Abstract
Approximately 25% of US adults are estimated to have hypertriglyceridemia (triglyceride [TG] level ≥150 mg/dL [≥1.7 mmol/L]). Elevated TG levels are associated with increased cardiovascular disease (CVD) risk, and severe hypertriglyceridemia (TG levels ≥500 mg/dL [≥5.6 mmol/L]) is a well-established risk factor for acute pancreatitis. Plasma TG levels correspond to the sum of the TG content in TG-rich lipoproteins (TRLs; ie, very low-density lipoproteins plus chylomicrons) and their remnants. There remains some uncertainty regarding the direct causal role of TRLs in the progression of atherosclerosis and CVD, with cardiovascular outcome studies of TG-lowering agents, to date, having produced inconsistent results. Although low-density lipoprotein cholesterol (LDL-C) remains the primary treatment target to reduce CVD risk, a number of large-scale epidemiological studies have shown that elevated TG levels are independently associated with increased incidence of cardiovascular events, even in patients treated effectively with statins. Genetic studies have further clarified the causal association between TRLs and CVD. Variants in several key genes involved in TRL metabolism are strongly associated with CVD risk, with the strength of a variant’s effect on TG levels correlating with the magnitude of the variant’s effect on CVD. TRLs are thought to contribute to the progression of atherosclerosis and CVD via a number of direct and indirect mechanisms. They directly contribute to intimal cholesterol deposition and are also involved in the activation and enhancement of several proinflammatory, proapoptotic, and procoagulant pathways. Evidence suggests that non-high-density lipoprotein cholesterol, the sum of the total cholesterol carried by atherogenic lipoproteins (including LDL, TRL, and TRL remnants), provides a better indication of CVD risk than LDL-C, particularly in patients with hypertriglyceridemia. This article aims to provide an overview of the available epidemiological, clinical, and genetic evidence relating to the atherogenicity of TRLs and their role in the progression of CVD.
Introduction
Robust clinical evidence demonstrates that statin-induced reductions in low-density lipoprotein cholesterol (LDL-C) lead to substantial reductions in cardiovascular disease (CVD) risk in both the primary and secondary prevention settings.Citation1 Therefore, in patients at high cardiovascular risk due to dyslipidemia, the majority of current guidelines recommend LDL-C as a primary treatment target, with statins as first-line therapy.Citation2–Citation4 Despite significant LDL-C lowering with statin therapy, substantial residual cardiovascular risk often remains.Citation5 This residual risk is thought to be due, in part, to inadequate reduction of LDL-C for a given level of risk, low levels of high-density lipoprotein cholesterol (HDL-C), and/or high levels of triglycerides (TG).Citation5 Additionally, there is increasing evidence that non-high-density lipoprotein cholesterol (non-HDL-C), which is calculated for individuals by subtracting their HDL-C value from total cholesterol (including TG-rich lipoproteins [TRLs]), may be more strongly associated with atherosclerotic risk than LDL-C alone.Citation6
Approximately 25% of US adults are estimated to have hypertriglyceridemia, defined as a TG level of ≥150 mg/dL (≥1.7 mmol/L).Citation7 Hypertriglyceridemia often coexists with secondary disorders that are independently associated with increased plasma TG levels, such as type 2 diabetes mellitus, chronic kidney disease, metabolic syndrome, and obesity.Citation8 Severe hypertriglyceridemia, defined by the 2014 National Lipid Association guidelines as a TG level of ≥500 mg/dL (≥5.6 mmol/L), is a well-established risk factor for acute pancreatitis,Citation9 and moderately elevated TG levels have been shown to be independently associated with increased CVD risk, even in patients treated effectively with statins to reduce LDL-C.Citation10,Citation11
Plasma TG levels are known to correspond with the levels of TRLs and their remnants.Citation2 However, hypertriglyceridemia is also often accompanied by further lipoprotein disturbances, including increased very low-density lipoproteins (VLDLs) and total apolipoprotein (apo) C-III, elevated levels of small, dense LDL-C and total LDL particles, and decreased levels of HDL-C, all of which have been shown to be associated with increased CVD risk.Citation12 This, along with the fact that cardiovascular outcome studies of TG-lowering agents have produced inconsistent results,Citation13,Citation14 means that there remains some uncertainty regarding the direct causal role of TRLs in the progression of atherosclerosis and CVD.Citation8
This review aims to provide an overview of the available genetic, epidemiological, and clinical evidence relating to the atherogenicity of TRLs and their role in the progression of CVD.
Search strategy
A search of PubMed was performed using the following search strategy: (“triglyceride-rich lipoproteins” OR “apolipoprotein C-III” OR “remnant lipoproteins” OR “intermediate-density lipoproteins”) AND (“Cardiovascular Diseases”[MeSH]).
The search was limited to English-language publications published between July 2005 and July 2015. The reference lists of articles identified using this search strategy was also searched such that widely referenced, older publications were also screened.
