Abstract
Background
Acute coronary syndrome (ACS) is a fatal cardiovascular disease caused by atherosclerotic plaque erosion or rupture and formation of coronary thrombus. The latest guidelines for ACS recommend the combined drug regimen, comprising aspirin, P2Y12 inhibitor, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, β-blocker, and statin, at discharge after ACS treatment to reduce recurrent ischemic cardiovascular events. This study aimed to examine prescription patterns of secondary prevention drugs in Korean patients with ACS after hospital discharge, to access the appropriateness of secondary prevention drug therapy for ACS, and to evaluate whether to persistently use discharge medications for 18 months.
Methods
This study was retrospectively conducted with the patients who were discharged from the tertiary hospital, located in South Korea, after ACS treatment between September 2009 and August 2013. Data were collected through electronic medical record.
Results
Among 3,676 patients during the study period, 494 were selected based on inclusion and exclusion criteria. The regimen of aspirin + clopidogrel + β-blocker + angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker + statin was prescribed to 374 (75.71%) patients with ACS at discharge. Specifically, this regimen was used in 177 (69.69%) unstable angina patients, 44 (70.97%) non-ST-segment elevation myocardial infarction patients, and 153 (85.96%) ST-segment elevation myocardial infarction patients. Compared with the number of ACS patients with all five guideline-recommended drugs at discharge, the number of ACS patients using them 12 (n=169, 34.21%) and 18 (n=105, 21.26%) months after discharge tended to be gradually decreased.
Conclusion
The majority of ACS patients in this study received all five guideline-recommended medications at discharge from the hospital. However, the frequency of using all of them had been gradually decreased 3, 6, 12, and 18 months after discharge compared with that at discharge. Careful monitoring of adherence on ACS secondary prevention medications may help improve the outcomes of ACS patients in terms of recurrent ischemic cardiovascular events.
Introduction
Acute coronary syndrome (ACS) is a serious cardiovascular disease, which is usually caused by atherosclerotic plaque erosion or rupture and subsequent coronary thrombus formation due to platelet activation.Citation1,Citation2 ACS is classified into three different types: unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation MI (STEMI).Citation3 UA is referred to as the presence of ischemic symptoms without an increase in biomarkers and shows a transient change in electrocardiogram.Citation3 The term MI is employed to indicate myocardial necrosis in the condition of acute myocardial ischemia.Citation3 NSTEMI and STEMI are distinguished according to whether to present persistent ST-segment elevation on electrocardiogram.Citation3
According to the latest ACS guidelines and clinical trials, it is strongly recommended to follow the ACS treatment guidelines in order to prevent the recurrence of ischemic diseases and to improve the quality of life in patients discharged from hospitals after ACS treatment.Citation4–Citation6 The American Heart Association/American College of Cardiology guidelines published in 2014 recommend the long-term prescription of the combined drug regimens, including aspirin, P2Y12 inhibitor, angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB), β-blocker, and statin, in discharging patients after the completion of ACS treatment.Citation4 In particular, early initiation of reperfusion by using either thrombolytic therapy or percutaneous coronary intervention (PCI) in patients with STEMI is necessary to reduce myocardial infarct size and to enhance a survival rate.Citation7–Citation9
In case the recommended drugs (eg, aspirin, ACE-I, β-blocker, and statin) were persistently administered to ACS patients, the risk rate of future cardiovascular diseases and death would be likely to decrease by 75% within 2 years after ACS incidence.Citation6,Citation10 In the study conducted by Allonen et al,Citation11 the mortality rate of ACS patients who had regularly taken statins was reduced by nearly three times as compared with that of ACS patients who had not taken statins (4.9% vs 14.9%). Especially, the cardiovascular-related mortality rate was 2.9% in ACS patients with statins regularly administered, whereas the rate was 7.4% in those who had not taken statins. In the 1-year follow-up study conducted with 5,833 ACS patients by Yan et al,Citation12 the mortality rate after 1 year was significantly reduced in ACS patients discharged with antiplatelet or anticoagulant, β-blocker, ACE-I, and statin as compared with ACS patients discharged without them (odds ratio: 0.54; 95% confidence interval: 0.36–0.81; P=0.003). Additionally, Bi et alCitation13 reported that the recommended drug use rates in patients with acute MI or UA pectoris were high at discharge, but those were gradually decreased after 6 and 12 months.
