Abstract
Antibiotics are prescribed by dentists for treatment as well as prevention of infection. Indications for the use of systemic antibiotics in dentistry are limited, since most dental and periodontal diseases are best managed by operative intervention and oral hygiene measures. However, the literature provides evidence of inadequate prescribing practices by dentists, due to a number of factors ranging from inadequate knowledge to social factors. Here we review studies that investigated the pattern of antibiotic use by dentists worldwide. The main defects in the knowledge of antibiotic prescribing are outlined. The main conclusion is that, unfortunately, the prescribing practices of dentists are inadequate and this is manifested by over-prescribing. Recommendations to improve antibiotic prescribing practices are presented in an attempt to curb the increasing incidence of antibiotic resistance and other side effects of antibiotic abuse.
Introduction
Dentists prescribe medications for the management of a number of oral conditions, mainly orofacial infections.Citation1 Since most human orofacial infections originate from odontogenic infections,Citation2 the prescribing of antibiotics by dental practitioners has become an important aspect of dental practice. For this reason, antibiotics account for the vast majority of medicines prescribed by dentists.Citation3 Dentists prescribe between 7% and 11% of all common antibiotics (betalactams, macrolides, tetracyclines, clindamycin, metronidazole).Citation4 In the UK, for instance, dentists accounted for 7% of all community prescriptions of antimicrobials.Citation5 On the other hand, the National Center for Disease Control and Prevention estimate that approximately one-third of all outpatient antibiotic prescriptions are unnecessary.Citation6
Antibiotic prescribing may be associated with unfavorable side effects ranging from gastrointestinal disturbances to fatal anaphylactic shock and development of resistance. The increasing resistance problems of recent years are probably related to over- or mis-use of broad-spectrum agents such as cephalosporins and fluoro-quinolones.Citation7 We have now entered an era where some bacterial species are resistant to the full range of antibiotics presently available, with the methicillin-resistant Staphylococcus aureus being the most widely known example of extensive resistance.Citation3
These serious complications associated with antibiotics use have encouraged studies investigating antibiotic prescribing practices of dentists.Citation8–Citation23
The PubMed database was searched in February 2010, and search criteria included: antibiotic, dentist, and prescribing. Searches were limited to human studies published in English. Over 400 references were retrieved; 33 of them investigated antibiotic prescribing by dentists. These references were reviewed to evaluate the therapeutic and prophylactic antibiotic prescribing practices of dentists with a focus on indications of antibiotics, type of antibiotic prescribed, and the duration of prescription.
Therapeutic antibiotic prescribing by dentists
Most oral diseases presented to the dentist are primarily inflammatory conditions that are associated with pain. These inflammatory conditions and their associated clinical features are shown in . A considerable percentage of dental pain originates from acute and chronic infections of pulpal origin, which necessitates operative intervention,Citation24 rather than antibiotics. Non-indicated clinical cases for antibiotic use include acute periapical infection, dry socket, and pulpitis.Citation15 Chronic inflammatory periodontal conditions are also not indicated for antibiotics; systemic antimicrobials should only be used in acute periodontal conditions where drainage or debridement is impossible, where there is local spread of the infection or where systemic upset has occurred.Citation10
Table 1 Orofacial painful/inflammatory conditions that may be encountered in dental practice and their important features
Data reported from different countries indicate differences in dentists’ knowledge of clinical situations indicated for antibiotics. Almost half or more of the dentists investigated in England,Citation8 Kuwait,Citation15 and TurkeyCitation19 would prescribe for dry socket. Another non-indicated condition is localized swelling, which was also among the conditions for which antibiotics were prescribed in Norway,Citation7 South Australia,Citation14 Kuwait,Citation15 and England.Citation25 On the other hand, the figures for England show that admissions for ‘drainage of an abscess related to tooth has doubled from just under 800 in 1998 to almost 1600 in 2006.Citation3
More common dental infections present in the form of pulpitis and periapical periodontitis, which require only operative measures like fillings, root canal therapy, or extraction if the tooth is not restorable. Unfortunately, dentists still prescribe antibiotics for this condition.Citation8,Citation11,Citation15,Citation17, Citation19,Citation23,Citation26,Citation27 A distressing finding was that a number of dentists prescribe antibiotics for viral infections like herpes simplex virus-1 infections.Citation11
Clinical situations that require antibiotic therapy on empirical basis are limited, and they include oral infection accompanied by elevated body temperature and evidence of systemic spread like lymphadenopathy and trismus.Citation6 Facial cellulitis that may or may not be associated with dysphagia,Citation15 is a serious disease that should be treated by antibiotics promptly because of the possibility of infection spread via lymph and blood circulation, with development of septicemia.