TRL metabolism
Cholesterol esters and TG are the two most important circulating lipids.Citation15 Owing to their hydrophobic nature, they are combined into lipoprotein particles in association with proteins that allow them to be transported in the plasma. Cholesterol is transported by all lipoproteins and is particularly concentrated in HDL and LDL particles. In general, TGs are transported in the plasma in VLDL, chylomicrons, and their remnants created during metabolism.Citation16 These TRLs are the largest lipoprotein particles. In addition to size, lipoproteins may also be characterized by the apos they contain, with apoB100 (also known as apoB) being associated with VLDL and LDL and apoA-I associated with HDL.Citation17 There are several other apos involved in lipoprotein metabolism, many of which are thought to potentially contribute to a number of diseases, such as CVD, multiple sclerosis, and Alzheimer’s disease.Citation18–Citation20
TRLs are highly heterogeneous, differing in size, density, composition, and associated cardiovascular risk.Citation21 They are composed of a neutral core of TG and cholesterol esters and a surface monolayer comprising phospholipids, free cholesterol, and apos, which participate in the regulation of transport and metabolism of the TRL.Citation22 The metabolism of TRLs occurs via two principal pathways, an exogenous pathway that originates in the small intestine and an endogenous pathway regulated by the liver (). During the exogenous pathway, TGs from dietary fat are absorbed by enterocytes following the ingestion of a meal. Here, they are incorporated into chylomicrons, which are large apoB48-containing lipoproteins with a large TG core (80%–95%).Citation23 Newly synthesized chylomicrons are then exported via perimesenteric lymphatics before entering the circulation, where they acquire apoC-II, apoC-III, and apoE. Once in circulation, chylomicrons are quickly hydrolyzed by lipoprotein lipase (LPL) along the luminal surface of the capillaries. LPL is synthesized by myocytes and adipocytes before being transported to the lumen of the capillaries via GPIHBPI, a small glycosylphosphatidylinositol-anchored protein synthesized by the capillary endothelial cells.Citation24,Citation25 LPL requires activation via apoC-II,Citation26 and its activity is highly regulated by various proteins, including apoC-III, apoA5, and angiopoietin-like proteins 3 and 4.Citation27 The hydrolysis of chylomicrons via LPL results in the production of free fatty acids and chylomicron remnants. The free fatty acids liberated by lipolysis are oxidized by a variety of cell types, such as skeletal and myocardial myocytes, or stored in adipose tissue. The chylomicron remnants, which are rich in cholesterol esters and apoE, are removed from circulation by the liver via binding to the LDL receptor or the LDL receptor-related protein.Citation28
Figure 1 Overview of triglyceride-rich lipoprotein metabolism.
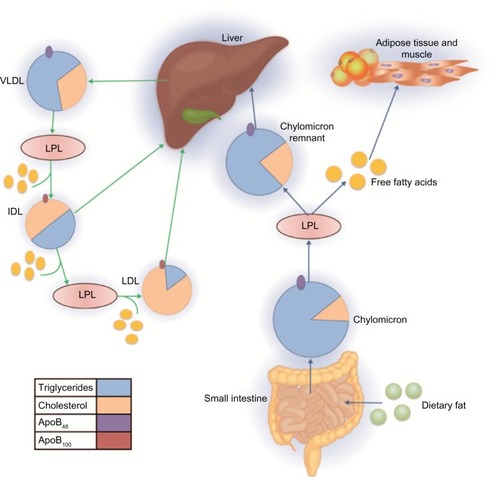
During the endogenous pathway, TGs are synthesized in hepatocytes from free fatty acids and glycerol and then incorporated into the core of apoB-containing VLDL particles. ApoC-I, apoC-II, apoC-III, and apoE are added to the surface of VLDL particles during secretion. Following secretion, VLDLs undergo LPL-mediated hydrolysis in the plasma, generating in succession progressively smaller VLDLs and then intermediate-density lipoproteins (IDLs). Some IDL particles are taken up by the liver, and some undergo further catabolism by LPL and hepatic TG lipase to produce LDL particles.Citation28
Importantly, polymorphisms in genes encoding key components of the TRL biosynthetic pathways are known to be strongly associated with CVD risk.Citation16,Citation29–Citation31
Proposed pathophysiology of TRLs in the progression of atherosclerosis
Evidence suggests that TRLs and their remnants and specific markers of TG metabolism, such as LPL and apoC-III, contribute to the progression of atherosclerosis and CVD both directly and indirectly.Citation12,Citation32 These proposed pathophysiological mechanisms are summarized in .
Figure 2 Proposed pathophysiology of triglyceride-rich lipoproteins in the progression of atherosclerosis.
Abbreviations: LPL, lipoprotein lipase; TRL, triglyceride-rich lipoproteins; TRL-R, triglyceride-rich lipoprotein remnants.