The morbidity and mortality rates of ACS patients due to atherosclerotic plaque erosion or rupture can be reduced with the uses of antithrombotic agents and early revascularization.Citation1 Aspirin is a basic antithrombotic agent to be prescribed to patients with ACS, and P2Y12 receptor inhibitors such as clopidogrel, prasugrel, and ticagrelor are also prescribed to ACS patients as single or combined antithrombotic agents.Citation1 Besides these drugs, anticoagulants such as bivalirudin, unfractionated heparin, enoxaparin, and fondaparinux are administered to patients with ACS.Citation1 For example, in case of using enoxaparin in patients with ACS, the incidence rates of death, reinfarction, and recurrent angina were reduced after 30 days from 21% to 13% (P=0.03).Citation14
So far, almost all of the studies regarding discharge medication patterns and their follow-up evaluations after ACS treatment were conducted in foreign countries, and these studies were usually followed up until 12 months.Citation6,Citation10–Citation13,Citation15–Citation21 However, to our knowledge, similar studies have rarely been implemented in Korea. Thus, the objectives of this study were to examine prescription patterns of secondary prevention drugs in ACS patients after hospital discharge, to access the appropriateness of secondary prevention drug therapy for ACS, and to evaluate whether to persistently use discharge medications for 18 months.
Methods
Ethical approval for the study was received by the Institutional Review Board of Chosun University Hospital (CHOSUN 2014-10-015). Informed consents were not acquired from the study patients because their data were deidentified and encoded anonymously before analysis. This study was implemented retrospectively with the patients who were discharged from the same hospital after ACS treatment between September 2009 and August 2013. Chosun University Hospital is a tertiary health care institution located at Gwangju in South Korea, and it is equipped with 715 beds.
Study population
Among patients who were diagnosed with UA, NSTEMI, or STEMI during the study period and were discharged after ACS treatment, those who had outpatient clinic visits as part of routine care and met the following inclusion criteria were selected for this study: patients with ≥18 years of age and patients who had prescription information of ACS medications at discharge and 3, 6, 12, and 18 months after discharge.Citation5,Citation15 However, the following exclusion criteria were applied: patients who were not diagnosed with ACS at hospital admission, patients whose types of ACS were not recorded, patients without prescription information at discharge, patients who died during hospitalization, patients who were transferred from other hospitals, patients who did not have acute or current clinical symptoms, patients with secondary infarction due to anemia, and the same patients who were readmitted to the hospital during the study period.Citation5,Citation6,Citation15,Citation22
Data collection and processing
Through retrospective chart review of electronic medical records (EMRs) of patients, the following information was collected by a trained hospital pharmacist with paper case report forms: demographic characteristics (eg, date of birth, age, sex, height, weight, body mass index, and types of ACS), risk factors for ACS (eg, diabetes, hypertension, hyperlipidemia, renal failure, current smoker, family history, and obesity), underlying diseases (eg, MI, heart failure [HF], coronary artery bypass graft, PCI, transient ischemic attack, and stroke), and prescribed medications (eg, aspirin, clopidogrel, β-blocker, ACE-I or ARB, and statin) at discharge and during the follow-up period.Citation5,Citation15,Citation22,Citation23
In this study, the adherence of ACS guidelines to medications was defined as the combined prescription of the following five drugs: acetylsalicylic acid, P2Y12 inhibitor, β-blocker, ACE-I or ARB, and statin.Citation4,Citation7,Citation8 From the EMRs, information with regard to the prescription of them was abstracted at discharge and 3, 6, 12, and 18 months after discharge. When assessing the prescription at each time point, only prescription information available was collected. Since the pharmacists in the hospital were not allowed to fully access the EMRs of patients, information about contraindications to antiplatelet therapy (eg, active peptic ulceration and bleeding disorders), β-blocker (eg, bradycardia, hypotension, and uncontrolled HF), ACE-I or ARB (eg, angioedema and renal artery stenosis), and statins (eg, allergy) could not be obtained.Citation22 Any additional contraindications documented by the treating clinicians in the EMRs could not be recorded.
