with CLL and coexisting conditionsN Engl J Med20143701101111024401022
- GoedeVFischerKEngelkeAObinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of CLL11 studyLeukemia20152971602160425634683
- GoedeVEngelkeAFischerKSalvage therapy with obinutuzumab (GA101) plus chlorambucil (Clb) after treatment failure of Clb alone in patients with chronic lymphocytic leukemia and comorbidities: results of CLL11 study abstract 332756th American Society of Hematology Annual Meeting and ExpositionSan Francisco2014
- FreemanCLMorschhauserFSehnLHPattern of cytokine release in patients with chronic lymphocytic leukemia treated with obinutuzumab and possible relationship with development of infusion related reactions (IRR)Blood2014124214674
- SachdevaMDhingraSObinutuzumab: a FDA approved monoclonal antibody in the treatment of untreated chronic lymphocytic leukemiaInt J Appl Basic Med Res201551545725664270
- PerrilloRMartinPLokAPreventing hepatitis B reactivation due to immunosuppressive drug treatmentsJAMA2015313161617161825790287
- Redbook [online database] Available from: http://www.redbook.com/redbook/onlineAccessed June 20, 2014
- Kharfan-DabajaMAWierdaWGCooperLJNImmunotherapy for chronic lymphocytic leukemia in the era of BTK inhibitorsLeukemia20142550751724157582
- HillmenPSkotnickiABRobakTAlemtuzuamb compared with chlorambucil as first-line therapy for chronic lymphocytic leukemiaJ Clinc Oncol2007253556165623
- StilgenbauerSDohnerHCampath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapyN Engl J Med200234745245312167696
- LozanskiGHeeremaNAFlinnIWAlemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletionsBlood20041033278328114726385
- HillmenPRobakTJanssensAChlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukemia (COMPLEMENT 1): a randomized, multicentre, open-label phase 3 trialLancet20153851873188325882396
- LadyzynskiPMolikMFoltynskiPA network meta-analysis of progression free survival and overall survival in first-line treatment of chronic lymphocytic leukemiaCancer Treat Rev2015412779325512118
- ReyesCGazauskasGBeckerUCost-effectiveness analysis of obinutuzumab versus ofatumumab for previously untreated chronic lymphocytic leukemia (CLL) [abstract 1324]56th American Society of Hematology Annual Meeting and ExpositionSan Francisco2014
- MottaGCeaMMoranEMonoclonal antibodies for non-Hodgkin’s lymphoma: state of the art and perspectivesClin Dev Immunol2010 Article ID 42825310.1155/2010/428253
- ShahAObinutuzumab: a novel anti-CD20 monoclonal antibody for previously untreated chronic lymphocytic leukemiaAnn Pharmacother201448101356136125037849