There are also a limited number of localized oral lesions that are indicated for antibiotic use and these include periodontal abscess, acute necrotizing ulcerative gingivitis, and pericoronitis.Citation15
Another aspect of antibiotic over-prescribing is prescribing based on non-clinical factors. Patient’s expectation of an antibiotic prescription, convenience, and demand necessitated by the social background of the patients are considered unscientific reasons for antibiotic prescription. Whereas English and Scottish dentists would not prescribe for non-clinical factors,Citation25 dentists in the Eastern Mediterranean region have shown a tendency to prescribe on a patient’s demand or socially, especially when short of time.Citation15,Citation17
The most commonly used antibiotic in dental practice, penicillins in general, were found to be the most commonly prescribed antibiotics by dentists,Citation16,Citation17,Citation20 the most popular one being amoxicillin,Citation8,Citation9,Citation16,Citation18,Citation21,Citation22 followed by penicillin V,Citation11,Citation12,Citation23 metronidazole,Citation8,Citation9 and amoxicillin and clavulanate.Citation28
Penicillin is still the gold standard in treating dental infections.Citation6 Among the group of penicillins, penicillin V,Citation6 amoxicillin,Citation29 and amoxicillin and clavulanateCitation30 have been advocated for the treatment of odontogenic infections. Kuriyama et al found no difference in clinical outcome between penicillin V, amoxicillin, or amoxicillin and clavulanate.Citation31
Frequency of prescribing is usually mentioned in the known resources for antibiotic prescribing,Citation32 whereas duration of treatment recommended in therapeutic guidelines is most commonly based on expert opinion.Citation33 A survey in Canada found that the average duration of antibiotic use prescribed by dentists is 6.92 days.Citation13 Another survey in the USA found that endodontists prescribe antibiotic use for an average of 7.58 days.Citation23 Recent studies on the attitudes of dentists in the Eastern Mediterranean region showed that dentists preferred to prescribe a lower dosage of an antibiotic over a longer period.Citation15,Citation18
In recent years, more attention has been given to short courses. Rubenstein explains that short-course antibiotic therapy requires that antibiotics have certain characteristics, such as: rapid onset of action, bactericidal activity, lack of propensity to induce resistant mutants, easy penetrability into tissues, activity against non-dividing bacteria, not being affected by adverse infection conditions (low pH, anaerobiasis, presence of pus, etc.), administration at an optimal dose, and optimal dosing regimen.Citation33 A two-dose, 3-gm regimen of amoxicillin has been shown to be effective in certain situations.Citation34 On the other hand, oral antibiotic use for 2 or 3 days has been advocated for the treatment of acute dentoalveolar infections, and in doses recommended by the British National Formulary (BNF).Citation24 Indeed, in some studies, patients improved after 2 or 3 days of antibiotic therapy.Citation31,Citation35–Citation37 In general, reducing the frequency of antibiotic intake (without compromising the dose) has yielded improved results: a twice-daily dosage of amoxicillin/clavulanate had several advantages over the three times-daily dosage, including increased convenience, improved compliance, and improved tolerability.Citation38,Citation39
Short courses are preferred to long courses particularly when treating children, since children’s compliance with conventional courses is poor.Citation40 A false conception about the use of antibiotics is that antibiotics should be used for a certain number of days to ‘kill the resistant strains’ as the vast majority of strains acquire resistance via transposable elements that are preferentially transferred when antibiotics are used in sub-therapeutic doses or for long durations.Citation41
In summary, antibiotics should be prescribed at the correct frequency, dose, and duration so that the minimal inhibitory concentration is exceeded, and so that side effects and the selection of resistant bacteria are prevented.Citation8 Prolonged courses of antibiotics destroy the commensal flora.Citation38 In addition, longer durations of up to 21 days may result in the selection of resistant strains and a reduction in the ability of the oral flora to resist the colonization by harmful micro-organisms that are not normal residents,Citation42 leading to superimposed infections by multi-resistant bacteria and yeasts.Citation15
Prophylactic antibiotic prescribing
Prophylactic antibiotics, taken prior to a number of dental procedures, have been advocated to reduce the likelihood of postoperative local complications, like infection, dry socket, or serious systemic complications like infective endocarditis. The evidence for antibiotics acting to prevent infection from surgical wounds in the mouth is poor to non-existent,Citation14 indicating that preoperative parenteral antibiotic prophylaxis for routine third molar surgery in medically fit patients is unwarranted.Citation43,Citation44 It was also found that a single dose of metronidazole was ineffective in preventing the development of dry socket.Citation45 For most dentoalveolar surgical procedures in fit, non-medically compromised patients, antibiotic prophylaxis is not required or recommended.Citation46
In the case of bacterial endocarditis (IE), the absolute risk rate after dental treatment, even in at-risk patients, is considered very low.Citation41 This is consistent with recent guidelines from the British Society for Antimicrobial Chemotherapy,Citation47 and the American Heart Association,Citation48 which recommend that only patients in the high risk category require cover. The basis for this recommendation is:
There is no consistent association between having an intervention, dental or non-dental, and the development of IE.