Statistical analysis
Demographic variables and clinical characteristics of patients selected in this study as well as prescribing rates were examined using descriptive statistics. Frequencies (n) and percentages (%) were utilized to present categorical variables, whereas mean and standard deviation were used for continuous variables. Chi-square test or Fisher’s exact test was performed to assess the differences in proportions. Student’s t-test or Wilcoxon rank sum test and analysis of variance test or Kruskal–Wallis test were used to compare means for between groups. However, before comparing means between groups, Shapiro–Wilk test was conducted in order to determine the normality of data. All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to be statistically significant.
Results
A total of 3,676 patients were hospitalized during the study period; 494 ACS patients met the inclusion and exclusion criteria and were included in the analysis (). summarizes the characteristics of ACS patients participating in the study. Three hundred and twenty-seven (66.19%) patients were males and 167 (33.81%) were females. The mean age of total patients was 67.71±10.92 years. Those patients with UA, NSTEMI, and STEMI were 68.17±10.48, 70.69±10.54, and 66.01±11.43 years, respectively. There was a statistically significant difference in age between three groups (P=0.0077). According to the analysis of risk factors for ACS, hypertension was reported in 306 (61.94%) patients, diabetes in 199 (40.28%) patients, and hyperlipidemia in 176 (35.63%) patients. In particular, 158 (31.98%) patients were current smokers. Of them, the numbers of patients with UA, NSTEMI, and STEMI were 55 (21.65%), 18 (29.03%), and 85 (47.75%), respectively. There was a statistically significant difference in current smoker between three groups (P<0.0001). According to the analysis of underlying diseases by types of ACS, there were statistically significant differences in previous MI (P=0.0095) and PCI (P<0.0001) between three groups.
Figure 1 Flow diagram of steps in the selection of study subjects.
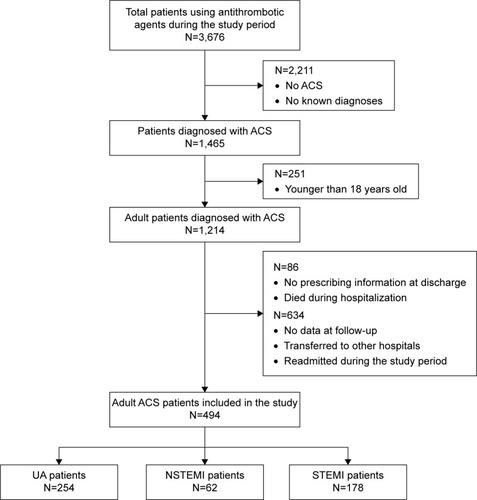
Table 1 Characteristics of study subjects according to types of ACS
The results of the analysis of the medications prescribed to ACS patients at discharge are summarized in . According to the classification of discharge medications by age and sex, aspirin had been most often prescribed compared with other discharge medications. Aspirin was also the discharge medication, which had been most often prescribed in UA, NSTEMI, and STEMI groups.
Table 2 Prescription frequency of the five medications for ACS secondary prevention according to the characteristics of study subjects
presents the drug regimens used in ACS patients at discharge based on types of ACS. The regimen of aspirin + clopidogrel + β-blocker + ACE-I/ARB + statin was prescribed to 374 (75.71%) patients with ACS at discharge. Specifically, this regimen was used in 177 (69.69%) UA patients, 44 (70.97%) NSTEMI patients, and 153 (85.96%) STEMI patients.