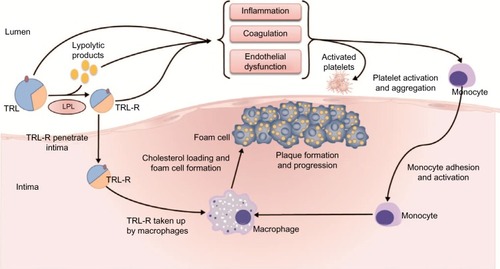
It has been suggested that it is primarily the cholesterol content of TRL remnants that directly contributes to the progression of atherosclerosis, rather than the TGs themselves.Citation15 Like LDL, cholesterol-enriched, TG-depleted TRL remnants are able to penetrate the arterial intima, where they become selectively bound to the connective tissue matrix. Once entrapped in the subendothelial space, TRLs can be scavenged by resident macrophages, thereby contributing to macrophage foam cell formation as well as plaque formation and progression.Citation12,Citation33–Citation36 TRLs are thought to be equally or more atherogenic than LDL. In contrast to LDL, TRL remnants can be taken up directly by arterial macrophages without oxidative modificationCitation37–Citation39 and, due to their larger size, carry more cholesterol per particle than LDL.Citation12 TRL remnants have also been shown to promote endothelial dysfunction, which potentiates atherogenesis.Citation40
LPL-mediated TRL hydrolysis results in a high concentration of lipolytic products, such as oxidized free fatty acids, along the vascular endothelium or within the arterial intima. These lipolytic products, along with TRLs themselves, are also known to activate a number of proinflammatory, procoagulant, and proapoptotic signaling pathways that play a fundamental role in the pathogenesis of atherosclerosis.Citation12 Oxidized free fatty acids are known to increase the expression of inflammatory interleukins and cytokines, leading to endothelial inflammation,Citation41–Citation43 while TRL remnants have been shown to upregulate the endothelial expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1.Citation44,Citation45 These proatherogenic adhesion molecules facilitate the transendothelial migration of leukocytes to sites of inflammation.Citation46 Consequently, their TRL remnant-mediated activation leads to endothelial monocyte adhesion and an enhanced inflammatory response.Citation44,Citation45 TRL remnants have also been shown to induce early monocyte and neutrophil activation.Citation47
Additionally, TRL remnants are known to increase the production of reactive oxygen species, which can increase vascular endothelial permeability, promote leukocyte adhesion, and, at high concentrations, cause cellular injury and death.Citation43,Citation48 TRL remnants have also been shown to induce endothelial cell apoptosis via increased secretion of the proapoptotic cytokines, tumor necrosis factor-α, and interleukin-1β, a process that is known to contribute to vascular injury and atherosclerosis.Citation49 TRLs and their remnants enhance platelet aggregation and clot formation and amplify the coagulation cascade by 1) supporting the assembly of the prothombinase complex and 2) upregulating the expression of plasminogen activator inhibitor-1 and plasminogen activator inhibitor-1 antigen.Citation50 They have also been shown to upregulate the endothelial expression of tissue factor, a key initiator of the coagulation cascade.Citation44,Citation45,Citation51 Finally, TRL remnants suppress the atheroprotective and anti-inflammatory effects of HDLCitation52 and have been shown to significantly correlate with impairment of coronary vasodilation.Citation53
Key components of TRL metabolism have also been shown to be associated with CVD, in particular apoC-III, a key contributor to hypertriglyceridemia due to its inhibitory effects on LPL.Citation30,Citation31,Citation54 ApoC-III is thought to contribute to the progression of atherosclerosis via a number of mechanisms:Citation54 it can impair VLDL binding to cellular receptors, resulting in small, dense LDL particle formation,Citation12 and has been shown to induce the expression of proinflammatory mediators and to stimulate monocyte activation and the adhesion of monocytes to endothelial cells.Citation55 It is also thought to induce apoptosisCitation56,Citation57 and may accumulate on atheroprotective HDL particles on secretion, thus rendering them dysfunctional.Citation58
Genetic evidence
The fact that monogenic disorders of TG metabolism, such as hyperlipoproteinemia type 3, predispose individuals to CVD suggests that raised TG and remnant cholesterol levels contribute to this process.Citation29 Conversely, a recent Mendelian randomization study based on data from 10,208 individuals included in the Copenhagen City Heart Study found that subjects with genetically confirmed reduction in non-fasting plasma TG levels had reduced all-cause mortality.Citation31 An additional meta-analysis involving 188,578 genotyped individuals with 185 different single nucleotide polymorphisms found that the strength of a variant’s effect on TG levels strongly correlated with the magnitude of its effect on coronary artery disease, even after adjustment for effects on LDL-C and HDL-C. These results support the hypothesis that TRLs causally influence cardiovascular risk.Citation16
Sequence variants in several key genes involved in the metabolism of TRLs, such as those encoding LPL and the proteins that regulate it, appear to be strongly associated with CVD risk.Citation30,Citation31 For example, apoC-III, an apolipoprotein playing a central role in TG metabolism by inhibiting LPL, is overexpressed in hypertriglyceridemia and is significantly associated with cardiovascular risk.Citation54 In one study, which evaluated 18,666 genes in 3,734 participants, four loss-of-function mutations were identified in APOC3, the gene encoding apoC-III. Heterozygous carriers of any of these mutations had 46% lower circulating levels of apoC-III, corresponding to 39% lower plasma TG levels, and a 40% lower risk of coronary heart disease than noncarriers.Citation59 Similarly, a second study, which analyzed data from 75,725 participants, found that heterozygosity of loss-of-function mutations in APOC3 were associated with a mean reduction in nonfasting TG levels of 44% and a corresponding 41% decrease in the incidence of ischemic vascular disease compared with wild-type individuals.Citation60 In addition, mutations in the gene encoding apoAV (APOA5), an activator of LPL, have also been shown to be associated with CVD risk. Carriers of nonsynonymous APOA5 mutations have higher plasma TG levels, lower HDL-C levels, but similar overall cholesterol levels, compared with noncarriers. Carriers of these mutations were shown to have a 2.2-fold higher risk of myocardial infarction (MI) and coronary artery disease compared with noncarriers.Citation20 Furthermore, polymorphisms in the APOA5 promoter region, which led to decreased APOA5 expression, were strongly related to increased plasma TG levels and a concordant increase in coronary heart disease risk.Citation61 Finally, loss-of-function variants in the gene encoding angiopoietin-like protein 4 (ANGPTL4), an inhibitor of LPL, were found to be associated with substantially decreased TG levels and decreased coronary heart disease risk.Citation62
Genetically elevated levels of TG and remnant lipoprotein cholesterol are also associated with increased low-grade inflammation, marked by elevated C-reactive protein levels. As this association was not observed for genetically elevated LDL-C, this suggests that the inflammatory component of atherosclerosis may be driven by elevated TRLs and remnant cholesterol.Citation63 Taken together, these findings are consistent with the hypothesis that elevated TG levels, and consequently TRLs, are causally associated with CVD.