Regular tooth brushing almost certainly presents a greater risk of IE than a single dental procedure because of repetitive exposure to bacteremia with oral flora.
The clinical effectiveness of antibiotic prophylaxis is not proven.
Antibiotic prophylaxis against IE for dental procedures may lead to a greater number of deaths through fatal anaphylaxis than would a strategy of no antibiotic prophylaxis. Nor is antibiotic prophylaxis against IE cost effective.Citation49
Most studies on prophylactic antibiotic use were carried out in developed countries,Citation8,Citation9,Citation11,Citation13,Citation14 and the results generally indicated that dentists have a good knowledge of prescribing.
The few studies done in developing countriesCitation15,Citation17 reported that abuse of prophylactic antibiotics was to prevent postoperative infection following surgical dental manipulationsCitation15 or to cover either a defect in aseptic clinical technique or improperly sterilized equipment; thus, a ‘just in case’ principle is practiced.Citation17
Recommendations
Recommended treatment modalities for common inflammatory oral conditions are shown in .
Figure 1 Recommended treatment modalities for common inflammatory oral lesions.
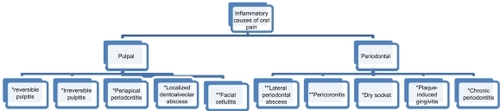
Drainage is the recommended treatment for periapical periodontitis and for localized dentoalveolar abscess, with incisional drainage rather than via the root canal preferred.Citation31
Empirical antibiotic therapy and drainage are recommended for more severe infections such as facial cellulitis, pericoronitis, lateral periodontal abscess, and necrotizing ulcerative gingivitis.
The type of antibiotic chosen and its dosing regimen are dependent upon the severity of infection and the predominant type of causative bacteria.
According to the BNF, amoxicillin is recommended for dental infections in doses ranging from 250 mg to 500 mg, every 8 hours.Citation50 The use of 3 g amoxicillin repeated after 8 hours is also mentioned, as a short course of oral therapy.Citation50 Another antibiotic that is also recommended by the BNF is co-amoxiclav, which can be used in doses ranging from 375 mg to 625 mg every 8 hours.Citation51 In patients allergic to penicillin, clindamycin can be used in doses ranging from 150 mg to 450 mg every 6 hours.Citation52 Another option for penicillin-allergic patients (as recommended by the BNF) is metronidazole, which can be used in a dose of 200 mg every 8 hours for 3–7 days.Citation53
For severe odontogenic infections, higher doses of a broad-spectrum antibiotic may be required. Lewis et al have shown that only 5% of the main isolates from dental abscesses are resistant to amoxicillin/clavulinic acid.Citation54 A more recent study found that bacteria associated with endodontic infections are completely susceptible to amoxicillin/clavulinic acid.Citation55 Furthermore, some researchers observed that amoxicillin/clavulinic acid and clindamycin are the only orally administered antimicrobials with adequate pharmacokinetic/pharmacodynamic properties to be effective against the most commonly isolated oral pathogens for the treatment of orofacial infections.Citation39 When amoxicillin/clavulinic acid is used, a dosing regimen of 1 g twice daily provides a successful clinical outcome, better patient convenience and compliance, and less gastrointestinal upset owing to the minimizing of the clavulinic acid dose.Citation39 As mentioned previously, patients can be seen after 2 or 3 days to determine whether treatment should be stopped or continued.
