Abstract
Background
Video-assisted thoracic surgery (VATS) has been shown to effectively reduce postoperative pain, enhance mobilization of the patients, shorten in-hospital length of stay, and minimize postoperative morbidity rates. The aim of this prospective study is to evaluate neuroendocrine and respiratory parameters as stress markers in cancer patients who underwent lung wedge resections, using both mini muscle-sparing thoracotomy and VATS approach.
Methods
The patients were randomly allocated into two groups: Group A (n=30) involved patients who were operated on using the VATS approach, while in group B (n=30), the mini muscle-sparing thoracotomy approach was used. Neuroendocrine and biological variables assessed included blood glucose levels, C-reactive protein (CRP) levels, cortisol, epinephrine, and adrenocorticotropic hormone (ACTH) levels. Arterial oxygen (PaO2) and carbon dioxide (PaCO2) partial pressure were also evaluated. All parameters were measured at the following time points: 24 hours preoperatively (T1), 4 hours (T2), 24 hours (T3), 48 hours (T4), and 72 hours (T5), after the procedure.
Results
PaO2 levels were significantly higher 4 and 24 hours postoperatively in group A vs group B, respectively (T2: 94.3 vs 77.9 mmHg, P=0.015, T3: 96.4 vs 88.7 mmHg, P=0.034). Blood glucose (T2: 148 vs 163 mg/dL, P=0.045, T3: 133 vs 159 mg/dL, P=0.009) and CRP values (T2: 1.6 vs 2.5 mg/dL, P=0.024, T3: 1.5 vs 2.1 mg/dL, P=0.044) were found increased in both groups 4 and 24 hours after the procedure. However, their levels were significantly lower in the VATS group of patients. ACTH and cortisol values were elevated immediately after the operation and became normal after 48 hours in both groups, without significant difference. Postoperative epinephrine levels measured in group A vs group B, respectively, (T2: 78.9 vs 115.6 ng/L, P=0.007, T3: 83.4 vs 122.5 ng/L, P=0.012, T4: 67.4 vs 102.6 ng/L, P=0.021). The levels were significantly higher in group B.
Conclusion
This study confirmed that minimally invasive thoracic surgery, by means of VATS, significantly reduces the acute-phase response and surgical stress, while enables better postoperative oxygenation.
Introduction
Not many people may argue that in the last decades thoracic surgery has made a very important step toward minimally invasive techniques by utilizing video-assisted thoracic surgery (VATS), with respect to both diagnosis and treatment of early-stage lung cancer, while numerous studies support the prevalence of VATS over the classic open thoracotomy approach.Citation1–Citation5 VATS lung resections are safe and equally effective as lung resections achieved using thoracotomy.Citation6,Citation7
The role of minimally invasive techniques in abdominal surgery and the impact they exert in metabolism, inflammatory, and immunological response has been well-described over the last years in the international literature.Citation8 Moreover, certain studies focused on their importance in thoracic surgery.Citation9 Increased surgical stress and systemic inflammatory response are associated with higher rates of morbidity and mortality. Furthermore, in cancer patients, open thoracotomy approach induces large immunologic changes, which may increase the rate of complications.Citation10,Citation11
The aim of this study was to evaluate in a prospective manner certain neuroendocrine and respiratory parameters, such as postoperative oxygenation and stress markers, in cancer patients who underwent lung wedge resections, using both mini muscle-sparing thoracotomy and VATS approach.
Patients and methods
From September 2012 to January 2014, 60 patients undergoing elective pulmonary sublobar wedge resections were enrolled in this prospective, single-institutional, randomized study. After study approval by the Theagenio Cancer Hospital Institutional Research Ethics Committee and after written informed consent was obtained from all patients, blinded randomization, using computer-generated random allocations, was performed. The patients were randomly assigned to one of two groups: Group A (n=30) involved patients who were operated on using the VATS approach, while in group B (n=30), the mini muscle-sparing thoracotomy approach was used ().
Demographic and preoperative patient data are shown in . Age <75 years, forced expiratory volume in 1 second ≥1 L, and American Society of Anesthesiologists Classification System ≤3 were defined as the inclusion criteria. Exclusion criteria involved emergency surgery and reoperation. The study is registered at ClinicalTrials.gov, number NCT01397045.