Epidemiological evidence
In the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22 (PROVE IT-TIMI 22) trial, it was demonstrated that, among patients receiving statin therapy following acute coronary syndrome, an on-treatment fasting TG level <150 mg/dL (<1.7 mmol/L) was associated with a reduction in recurrent coronary heart disease risk versus higher TG levels (), even after adjustment for HDL-C and LDL-C levels (hazard ratio 0.8; P=0.025).Citation10 A wealth of epidemiological evidence exists, demonstrating that both fasting and nonfasting TG levels are significant predictors of cardiovascular events, even in individuals who have already achieved guideline-recommended LDL-C levels with lipid-lowering therapy.Citation64–Citation68
Table 1 Key studies investigating the association between triglycerides and cardiovascular disease
However, nonfasting TG levels are thought to be a much stronger predictor of cardiovascular events than fasting TG levels. The Women’s Health Study (n=26,509) showed that both fasting and nonfasting TG levels were strongly associated with an increased risk of cardiovascular events, independent of baseline cardiovascular risk factors (age, blood pressure, smoking, and use of hormone therapy). However, after adjustment for TC and HDL-C levels and indicators of insulin resistance, the association between fasting TG levels and the risk of cardiovascular events was no longer significant (P=0.90). In contrast, the association between nonfasting TG levels and cardiovascular risk remained strong even after adjustment for other lipid levels and markers of insulin resistance (P=0.006).Citation67 Likewise, two prospective cohort studies using data from the Copenhagen City Heart Study found that the cumulative incidence of cardiovascular events (ischemic stroke, MI, ischemic heart disease) and all-cause mortality were strongly associated with increasing nonfasting TG levels (all P<0.001) (). That being said, these associations were not adjusted for other lipid parameters.Citation66,Citation68
A number of studies have found that the association between plasma TG levels (both fasting and nonfasting) and cardiovascular risk is often attenuated once adjusted for other lipid parameters, including HDL-C and non-HDL-C. An analysis conducted by the Emerging Risk Factors Collaboration, which included data from 302,430 individuals from 68 long-term prospective studies, demonstrated that there was a significant and stepwise association between fasting and nonfasting TG levels and CVD risk. However, this association was no longer significant after adjustment for HDL-C and non-HDL-C ().Citation64 Likewise, in a combined analysis of the Incremental Decrease in End Points through Aggressive Lipid Lowering (IDEAL) and Treating to New Targets (TNT) trials in patients achieving low LDL-C (<70 mg/dL [1.8 mmol/L]), CVD risk increased incrementally with increasing on-treatment fasting TG level, with patients in the highest quintile experiencing a 63% higher rate of cardiovascular events than those in the lowest quintile (P<0.001). However, this association was also attenuated (P=0.044) after adjustment for HDL-C and apoB/apoA1 ().Citation65 Elevated TG levels are closely associated with higher levels of non-HDL-C and apoB and low levels of HDL-C,Citation2 and this may explain why this association is weakened after adjustment for these parameters.
Elevated remnant cholesterol levels, which directly correlate with elevated levels of TRLs, have also been shown to be associated with CVD. Using data from 73,513 subjects, Varbo et alCitation30 found that every 88.6 mg/dL (1 mmol/L) increase in remnant cholesterol was associated with a 2.8-fold increase in CVD risk, independent of low HDL-C.
Available treatment options to reduce TG levels and their potential impact on cardiovascular outcomes
Although the clinical definition of the severity of hypertriglyceridemia differs among guidelines,Citation2,Citation7,Citation28,Citation69–Citation71 the majority of guidelines define severe hypertriglyceridemia as a TG level of ≥500 mg/dL (≥5.6 mmol/L).Citation28,Citation70 In such cases, guidelines recommend the initiation of TG-lowering therapy to reduce the risk of pancreatitis.Citation3,Citation4,Citation70 Guidelines also acknowledge that a TG level of <150 mg/dL (<1.7 mmol/L) is desirable, and if elevated TG or non-HDL-C levels remain following lifestyle intervention and statin therapy, a number of guidelines recommend the use of TG-lowering agents, primarily fibrates, niacin, or omega-3 fatty acids ().Citation2,Citation4,Citation8,Citation70
Table 2 Summary of available triglyceride-lowering therapies
Fibrates
Fibrates decrease TG levels by ∼36%, non-HDL-C levels by ∼6%–16%, and LDL-C levels by ∼8% and increase HDL C levels by ∼10%.Citation72,Citation73 Of note, however, fibrate-induced increases in LDL-C may occur in patients with severe hypertriglyceridemia.Citation74,Citation75 To date, cardiovascular outcome studies of fibrates have produced varied results, with some studies suggesting a small benefit, particularly in patients with other factors besides hypertriglyceridemia, such as low HDL-C or metabolic syndrome, and others showing no benefit.Citation76–Citation79 A meta-analysis including data from 45,058 participants from 18 clinical trials showed that fibrate therapy was associated with a significant decrease in major cardiovascular events (relative risk [RR] reduction 10%; P=0.048), although this did not translate into a benefit for all-cause mortality (RR reduction 0%; P=0.92).Citation14 In another meta-analysis of 7,389 patients with high TG levels (>200 mg/dL [2.3 mmol/L]), fibrate therapy was associated with a 25% decrease in vascular events, and in 5,068 patients with both high TG and low HDL-C levels (<40 mg/dL [1 mmol/L]), a 29% decrease in vascular events was observed.Citation80