Patients who are allergic to penicillin should benefit from clindamycin; it is active against some oral anaerobes and facultative bacteria, and has the advantage of good bone penetration. However, increasing the dose may increase the possibility of serious side effects such as pseudomembranous colitis,Citation56–Citation58 Sweet’s syndrome,Citation59 and neutropenia.Citation60
Infections in which anaerobic bacteria are implicated (such as pericoronitis, periodontal abscess and necrotizing ulcerative gingivitis) are better treated with metronidazole; the best dosage regimen in terms of pharmacodynamic/pharmacokinetic aspect is 250 mg every 8 hours.Citation39
Other inflammatory/painful oral conditions such as cracked tooth, dentine hypersensitivity, and bacterial sialad-entitis are outside the scope of this review and their management is thoroughly explained in specialized references.
In addition to the proper dosing regimens and professionally responsible prescribing practices, the general public needs to be educated about the importance of restricting the use of antibiotics to only cases of severe infection. Patients have become accustomed to being given an antibiotic for a range of medical complaints. Unfortunately, patients presenting at dental surgeries also routinely expect an antibiotic for the treatment of ‘toothache’.Citation3
Dental patients not only pressure their dentist to get an antibiotic prescription, they also self-medicate. Self-medication with antibiotics was found to be alarmingly high in some developing countries.Citation61–Citation64 Also in Europe, self-prescription of antibiotics was reported, particularly in eastern and southern parts.Citation65,Citation66
In conclusion, prescribing practices of dentists can be improved by increasing awareness among dental practitioners of the recommended guidelines. Furthermore, the importance of initiating awareness programs among the general public should not be overlooked.
Disclosure
The authors report no conflicts of interest in this work.
References
- Dar-OdehNRyalatSShayyabMAbu-HammadOAnalysis of clinical records of dental patients attending Jordan University Hospital: documentation of drug prescriptions and local anesthetic injectionsTher Clin Risk Manag2008451111111719209291
- LaskinDMLaskinJLOdontogenic infections of the head and neckLaskinDMOral and maxillofacial surgerySt LouisMosby1985219252
- LewisMAWhy we must reduce dental prescription of antibiotics: European Union Antibiotic Awareness DayBr Dent J20082051053753819023306
- ClevelandJIKohnWCAntimicrobial resistance and dental care: a CDC perspectiveDent Abstr1998108110
- SweeneyLCDaveJChambersPAHeritageJAntibiotic resistance in general dental practice – a cause for concernJ Antimicrob Chemother20045356757614985274
- SwiftJQGuldenWSAntibiotic therapy – managing odontogenic infectionsDent Clin N Am20024662363312436820
- WiseRHartTCarrsOAntimicrobial resistance is a major threat to public healthBMJ19983176096109727981
- PalmerNOMartinMVPealingRIrelandRSAn analysis of antibiotic prescriptions from general dental practitioners in EnglandJ Antimicrob Chemother2000461033103511102428
- PalmerNOMartinMVPealingRIrelandRSPaediatric antibiotic prescribing by general dental practitioners in EnglandInter J Paediatr Dent200111242248
- AddyMMartinMVSystemic antimicrobials in the treatment of chronic periodontal diseases: a dilemmaOral Dis20039Suppl 1384412974529
- DemirbasFGjermoPEPreusHRAntibiotic prescribing practices among Norwegian dentistsActa Odontol Scand200664635535917123912
- Al-HaroniMSkaugNIncidence of antibiotic prescribing in dental practice in Norway and its contribution to national consumptionJ Antimicrob Chemother2007591161116617446241
- EpsteinJBChongSLeNDA survey of antibiotic use in dentistryJ Am Dent Assoc2000131111600160911103580