Table 3 Prescription patterns for the five medications for ACS secondary prevention according to types of ACS
shows the appropriate prescription of discharge mediations based on age, sex, and types of ACS. Overall, 374 (75.71%) of 494 patients with ACS received all five guideline-recommended medications at discharge from the hospital. When analyzed as the definition of ACS guideline adherence to medication by age, there was a statistically significant difference between guideline adherence and incomplete guideline adherence groups (P=0.0117). Two hundred and fifty-seven (68.72%) patients who received all five guideline-recommended drugs were males, and 117 (31.28%) were females, and there was a significant difference in sex between guideline adherence and incomplete guideline adherence groups (P=0.0364). The UA, NSTEMI, and STEMI patients with all five guideline-recommended drugs at discharge were 177 (47.33%), 44 (11.76%), and 153 (40.91%), respectively, and there was also a significant difference in types of ACS between guideline adherence and incomplete guideline adherence groups (P=0.0003).
Table 4 Appropriateness of discharge medications for ACS secondary prevention according to the characteristics of study subjects
summarizes the prescription patterns of secondary prevention medications for ACS at discharge and 3, 6, 12, and 18 months after discharge. Compared with the number of ACS patients with all five guideline-recommended drugs at discharge (n=374, 75.71%), the number of ACS patients using them 12 (n=169, 34.21%) and 18 (n=105, 21.26%) months after discharge tended to be gradually decreased. In particular, clopidogrel was prescribed to 445 (90.08%) ACS patients at discharge; however, it was used in 247 (50.00%) and 173 (35.02%) ACS patients 12 and 18 months after discharge, respectively. presents the regimens to be used for the purpose of secondary prevention after ACS at discharge and 3, 6, 12, and 18 months after discharge.
Table 5 Prescription frequency of the five medications for ACS secondary prevention at discharge and 3, 6, 12, and 18 months after discharge
Table 6 Prescription patterns for the five medications for ACS secondary prevention at discharge and 3, 6, 12, and 18 months after discharge
Discussion
In this study, the guideline adherence to secondary prevention medications for ACS patients at discharge and at 3, 6, 12, and 18 months between September 2009 and August 2013 was investigated. The majority of ACS patients included in this study received all five guideline-recommended medications at discharge from the hospital. However, the frequency of using all of them had been gradually decreased 3, 6, 12, and 18 months after discharge.
Cardiovascular diseases may be increased continuously due to increase in the prevalence of metabolic syndrome over time, since the aging index in Korea is expected to rise from 67.7% in 2010 to 213.8% in 2030.Citation24,Citation25 Therefore, in Korea, ACS is expected to become one of the most common conditions associated with ischemic heart disease, and ACS-related costs will be likely to increase steadily. It is essential to manage ACS early and effectively to prevent ACS-associated mortality and morbidity. According to the ACS guidelines, aspirin, β-blockers, and statins are recommended for life, whereas ACE-I/ARB should be administered to ACS patients with anterior infarction, HF, or ejection fraction ≤40%.Citation4,Citation7,Citation8 The ACS guidelines also recommend P2Y12 inhibitors for at least 12 months depending on stents placed.Citation8 Since the study on discharge medication patterns and their follow-up assessments after ACS treatment has been rarely conducted in Korea, it is meaningful in that this study could be useful in identifying the gaps between recommended and prescribed ACS medications in Koreans by 18 months from discharge.
Approximately 76% of ACS patients included in this study received all five guideline-recommended medications at discharge from the hospital. This level was a little higher than those found in other studies. According to the studies conducted in the Netherlands, about 65% and 69% of ACS patients were discharged with the guideline-recommended medications for ACS secondary prevention.Citation20,Citation23 The study conducted in Australia and New Zealand also reported that the rate of ACS patients discharged on secondary prevention medications was about 71%.Citation26 However, this study applied the different definition about an optimal medication therapy for secondary prevention after ACS. It was defined as prescribing any four mediations of the following: aspirin, other antiplatelet, ACE-I/ARB, β-blocker, or statin/lipid-lowering agent.Citation26 Consequently, if the definition used in our study was applied, the rate may be expected to be lower than observed. In particular, patients with NSTEMI showed much lower rates of prescription compared with patients with UA and STEMI, which was similar to the results of the Taiwan Acute Coronary Syndrome Descriptive Registry.Citation27 This could suggest that the treating physicians had a lower perception to detect disease severity in case of NSTEMI.