Fibrate therapy is associated with a number of adverse effects, including increases in creatinine levels, myopathy, and, in rare cases, rhabdomyolysis, especially when used in combination with other lipid-lowering therapies.Citation79,Citation81,Citation82 Gemfibrozil, in particular, has been shown to increase exposure to and reduce the renal clearance of statins by inhibiting their glucuronidation,Citation83 potentially leading to severe side effects.Citation82 However, fenofibrate appears to be better tolerated than other fibrates, with no cases of rhabdomyolysis observed with fenofibrate–statin combination therapy in two large-scale clinical trials.Citation79,Citation84 Fenofibrate is often recommended for use in combination with statin therapy in patients requiring additional non-HDL-C lowering.Citation2,Citation4,Citation69 Studies have so far failed to demonstrate any significant reduction in cardiovascular risk with fenofibrate–simvastatin combination therapy, compared with simvastatin monotherapy. However, in a subgroup of patients with a TG level in the upper third (≥204 mg/dL [≥2.30 mmol/L]) and an HDL-C level in the lower third (≤34 mg/dL [≤0.88 mmol/L]), there was a nonsignificant trend (P=0.06) for reduction in cardiovascular risk.Citation84
Niacin
Prescription strength niacin is indicated to reduce elevated total cholesterol, LDL-C, apoB, and TG levels and to increase HDL-C in patients with primary hyperlipidemia, severe hypertriglyceridemia, and mixed dyslipidemia. Niacin has been shown to decrease TG levels by 20%, LDL-C levels by 12%, and non-HDL-C levels by 7%–39% and to increase HDL-C levels by an average of 16%.Citation72,Citation73 Despite improve ments in coronary atherosclerosis and carotid intima-media thickness, niacin does not appear to impact risk for cardiovascular events when added to statin therapy.Citation85–Citation89 That being said, one post hoc analysis showed that among patients with TG >200 mg/dL (>2.3 mmol/L) and HDL-C <32 mg/dL (<0.8 mmol/L), niacin may reduce cardiovascular events by 37% (P<0.05).Citation90
The use of niacin is often limited due to the high incidence of associated adverse effects.Citation91 The most common adverse effect, cutaneous vasodilatation or “flushing”, reportedly occurs in up to 70% of patients receiving niacin therapyCitation72 and often leads to treatment discontinuation.Citation4,Citation91 Although attempts have been made to reduce the incidence of niacin-induced flushing using laropiprant, a specific antagonist of the prostaglandin D2 receptor, this combination therapy does not significantly reduce the risk of major cardio vascular events and has been shown to actually increase the incidence of adverse effects.Citation88 Additional adverse effects, including hyperglycemia, insulin resistance, hyperuricemia, myopathy, pruritus, and elevations in liver enzymes, are also associated with niacin monotherapy or statin–niacin combination therapy.Citation88,Citation89
Omega-3 fatty acids
Three prescription omega-3 fatty acid formulations are currently approved in the US, including formulations comprising omega-3 carboxylic acids, a mixture of long-chain omega-3 fatty acids in free fatty acid form, primarily eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (Epanova®);Citation92 omega-3 fatty acid ethyl esters, a mixture of long-chain omega-3 fatty acid ethyl esters, primarily EPA and DHA (Lovaza®, Omtryg®, and some generics);Citation93,Citation94 and icosapent ethyl (EPA ethyl esters) (Vascepa®).Citation95 These prescription omega-3 fatty acids have been shown to reduce plasma TG levels by 25%–45%, VLDL-C levels by 20%–42%, and non-HDL-C levels by 8%–14% in patients with severe hypertriglyceridemia.Citation92,Citation93,Citation95 Increases in HDL-C levels of 5%–9% have also been observed with DHA-containing formulations.Citation92,Citation93 Higher doses of each formulation and higher baseline TG levels are associated with greater TG reductions.Citation96 Of note, DHA-containing formulations are thought to increase LPL expression, leading to increased TG removal from circulating VLDL and chylomicron particles. This results in the increased production of IDL particles, some of which undergo further catabolism by LPL to produce LDL particles.Citation28 Consequently, DHA-containing formulations have been shown to significantly increase LDL-C levels in patients with severe hypertriglyceridemia by up to 45%.Citation92,Citation93 However, these increases are accompanied by reductions in non-HDL-C. This may be of particular importance, as non-HDL-C levels are more strongly associated with the risk of cardiovascular events than LDL-C,Citation6 particularly in patients with hypertriglyceridemia.Citation70
Like fibrates, cardiovascular outcome studies of omega-3 fatty acids have also produced inconsistent results.Citation97–Citation100 A meta-analysis including data from 63,030 individuals from 20 clinical trials demonstrated that omega-3 fatty acid therapy did not have an impact on a composite cardiovascular end point or total mortality (P=0.24 and P=0.28, respectively) but was associated with a significantly decreased rate of vascular death (RR 0.86; 95% confidence interval, 0.75–0.99; P=0.03).Citation13 In subgroup analysis, protection against the composite cardiovascular end point with omega-3 fatty acid use was observed in those trials that enrolled patients with high baseline TG levels (≥150 mg/dL [≥1.7 mmol/L]; RR 0.82; 95% confidence interval 0.74–0.91) versus those with lower baseline TG levels (P=0.006).Citation13 Notably, many of the clinical trials included in the meta-analysis did not use recommended prescription doses of omega-3 fatty acids. The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardio (GISSI)-Prevenzione Trial showed that in post-MI patients, EPA and DHA significantly reduced the risk of reinfarction and death over a 3-year follow-up period.Citation97 Additionally, in the Japan EPA Lipid Intervention Study (JELIS), the addition of 1.8 mg EPA provided an incremental 18% reduction in major coronary events. In patients with TG >200 mg/dL (>2.3 mmol/L) and HDL-C <40 mg/dL (<1.0 mmol/L), risk reduction was 53% compared with statin monotherapy.Citation98
Heterogeneity in results observed in cardiovascular outcome studies of TG-lowering agents may be due, in part, to the inclusion of subjects with normal baseline TG levels (<150 mg/dL [<1.7 mmol/L]). Therefore, additional large-scale cardiovascular outcome studies in patients with clinically defined hypertriglyceridemia may be beneficial. In this regard, the Reduction of Cardiovascular Events with EPA – Intervention Trial (REDUCE-IT; NCT01492361)Citation101 and the STatin Residual risk reduction with EpaNova in hiGh cardiovascular risk paTients with Hypertriglyceridemia (STRENGTH; NCT02104817) trialCitation102 are currently under way. These studies should provide valuable information on the utility of omega-3 fatty acids in combination with statin therapy in high-risk patients with TG levels of 200–500 mg/dL.