- JaunayTSambrookPGossAAntibiotic prescribing practices by South Australian general dental practitionersAust Dent J200045317918611062935
- SalakoNRotimiVOAdibSMAl-MutawaSPattern of antibiotic prescription in the management of oral diseases among dentists in KuwaitJ Dent20043250350915304295
- Al-MubarakSAl-NowaiserARassMAAntibiotic prescription and dental practice within Saudi Arabia; the need to reinforce guidelines and implement specialty needsJ Int Acad Periodontol200462475515125015
- Al-HaroniMSkaugNKnowledge of prescribing antimicrobials among Yemeni general dentistsActa Odontol Scand200664527428016945892
- Dar-OdehNSAbu-HammadOAKhraisatASEl MaaytahMAShehabiAAn analysis of therapeutic, adult antibiotic prescriptions issued by dental practitioners in JordanChemotherapy2008541172218063860
- ÖcekZSahinHBaksiGApaydinSDevelopment of a rational antibiotic usage course for dentistsEur J Dent Educ200812414718257764
- OgunbodedeEOFatusiOAFolayanMOOlayiwolaGRetrospective survey of antibiotic prescriptions in dentistryJ Contemp Dent Pract200562647115915205
- SarkarCDasBBaralPAn audit of drug prescribing practices of dentistsIndian J Dent Res2004152586115751782
- MurtiAMorseZDental antibiotic prescription in Fijian adultsInt Dent J2007572657017506464
- YinglingNMByrneBEHartwellGRAntibiotic use by members of the American Association of Endodontists in the year 2000: report of a national surveyJ Endod200228539640412026927
- LongmanLPPrestonAJMartinMVWilsonNHFEndodontics in the adult patient: the role of antibioticsJ Dent20002853954811082521
- PalmerNOMartinMVPealingRAntibiotic prescribing knowledge of National Health Service general dental practitioners in England and ScotlandJ Antimicrob Chemother200147223323711157915
- Rodriguez-NúnezACisneros-CabelloRVelasco-OrtegaELlamas-CarrerasJMTórres-LagaresDSegura-EgeaJJAntibiotic use by members of the Spanish Endodontic SocietyJ Endod20093591198120319720216
- MainjotAD’HooreWVanheusdenAVan NieuwenhuysenJPAntibiotic prescribing in dental practice in BelgiumInt Endod J200942121112111719912383
- Poveda RodaRBaganJVSanchis BielsaJMCarbonell PastorEAntibiotic use in dental practice. A reviewMed Oral Patol Oral Cir Bucal2007123E18619217468711
- EllisonSJThe role of phenoxymethylpenicillin, amoxicillin, metronidazole and clindamycin in the management of acute dentoalveolar abscesses – a reviewBr Dent J2009206735736219357666
- FarrierJNKitturMASugarAWNecrotising fasciitis of the sub-mandibular region; a complication of odontogenic originBr Dent J20072021060760917534319
- KuriyamaTAbsiEGWilliamsDWLewisMAAn outcome audit of the treatment of acute dentoalveolar infection: impact of penicillin resistanceBr Dent J20051981275976315980845
- British Dental Association; British Medical Association; Royal Pharmaceutical Society of Great BritainDental Practitioner’s FormularyLondon UKBritish Medical Association and the Royal Pharmaceutical Society of Great Britain2006
- RubinsteinEShort antibiotic treatment courses or how short is short?Inter J Antimicrob Agents200730SS76S79
- SwiftJQGuldenWSAntibiotic therapy – managing odontogenic infectionsDent Clin N Am20024662363312436820
- LewisMAMcGowanDAMacFarlaneTWShort-course high-dosage andomized in the treatment of acute dento-alveolar abscessBr Dent J198625161(8)2993023535857
- PatersonSACurzonMEThe effect of andomized versus penicillin V in the treatment of acutely abscessed primary teethBr Dent J199319174(12)4434498518048
- MartinMVLongmanLPHillJBHardyPAcute dentoalveolar infections: an investigation of the duration of antibiotic therapyBr Dent J199723183(4)1351379293130
- BaxRDevelopment of a twice daily dosing regimen of amoxicillin/clavulanateInt J Antimicrob Agents200730Suppl 2S118S12117983732
- IslaACanutAGascónARLaboraAArdanza-TrevijanoBSolinísMAPedrazJLPharmacokinetic/pharmacodynamic evaluation of antimicrobial treatments of orofacial odontogenic infectionsClin Pharmacokinet200544330531615762771