Table 1 Preoperative patients’ characteristics per treatment group assigned
Surgical strategy
In group B, access to the pleural cavity was obtained by a limited (4–5 cm) posterolateral thoracotomy, preserving latissimus dorsi and partially dividing serratus anterior muscle. In VATS approach group, three stab incisions were made over the lateral thoracic wall (three holes technique). The lowest hole was located at the middle axillary line just above the level of the diaphragm and served as the entrance point for a 0° fiber-optic camera. The other two incisions were carried out at the posterolateral and the anterolateral thoracic wall, under direct thoracoscopic vision, in such a way as to facilitate the access to the pulmonary lesions. In both groups, a 60 mm endostapler was used in order to perform pulmonary wedge sublobar resection. Two 32 French chest tubes were placed upon the completion of the operation.
Anesthetic and postoperative management
All patients received premedication and anesthesia induction using the same method. Tracheal intubation was achieved with the use of a double-lumen tube under bronchoscopic guidance in all cases. Patients’ arterial blood pressure, electrocardiogram, central venous pressure, oxygen saturation (SpO2), end-tidal carbon dioxide (ETCO2), and blood gas values were constantly monitored. Postoperative systemic analgesia was identical for both groups. All patients were mobilized from the first postoperative day, and the thoracic tubes were removed when output was <200 mL in 24 hours and no air leakage was present.
Study endpoints
Neuroendocrine and biological variables assessed included blood glucose levels, C-reactive protein (CRP) levels, cortisol, epinephrine, and adrenocorticotropic hormone (ACTH) levels. Arterial oxygen (PaO2) and carbon dioxide (PaCO2) partial pressure were also evaluated. All parameters were measured at the following time points: 24 hours preoperatively (T1) and 4 hours (T2), 24 hours (T3), 48 hours (T4), and 72 hours (T5), after the procedure. Significant perioperative variables were also recorded and analyzed: number of the segments resected, morphology of the lesion, major cardiopulmonary complications, in-hospital length of stay, and 30-day mortality. The primary endpoint of the study was the effect of VATS on epinephrine levels. Cortisol, ACTH, blood glucose, and CRP levels served as secondary endpoints.
Statistical analysis
Power analysis was employed to detect appropriate sample size. We have calculated that the average morbidity rate in our institution using mini muscle-sparing thoracotomy approach is about 5%. We supposed that implementation of VATS approach is expected to reduce the corresponding rates up to 30%. Setting the level of type I error (α) at 0.05 and the desired power of the study (1-β) at 80%, we calculated that each group should include at least 30 subjects (G*Power v. 3.1.2, Heinrich-Heine University, Düsseldorf, Germany). Statistical analysis was performed using the SPSS 17.0 software (SPSS, Chicago, IL, USA) for windows. Continuous data are presented as mean ± standard deviation, while categorical variables are presented as absolute numbers and percentages. Normal distribution was assessed by the Kolmogorov–Smirnov test. Univariate comparison of means was made using Student’s t- and Mann–Whitney U-tests when appropriate. Comparisons between percentages were assessed using the chi-square test. The level of statistical significance was set at 0.05.
Results
Perioperative clinical data are shown in . Duration of operation was shorter in group A; however, this did not reach statistical significance. The number of pulmonary segments resected was similar in both groups, as was as the morphology of the lesions. The pathology of each resected nodule is shown in . Tumor nodes metastasis staging of lung cancer patients and regimen of neoadjuvant therapy used is shown in . Furthermore, the location of resections did not significantly differ among the groups. No major cardiopulmonary complications or death were documented in the VATS group, while one patient died from pulmonary embolism on the sixth postoperative day in group B. Mean duration of hospitalization was significantly shorter in group A (5.1 vs 6.7 days, P=0.011). No conversion from VATS to open thoracotomy was necessary.