As a result of the 18-month follow-up evaluation of discharge medications recommended by the latest guidelines for ACS secondary prevention, the frequency of using all five guideline-recommended discharge medications had been gradually decreased 3, 6, 12, and 18 months after discharge compared with that at discharge. Specifically, the frequency of prescribing them was reduced to about one-half after 12 months and to about one-third after 18 months compared with that at discharge. This result is similar to that from the prospective follow-up study conducted by Bi et al,Citation13 in which the frequency of using the discharge medications was decreased 6 and 12 months after discharge. The prescription rate of clopidogrel declined from 90.08% at discharge to 50.00% at 12 months and to 35.02% at 18 months. This result was similar to that of the Taiwan Acute Coronary Syndrome Descriptive Registry, wherein Cheng et alCitation27 reported that the prescription rate of aspirin and clopidogrel rapidly decreased from 61.8% at discharge to 12.6% at 12 months. They also reported physician’s judgment as the most common reason to discontinue clopidogrel.Citation27 However, this study could not observe the causes of clopidogrel discontinuation due to limited authority of pharmacists to access the EMRs of patients.
The use of antithrombotic agents is recommended in order to reduce the rates of morbidity and mortality in patients with ACS.Citation1 In this study, aspirin was an antithrombotic agent most frequently prescribed during the hospitalization followed by clopidogrel (93.93%) and enoxaparin (71.40%). In particular, compared with unfractionated heparin, enoxaparin has more predictable anticoagulant effects, better bioavailability, longer half-life, and less frequent laboratory monitoring so that enoxaparin has more advantages over unfractionated heparin in the treatment for ACS.Citation28 However, in spite of these benefits from using enoxaparin, its most important side effect is associated with bleeding complications.Citation28 For example, potentially fatal bleeding complications such as spontaneous retroperitoneal hematoma may occur in case of administering enoxaparin to patients with reduced renal functions.Citation29 Thus, it is necessary to carefully monitor side effects from enoxaparin as well as antiplatelet agents.
However, despite more improved management of ACS with antiplatelet and anticoagulant agents, a number of ACS patients keep suffering from the recurrence of ischemic cardiovascular events, which has contributed to the development of novel antithrombotic agents in order to more efficiently inhibit the formation of coronary thrombus.Citation30–Citation33 These novel agents targeting thrombin-mediated pathways consist of direct Xa inhibitors (apixaban, rivaroxaban, and darexaban), direct thrombin inhibitors (dabigatran), and protease-activated receptor 1 antagonists (vorapaxar and atopaxar).Citation30 The concept of ACS follow-up management using a novel oral anticoagulant (NOAC) together with standard antithrombotic therapy including aspirin and P2Y12 inhibitor may be expected to reduce the rate of future ischemic cardiovascular events.Citation32–Citation34 However, as shown in some clinical trials,Citation35–Citation39 adding a NOAC to standard antithrombotic therapy after ACS has led to modest reduction of ischemic events, but it has consistently caused increases in bleeding complications. Therefore, before using NOACs for ACS follow-up management, whether the clinical benefit outweighs the risk should be evaluated.
Our study has some limitations which should be mentioned. All necessary data for the analysis were retrospectively collected through the review of electronic patients’ medical charts, and the appropriateness of secondary prevention drug therapy for ACS was determined based on all five guideline-recommended medications prescribed most closely to 3, 6, 12, and 18 months after discharge. Therefore, this could affect assessment of the appropriate medical therapy for secondary prevention after ACS. In this study, only prescription patterns of discharge medications and their appropriateness in ACS patients were investigated, so it is necessary to conduct more studies regarding the outcomes, such as changes in laboratory parameters, of ACS patients according to the prescription patterns of discharge medications for ACS in the near future.