- CharneyEBynumREldredgeDHow well do patients take oral penicillin? A collaborative study in private practicePediatrics19674021881955006583
- PallaschTJAntibiotic resistanceDent Clin N Am20034762363914664456
- LongmanLPMartinMVThe use of antibiotics in the prevention of postoperative infection: a re-appraisalBr Dent J19911702572621827339
- ThomasDWHillCMAn audit of antibiotic prescribing in third molar surgeryBr J Oral Maxillofac Surg19973521261289146871
- KaczmarzykTWichlinskiJStypulkowskaJZaleskaMPanasMWoronJSingle-dose and multi-dose clindamycin therapy fails to demonstrate efficacy in preventing infectious and inflammatory complications in third molar surgeryInt J Oral Maxillofac Surg200736541742217408924
- BergdahlMHedströmLMetronidazole for the prevention of dry socket after removal of partially impacted mandibular third molar: a andomized controlled trialBr J Oral Maxillofac Surg200442655555815544888
- LawlerBSambrookPJGossANAntibiotic prophylaxis for dentoalveolar surgery: is it indicated?Aust Dent J200550Suppl 2S54S5916416719
- GouldFKElliottTSFowerakerJGuidelines for the prevention of endocarditis: report of the Working Party of the British Society for Antimicrobial ChemotherapyJ Antimicrob Chemother20065761035104216624872
- WilsonWTaubertKAGewitzMPrevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working GroupCirculation2007116151736175417446442
- NICEProphylaxis against infective ednocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional proceduresNice Clinical Guidelines No 64LondonNational Institute for Health and Clinical Excellence2008
- British National Formulary websitehttp://bnf.org/bnf/bnf/current/3724.htm Accessed Feb 22, 2010
- British National Formulary website http://bnf.org/bnf/bnf/current/3737.htm. Accessed Feb 22, 2010.
- British National Formulary website http://bnf.org/bnf/bnf/current/3863.htm. Accessed Feb 22, 2010.
- British National Formulary website http://bnf.org/bnf/bnf/current/3932.htm. Accessed Feb 22, 2010
- LewisMAParkhurstCLDouglasCWPrevalence of penicillin resistant bacteria in acute suppurative oral infectionJ Antimicrob Chemother1995357857917559190
- BaumgartnerJCXiaTAntibiotic susceptibility of bacteria associated with endodontic abscessesJ Endod200329444712540219
- TrexlerMFFraserTGJonesMPFulminant pseudomembranous colitis caused by clindamycin phosphate vaginal creamAm J Gastroenterol19979211211221139362204
- ParryMFRhaCKPseudomembranous colitis caused by topical clindamycin phosphateArch Dermatol198612255835842939805
- SurawiczCMAntibiotic-associated diarrhea and pseudomembranous colitis: are they less common with poorly absorbed antimicrobials?Chemotherapy200551Suppl 1818915855751
- ClarkBMHomeyerDGlassKRD’AvignonLCClindamycin-induced Sweet’s syndromePharmacotherapy20072791343134617723089
- BubaloJSBlasdelCSBeardenDTNeutropenia after single-dose clindamycin for dental prophylaxisPharmacotherapy200323110110312523467
- Al-AzzamSIAl-HuseinBAAlzoubiFMasadehMMAl-HoraniMASelf-medication with antibiotics in Jordanian populationInt J Occup Med Environ Health200720437338018165197
- AwadAIEltayebIBSelf-medication practices with antibiotics and antimalarials among Sudanese undergraduate university studentsAnn Pharmacother20074171249125517565044
- YousefAMAl-BakriAGBustanjiYWazaifyMSelf-medication patterns in Amman, JordanPharm World Sci2008301243017562220
- NajiLShakhatrehFShehabiAAntibiotic self-medication among health professional students at Jordan University Hospital MSc thesis, Faculty of Graduate studies, 2007. The University of Jordan, Amman, Jordan
- GrigoryanLHaaijer-RysjampFMBurgerhofJGSelf-medication with antimicrobial drugs in EuropeEmerg Infect Dis200612345245916704784
- VäänänenMHPietiläKAiraksinenMSelf-medication with antibiotics – does it really happen in EuropeHealth Policy20067716617116095749