Table 2 Perioperative clinical parameters
Table 3 Pathology of resected nodules
Table 4 TNM staging of lung cancer patients and regimen of neoadjuvant therapy used
Preoperative values of neuroendocrine, biological, and respiratory variables were similar in both groups. Regarding respiratory parameters, PaO2 levels were significantly higher 4 and 24 hours postoperatively in group A (T2: 94.3 vs 77.9 mmHg, P=0.015, T3: 96.4 vs 88.7 mmHg, P=0.034), while Paco2 levels did not differ between the groups (). Blood glucose (T2: 148 vs 163 mg/dL, P=0.045, T3: 133 vs 159 mg/dL, P=0.009) and CRP values (T2: 1.6 vs 2.5 mg/dL, P=0.024, T3: 1.5 vs 2.1 mg/dL, P=0.044) were found to be increased in both groups 4 and 24 hours after the procedure. However, their levels were significantly lower in the VATS group of patients (). ACTH and cortisol values were elevated immediately after the operation and became normal after 48 hours in both groups, without significant difference (). Postoperative epinephrine levels measured (T2: 78.9 vs 115.6 ng/L, P=0.007, T3: 83.4 vs 122.5 ng/L, P=0.012, T4: 67.4 vs 102.6 ng/L, P=0.021) were significantly higher in group B ().
Figure 2 Blood gas levels.
Abbreviation: VATS, video-assisted thoracic surgery.
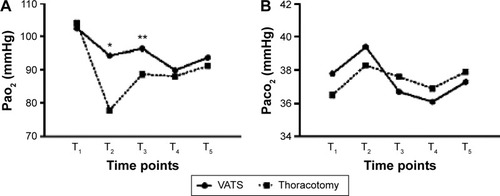
Figure 3 Glucose and c-reactive protein levels.
Abbreviations: VATS, video-assisted thoracic surgery; CRP, C-reactive protein.
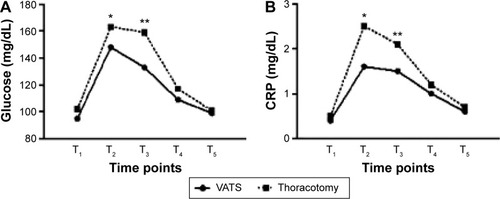
Figure 4 Hormone levels.
Abbreviations: VATS, video-assisted thoracic surgery; ACTH, adrenocorticotropic hormone.
Discussion
The role of VATS in the treatment of patients with pulmonary nodules has been upgraded during the last years, and the majority of thoracic surgeons consider it as the most suitable approach. The advantages of VATS over thoracotomy have been well-established by numerous studies. Less postoperative pain, better mobilization of the arm and shoulder, shorter duration of hospitalization, and less morbidity in contrast to thoracotomy characterize VATS procedures.Citation12–Citation14
All patients who received neoadjuvant therapy, as shown in , were initially operated on for malignancy located in organs other than the lungs (eg, breast, colon, etc). Therefore, these first operations were followed by neoadjuvant therapy when appropriate. Neoadjuvant therapy was performed before the pulmonary resection. The minimum time interval between the last therapy and the pulmonary resection was at least 1 month. It is unlikely that stress variables’ levels could be affected after this time interval. Moreover, baseline measurements were not statistically significant, and both groups included similar proportion of patients who had received neoadjuvant therapy.
This study confirmed that VATS is associated with significantly higher postoperative PaO2 values. Elevated PaO2 levels imply optimum oxygenation, possibly attributed to the reduced postoperative pain experienced in patients operated using the thoracoscopic approach. Lower PaO2 levels in the open-approach group correlate with ineffective postoperative respiratory function due to increased thoracotomy related pain.Citation15 PaCO2 levels recorded were similar in both groups. CO2 exchange in alveoli is independent from the approach used and does not relate to postoperative thoracotomy pain.Citation16
The stress response to surgery, with the production of mediators, cytokines, acute-phase proteins, adrenocorticoids, and catecholamines is influenced by the degree of surgical trauma, general anesthesia, and analgesics. Both general anesthesia and postoperative analgesic treatment were identical for all patients included in this study.
Lower insulin levels after induction of anesthesia and during surgery, along with decreased peripheral use of insulin due to perioperative increased “insulin resistance” effect, resulted in elevated concentration of blood glucose in surgical patients. Connection between elevated glucose levels after surgery and intensity of surgical injury has already been described.Citation17 In correlation with these findings, patients allocated to group B were characterized by significantly elevated blood glucose levels, which returned to normal on the third postoperative day. On the contrary, VATS group of patients experienced a milder postoperative hyperglycemia.
CRP is an acute-phase response marker produced in the liver. Along with interleukin-6, fibrinogen, and other proteins, CRP acts as an inflammatory mediator. Minimally invasive thoracic surgery, utilizing VATS, is associated with minor increase in serum levels of these mediators,Citation18 due to limited trauma. Reduced inflammatory response in group A was confirmed in this study.