Conclusion
The American Heart Association/American College of Cardiology guidelines for ACS recommend the long-term administration of aspirin, P2Y12 inhibitor, ACE-I/ARB, β-blocker, and statin from discharge to reduce recurrence of ischemic cardiovascular events and to improve the quality of life in patients with ACS. In this study, we examined the prescription patterns of these medications from discharge to 18 months after discharge. The majority of ACS patients included in this study received all five guideline-recommended medications at discharge from the hospital. However, the frequency of using all of them had been gradually decreased 3, 6, 12, and 18 months after discharge compared with that at discharge. It is also necessary to perform more studies about the outcomes of ACS patients according to the prescription patterns of those medications for ACS secondary prevention in the near future.
Acknowledgments
This study was supported by a research fund from Chosun University, 2015.
Disclosure
The authors report no conflicts of interest in this work.
References
- HuberKBatesERValgimigliMAntiplatelet and anticoagulant agents in acute coronary syndromes: what is the current status and what does the future hold?Am Heart J201416861162125440788
- AronowWSTreatment of unstable angina pectoris/non-ST-segment elevation myocardial infarction in elderly patientsJ Gerontol A Biol Sci Med Sci200358M927M93314570861
- SmithJNNegrelliJMManekMBHawesEMVieraAJDiagnosis and management of acute coronary syndrome: an evidence-based updateJ Am Board Fam Med20152828329325748771
- AmsterdamEAWengerNKBrindisRG2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesJ Am Coll Cardiol201464e139e22825260718
- TraJEngelJvan der WulpIde BruijneMCWagnerCMonitoring guideline adherence in the management of acute coronary syndrome in hospitals: design of a multicentre studyNeth Heart J20142234635324980680
- HassanYKassabYAbd AzizNAkramHIsmailOThe impact of pharmacist-initiated interventions in improving acute coronary syndrome secondary prevention pharmacotherapy prescribing upon dischargeJ Clin Pharm Ther2013389710023441979
- HuberKGershBJGoldsteinPGrangerCBArmstrongPWThe organization, function, and outcomes of ST-elevation myocardial infarction networks worldwide: current state, unmet needs and future directionsEur Heart J2014351526153224742888
- O’GaraPTKushnerFGAscheimDD2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice GuidelinesJ Am Coll Cardiol201361e78e14023256914
- JägerBFarhanSKallaKOne-year mortality in patients with acute ST-elevation myocardial infarction in the Vienna STEMI registryWien Klin Wochenschr201512753554226162464
- YusufSTwo decades of progress in preventing vascular diseaseLancet20023602312114031
- AllonenJNieminenMSLokkiMMortality rate increases steeply with nonadherence to statin therapy in patients with acute coronary syndromeClin Cardiol201235E22E2722961648
- YanATYanRTTanMOptimal medical therapy at discharge in patients with acute coronary syndromes: temporal changes, characteristics, and 1-year outcomeAm Heart J20071541108111518035083
- BiYGaoRPatelAEvidence-based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: results from the Clinical Pathways for Acute Coronary Syndromes in China (CPACS) studyAm Heart J2009157509.e1–516.e119249422
- GurmHSEagleKAUse of anticoagulants in ST-segment elevation myocardial infarction patients: a focus on low-molecular-weight heparinCardiovasc Drugs Ther200822596918165932
- LeeHYCookeCERobertsonTAUse of secondary prevention drug therapy in patients with acute coronary syndrome after hospital dischargeJ Manag Care Pharm20081427128018439049
- DoyleFDe La HarpeDMcGeeHShelleyEConroyRGender differences in the presentation and management of acute coronary syndromes: a national sample of 1365 admissionsEur J Cardiovasc Prev Rehabil20051237637916079646
- BirkheadJSWestonCLoweDImpact of specialty of admitting physician and type of hospital on care and outcome for myocardial infarction in England and Wales during 2004-5: observational studyBMJ20063321306131116705004