The most common adverse effects associated with omega-3 fatty acids are gastrointestinal (such as nausea and diarrhea).Citation103,Citation104 The rate of treatment discontinuation observed in clinical trials is similar between omega-3 fatty acid groups and placebo groups.Citation81,Citation105–Citation107 Moreover, omega-3 fatty acids do not affect liver function and do not exhibit drug–drug interactions with other lipid-lowering agents, including statins.Citation108,Citation109 They are metabolized by mitochondrial beta-oxidation.
Emerging TG-lowering therapies
As studies further elucidate the proatherogenic mechanisms of TRLs, new treatment targets are beginning to emerge, and a number of novel therapeutic agents are currently in clinical development. These agents include antisense apoC-III inhibitors and LPL gene replacement therapy. Volanesorsen (formerly ISIS-APOCIIIRx) is an antisense apoC-III inhibitor currently undergoing Phase III clinical trials. It inhibits hepatic apoC-III synthesis by binding to apoC-III messenger RNA, thereby promoting its degradation. Phase II clinical trials conducted in patients with LPL deficiency demonstrated that Volanesorsen successfully reduced levels of apoC-III, TG, and non-HDL-C.Citation110 However, concerns have been raised that the inhibition of hepatic apoC-III synthesis could lead to hepatic lipid accumulation.Citation111
Another agent with promise in reducing TG levels is alipogene tiparvovec (AAV1-LPLS447X), a nonreplicating and nonreplacing adeno-associated viral vector that delivers copies of the human LPL gene to muscle tissue. It has been approved in Europe for adult patients diagnosed with familial LPL deficiency, a disorder characterized by severe hypertriglyceridemia and increased risk of pancreatitis. However, it has not yet been approved in the US. Intramuscular administration of alipogene tiparvovec has been shown to be generally well tolerated and is associated with clinical improvement and reduced incidence and severity of acute pancreatitis.Citation112
Mipomersen and lomitapide are both approved by the US Food and Drug Administration for the treatment of homozygous familial hypercholesterolemia. However, as both agents interfere with TRL synthesis in the liver, along with substantial reductions in LDL-C, they have also been shown to diminish plasma TG levels.Citation113,Citation114 Mipomersen is an antisense oligonucleotide that blocks the translation of the apoB-100 gene, reducing its synthesis and consequently decreasing the circulation of atherogenic apoB-100-containing lipoproteins, including VLDL, IDL, and LDL.Citation113 Lomitapide is a microsomal TG transfer protein inhibitor that blocks the microsomal TG transfer protein-mediated transfer of lipids to apoB, and therefore, results in significant reductions in VLDL, LDL-C, TG, and non-HDL-C.Citation114 Currently, the sole indication for both mipomersen and lomitapide is homozygous familial hypercholesterolemia. However, in the future they may be found to have a place in the treatment of hypertriglyceridemia once their effect on TRLs has been fully defined.
Additional treatment considerations
Several guidelines recommend evaluating TG levels in the fasting state.Citation4,Citation69,Citation115 However, nonfasting TG levels significantly correlate with increased levels of remnant lipoprotein cholesterol, are associated with a stepwise increase in the incidence of cardiovascular events,Citation66,Citation68 and have been shown to be a superior predictor of cardiovascular risk compared with fasting TG levels.Citation67,Citation68 Therefore, the measurement of nonfasting TG levels in the absence of a high-fat meal is now suggested by the American Heart Association and the European Atherosclerosis Society.Citation2,Citation28
Additionally, although a number of guidelines recommend LDL-C as a primary treatment target, there is increasing evidence that non-HDL-C, the sum of the total cholesterol carried by all atherogenic lipoproteins (including LDL, IDL, and VLDL and chylomicrons and their remnants) provides a better indication of cardiovascular risk than LDL-C.Citation6 Therefore, the use of non-HDL-C as a treatment target is now advocated by several guidelines, particularly in patients with hypertriglyceridemia.Citation8,Citation69,Citation70 It is also worth noting that LDL-C levels are ordinarily estimated by the Friedewald equation.Citation116 The Friedewald equation is inapplicable for patients with fasting TG >400 mg/dL; however, it has also been shown to underestimate LDL-C levels in the presence of TG levels ≥150 mg/dL, particularly in patients with low LDL-C. Therefore, alternative evaluation is warranted in high-risk patients with elevated TG levels.Citation117,Citation118
Conclusion
Although some studies have failed to report a link between raised TG levels and CVD when adjusting for other lipid parameters, there is now a large body of evidence indicating that elevated TG levels are independently associated with an increased incidence of cardiovascular events. Genetic studies have further clarified the causal association between TRLs and CVD, with variants in several key genes involved in TRL metabolism, such as LPL and its regulators, shown to be strongly associated with cardiovascular risk. Moreover, it has been shown that the strength of a variant’s effect on TG levels correlates with the magnitude of the variant’s effect on CVD. TRLs are known to contribute to the progression of atherosclerosis and CVD via a number of direct and indirect mechanisms. For example, they directly contribute to intimal cholesterol deposition and are also involved in the activation and enhancement of several proinflammatory, proapoptotic, and procoagulant pathways. Consequently, a number of guidelines now recommend the use of TG-lowering agents, primarily fibrates, niacin, or omega-3 fatty acids, if elevated TG or non-HDL-C levels remain following lifestyle intervention and statin therapy. Although cardiovascular outcome studies of TG-lowering agents have produced inconsistent results, post hoc analyses of large-scale clinical trials have shown a clinical benefit of TG-lowering agents in patients with hypertriglyceridemia, those with atherogenic dyslipidemia, and patients with features of metabolic syndrome. However, further large-scale clinical trials conducted in patients with clinically defined hypertriglyceridemia are required to further clarify this clinical benefit.