Increase of ACTH during the operation results from stimulation of the anterior pituitary by hypothalamic releasing factors.Citation19 Surgery has been recognized as one of the most potent activators of ACTH secretion.Citation17 ACTH in turn stimulates the adrenal cortical secretion of glucocorticoids, thus contributing to elevated serum levels of cortisol. ACTH and cortisol levels are strongly related. No significant difference between two groups of this trial was recorded regarding postoperative measurements of these stress markers. As a result, the degree of surgical trauma itself does not significantly influence the stress response as far as ACTH and cortisol values are concerned. Identical changes in postoperative levels of these hormones in both groups are probably attributed to general anesthesia in combination with the extent of pulmonary resection.
Catecholamine levels increase as a result of the hypothalamic activation of the sympathetic autonomous nervous system and the stimulation of the adrenal medulla. Epinephrine is a strong stress indicator.Citation17 Postoperative epinephrine levels recorded in this study were significantly higher in group B in comparison with group A. In addition, even 72 hours after the procedure, these levels remained elevated. This finding is consistent with a strong correlation between the epinephrine concentration increase and the extent of the surgical trauma, which is also verified by several studies.Citation18
In conclusion, this study confirmed that minimally invasive thoracic surgery, by means of VATS, significantly reduces the acute-phase response and surgical stress, while enabling better postoperative oxygenation. Larger randomized trials are expected to illuminate the impact of these important findings on the treatment of cancer patients and establish VATS as the approach of choice.
Disclosure
The authors report no conflicts of interest in this work.
References
- CraigSRLeaverHAYapPLAcute phase responses following minimal access and conventional thoracic surgeryEur J Cardiothorac Surg20012045546311509263
- WhitsonBAD’CunhaJAndradeRSThoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunityAnn Thorac Surg2008861735174419021967
- KoizumiKHaraguchiSHirataTVideo-assisted lobectomy in elderly lung cancer patientsJpn J Thorac Cardiovasc Surg200250152211855094
- MuraokaMOkaTAkamineSVideo-assisted thoracic surgery lobectomy reduces the morbidity after surgery for stage I non-small cell lung cancerJpn J Thorac Cardiovasc Surg200654495516519128
- WhitsonBAAndradeRSBoettcherAVideo-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancerAnn Thorac Surg2007831965197017532379
- KirbyTJRiceTWThoracoscopic lobectomyAnn Thorac Surg1993567847868397498
- SugiKKanedaYEsatoKVideo-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancerWorld J Surg200024273010594199
- PuggioniAWongLLA metaanalysis of laparoscopic cholecystectomy in patients with cirrhosisJ Am Coll Surg200319792192614644279
- WalkerWSLeaverHAYapPLThe immune response to surgery: conventional and video assisted thorascopic pulmonary lobectomyYimAPCMinimal Access Cardiothoracic SurgeryPhiladelphia, PASaunders1999127134
- SietsesCHavenithCEEijsboutsQALaparoscopic surgery preserves monocyte-mediated tumor cell killing in contrast to the conventional approachSurg Endosc20001445646010858471
- SietsesCBeelenRHMeijerSImmunological consequences of laparoscopic surgery, speculations on the cause and clinical implicationsLangenbecks Arch Surg199938425025810437613
- WalkerWSMajor pulmonary resectionWalkerWSVideo-assisted Thoracic SurgeryOxford, UKIsis Medical Media1999135159
- YanTDBlackDBannonPGSystematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancerJ Clin Oncol2009272553256219289625
- YimAPWanSLeeTWVATS lobectomy reduces cytokine responses compared with conventional surgeryAnn Thorac Surg20007024324710921716
- CatleyDMThorntonCJordanCPronounced, episodic oxygen desaturation in the postoperative period: its association with ventilatory pattern and analgesic regimenAnesthesiology19856320284014768
- TschernkoEMHoferSBieglmayerCEarly postoperative stress: video-assisted resection/lobectomy vs conventional axillary thoracotomyChest1996109163616428769523
- DesboroughJPThe stress response to trauma and surgeryBr J Anaesth20008510911710927999
- WalkerWSLeaverHAImmunologic and stress responses following video-assisted thoracic surgery and open pulmonary lobectomy in early stage lung cancerThorac Surg Clin20071724124917626402
- LyonsFMMeeranKThe physiology of the endocrine systemInt Anesthesiol Clin1997351219444528