- DanchinNCambouJPHananiaGImpact of combined secondary prevention therapy after myocardial infarction: data from a nationwide French registryAm Heart J20051501147115316338251
- van der ElstMEBouvyMLde BlaeyCJde BoerAEffect of drug combinations on admission for recurrent myocardial infarctionHeart2007931226123017502329
- TraJvan der WulpIAppelmanYde BruijneMCWagnerCAdherence to guidelines for the prescription of secondary prevention medication at hospital discharge after acute coronary syndrome: a multicenter studyNeth Heart J20152321422125884093
- ChenHYSaczynskiJSLapaneKLKiefeCIGoldbergRJAdherence to evidence-based secondary prevention pharmacotherapy in patients after an acute coronary syndrome: a systematic reviewHeart Lung20154429930825766041
- VermeerNSBajorekBVUtilization of evidence-based therapy for the secondary prevention of acute coronary syndromes in Australian practiceJ Clin Pharm Ther20083359160119138236
- YetginTvan der LindenMMde VriesAGCurrent discharge management of acute coronary syndromes: data from the Rijnmond Collective Cardiology Research (CCR) studyNeth Heart J201422202724155103
- KimJLeeELeeTSohnAEconomic burden of acute coronary syndrome in South Korea: a national surveyBMC Cardiovasc Disord2013135523924508
- MoebusSBalijepalliCLöschCAge- and sex-specific prevalence and ten-year risk for cardiovascular disease of all 16 risk factor combinations of the metabolic syndrome: a cross-sectional studyCardiovasc Diabetol201093420696055
- RedfernJHyunKChewDPPrescription of secondary prevention medications, lifestyle advice, and referral to rehabilitation among acute coronary syndrome inpatients: results from a large prospective audit in Australia and New ZealandHeart20141001281128824914060
- ChengCIChenCPKuanPLThe causes and outcomes of inadequate implementation of existing guidelines for antiplatelet treatment in patients with acute coronary syndrome: the experience from Taiwan Acute Coronary Syndrome Descriptive Registry (T-ACCORD Registry)Clin Cardiol201033E40E4820552592
- JinatongthaiPKhaisombutNLikittanasombatKChaiyakunaprukNWatcharathanakijSNathisuwanSUse of the CRUSADE bleeding risk score in the prediction of major bleeding for patients with acute coronary syndrome receiving enoxaparin in ThailandHeart Lung Circ2014231051105824931064
- SalemisNSOikonomakisILagoudianakisEEnoxaparin-induced spontaneous massive retroperitoneal hematoma with fatal outcomeAm J Emerg Med2014321559e1e324972961
- CostopoulosCNiespialowska-SteudenMKukrejaNGorogDANovel oral anticoagulants in acute coronary syndromeInt J Cardiol20131672449245522989603
- De CaterinaRHustedSWallentinLNew oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC working group on thrombosis-task force on anticoagulants in heart disease position paperJ Am Coll Cardiol2012591413142522497820
- DeftereosSBourasGGiannopoulosGNovel oral anticoagulants in the treatment of acute coronary syndromes: is there any room for new anticoagulants?Curr Clin Pharmacol2012719520822564121
- AlfredssonJRoeMTRisks and benefits of triple oral anti-thrombotic therapies after acute coronary syndromes and percutaneous coronary interventionDrug Saf20153848149125829216
- GanetskyVSHadleyDEThomasTFRole of novel and emerging oral anticoagulants for secondary prevention of acute coronary syndromesPharmacotherapy20143459060424338703
- AlexanderJHLopesRDJamesSApixaban with antiplatelet therapy after acute coronary syndromeN Engl J Med201136569970821780946
- MegaJLBraunwaldEWiviottSDRivaroxaban in patients with a recent acute coronary syndromeN Engl J Med201236691922077192
- StegPGMehtaSRJukemaJWRUBY-1: a randomized, double-blind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM150) following acute coronary syndromeEur Heart J2011322541255421878434
- OldgrenJBudajAGrangerCBDabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized double-blind, phase II trialEur Heart J2011322781278921551462
- TricociPHuangZHeldCThrombin-receptor antagonist vorapaxar in acute coronary syndromesN Engl J Med2012366203322077816