Acknowledgments
Medical writing support was provided by Alex Mellors of Prime Medica Ltd, Knutsford, Cheshire, UK, and funded by AstraZeneca. The opinions, conclusion, and interpretation of the data are the responsibility of the author.
Disclosures
Doctor Toth received no compensation and was involved in all stages of manuscript development. Doctor Toth is a member of speakers bureaus for Amarin, Amgen, AstraZeneca, GSK, Kowa, and Merck & Co., Inc. and is a consultant/advisory board member for Amgen, AstraZeneca, Merck & Co., Inc. Novartis, Sanofi, Regeneron, and Kowa. The author reports no other conflicts of interest in this work.
References
- Cholesterol Treatment Trialists’ (CTT) CollaborationFulcherJO’ConnellREfficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trialsLancet201538599761397140525579834
- ChapmanMJGinsbergHNAmarencoPTriglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for managementEur Heart J201132111345136121531743
- JellingerPSSmithDAMehtaAEAmerican Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosisEndocr Pract201218suppl 117822522068
- CatapanoALReinerZDe BackerGESC/EAS guidelines for the management of dyslipidaemias. The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS)Atherosclerosis2011217134621882396
- SampsonUKFazioSLintonMFResidual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challengesCurr Atheroscler Rep201214111022102062
- BoekholdtSMArsenaultBJMoraSAssociation of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysisJAMA2012307121302130922453571
- CarrollMKitBLacherDTrends in elevated triglyceride in adults: United States, 2001–2012NCHS Data Brief201519819825973997
- HegeleRAGinsbergHNChapmanMJThe polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and managementLancet Diabetes Endocrinol20142865566624731657
- BrunzellJDSchrottHGThe interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitisJ Clin Lipidol20126540941223009776
- MillerMCannonCPMurphySAPROVE IT-TIMI 22 InvestigatorsImpact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trialJ Am Coll Cardiol200851772473018279736
- SchwartzGGAbtMBaoWFasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statinsJ Am Coll Cardiol201565212267227526022813
- RosensonRSDavidsonMHHirshBJKathiresanSGaudetDGenetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular diseaseJ Am Coll Cardiol201464232525254025500239
- KotwalSJunMSullivanDPerkovicVNealBOmega 3 fatty acids and cardiovascular outcomes: systematic review and meta-analysisCirc Cardiovasc Qual Outcomes20125680881823110790
- JunMFooteCLvJEffects of fibrates on cardiovascular outcomes: a systematic review and meta-analysisLancet201037597291875188420462635
- NordestgaardBGVarboATriglycerides and cardiovascular diseaseLancet2014384994362663525131982
- DoRWillerCJSchmidtEMCommon variants associated with plasma triglycerides and risk for coronary artery diseaseNat Genet201345111345135224097064
- ZhengCUpdates on apolipoprotein CIII: fulfilling promise as a therapeutic target for hypertriglyceridemia and cardiovascular diseaseCurr Opin Lipidol2014251353924345989
- FazekasFEnzingerCRopeleSSchmidtHSchmidtRStrasser-FuchsSThe impact of our genes: consequences of the apolipoprotein E polymorphism in Alzheimer disease and multiple sclerosisJ Neurol Sci20062451–2353916631796
- MahleyRWHuangYWeisgraberKHDetrimental effects of apolipoprotein E4: potential therapeutic targets in Alzheimer’s diseaseCurr Alzheimer Res20074553754018220516
- DoRStitzielNOWonHHExome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarctionNature2015518753710210625487149
- GinsbergHNNew perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolismCirculation2002106162137214212379586
- XiaoCHsiehJAdeliKLewisGFGut-liver interaction in triglyceride-rich lipoprotein metabolismAm J Physiol Endocrinol Metab20113013E429E44621693689
- GinsbergHNLipoprotein physiologyEndocrinol Metab Clin North Am19982735035199785050
- BeigneuxAPDaviesBSBensadounAFongLGYoungSGGPIHBP1, a GPI-anchored protein required for the lipolytic processing of triglyceride-rich lipoproteinsJ Lipid Res200950supplS57S6218854402
- DaviesBSBeigneuxAPBarnesRH2ndGPIHBP1 is responsible for the entry of lipoprotein lipase into capillariesCell Metab2010121425220620994
- GoldbergIJScheraldiCAYacoubLKSaxenaUBisgaierCLLipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IVJ Biol Chem19902658426642722307668
- JohansenCTKathiresanSHegeleRAGenetic determinants of plasma triglyceridesJ Lipid Res201152218920621041806
- MillerMStoneNJBallantyneCTriglycerides and cardiovascular disease: a scientific statement from the American Heart AssociationCirculation2011123202292233321502576
- BrahmAHegeleRAHypertriglyceridemiaNutrients201353981100123525082
- VarboABennMTybjaerg-HansenAJorgensenABFrikke- SchmidtRNordestgaardBGRemnant cholesterol as a causal risk factor for ischemic heart diseaseJ Am Coll Cardiol201361442743623265341
- ThomsenMVarboATybjaerg-HansenANordestgaardBGLow nonfasting triglycerides and reduced all-cause mortality: a mendelian randomization studyClin Chem201460573774624436475
- HodisHNTriglyceride-rich lipoprotein remnant particles and risk of atherosclerosisCirculation199999222852285410359725
- NordestgaardBGStenderSKjeldsenKReduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wallArteriosclerosis1988844214283395278
- RappJHLespineAHamiltonRLTriglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaqueArterioscler Thromb19941411176717747947602
- NordestgaardBGWoottonRLewisBSelective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner mediaArterioscler Thromb Vasc Biol19951545345427749867
- Yla-HerttualaSJaakkolaOEhnholmCCharacterization of two lipoproteins containing apolipoproteins B and E from lesion-free human aortic intimaJ Lipid Res19882955635723137302
- GoldsteinJLHoYKBrownMSInnerarityTLMahleyRWCholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteinsJ Biol Chem19802555183918487354064
- PitasREInnerarityTLMahleyRWFoam cells in explants of atherosclerotic rabbit aortas have receptors for beta-very low density lipoproteins and modified low density lipoproteinsArteriosclerosis1983312126297442
- BattKVPatelLBothamKMSucklingKEChylomicron remnants and oxidised low density lipoprotein have differential effects on the expression of mRNA for genes involved in human macrophage foam cell formationJ Mol Med (Berl)200482744945815156288
- AungHHLameMWGohilKAnCIWilsonDWRutledgeJCInduction of ATF3 gene network by triglyceride-rich lipoprotein lipolysis products increases vascular apoptosis and inflammationArterioscler Thromb Vasc Biol20133392088209623868936
- SunCAlkhouryKWangYIIRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat mealCirc Res201211181054106422874466
- GowerRMWuHFosterGACD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1Arterioscler Thromb Vasc Biol201131116016621030716
- WangLGillRPedersenTLHigginsLJNewmanJWRutledgeJCTriglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammationJ Lipid Res200950220421318812596
- DoiHKugiyamaKOkaHRemnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanismCirculation2000102667067610931808
- WangYIBettaiebASunCTriglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stressPLoS One2013810e7832224205197
- HuaSTargeting sites of inflammation: intercellular adhesion molecule-1 as a target for novel inflammatory therapiesFront Pharmacol2013412724109453
- AlipourAvan OostromAJIzraeljanALeukocyte activation by triglyceride-rich lipoproteinsArterioscler Thromb Vasc Biol200828479279718218988
- HadiHACarrCSAl SuwaidiJEndothelial dysfunction: cardiovascular risk factors, therapy, and outcomeVasc Health Risk Manag20051318319817319104
- ShinHKKimYKKimKYLeeJHHongKWRemnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazolCirculation200410981022102814967724
- OlufadiRByrneCDEffects of VLDL and remnant particles on plateletsPathophysiol Haemost Thromb2006353–428129116877877
- SteffelJLuscherTFTannerFCTissue factor in cardiovascular diseases: molecular mechanisms and clinical implicationsCirculation2006113572273116461845
- PatelSPuranikRNakhlaSAcute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteinsAtherosclerosis2009204242442819111829
- ZhengXYLiuLRemnant-like lipoprotein particles impair endothelial function: direct and indirect effects on nitric oxide synthaseJ Lipid Res20074881673168017496332
- Wyler von BallmoosMCHaringBSacksFMThe risk of cardiovascular events with increased apoplipoprotein CIII: a systematic review and meta-analysisJ Clin Lipidol2015949851026228667
- KawakamiAAikawaMAlcaidePLuscinskasFWLibbyPSacksFMApolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cellsCirculation2006114768168716894036
- Juntti-BerggrenLRefaiEAppelskogIApolipoprotein CIII promotes Ca2+-dependent beta cell death in type 1 diabetesProc Natl Acad Sci U S A200410127100901009415210953
- SolEMSundstenTBergstenPRole of MAPK in apolipoprotein CIII-induced apoptosis in INS-1E cellsLipids Health Dis20098319196457
- KonesRMolecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomesVasc Health Risk Manag2013961767024174878
- CrosbyJPelosoGMAuerPLLoss-of-function mutations in APOC3, triglycerides, and coronary diseaseN Engl J Med20143711223124941081
- JorgensenABFrikke-SchmidtRNordestgaardBGTybjaerg-HansenALoss-of-function mutations in APOC3 and risk of ischemic vascular diseaseN Engl J Med20143711324124941082
- Triglyceride Coronary Disease Genestics Consortium and Emerging Risk Factors CollaborationSarwarNSandhuMSTriglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studiesLancet201037597261634163920452521
- FolsomARPeacockJMDemerathEBoerwinkleEVariation in ANGPTL4 and risk of coronary heart disease: the Atherosclerosis Risk in Communities StudyMetabolism200857111591159618940399
- VarboABennMTybjaerg-HansenANordestgaardBGElevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammationCirculation2013128121298130923926208
- Di AngelantonioESarwarNPerryPMajor lipids, apolipoproteins, and risk of vascular diseaseJAMA2009302181993200019903920
- FaergemanOHolmeIFayyadRPlasma triglycerides and cardiovascular events in the treating to new targets and incremental decrease in end-points through aggressive lipid lowering trials of statins in patients with coronary artery diseaseAm J Cardiol2009104445946319660594
- FreibergJJTybjaerg-HansenAJensenJSNordestgaardBGNon fasting triglycerides and risk of ischemic stroke in the general populationJAMA2008300182142215219001625
- BansalSBuringJERifaiNMoraSSacksFMRidkerPMFasting compared with nonfasting triglycerides and risk of cardiovascular events in womenJAMA2007298330931617635891
- NordestgaardBGBennMSchnohrPTybjaerg-HansenANonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and womenJAMA2007298329930817635890
- BerglundLBrunzellJDGoldbergACEvaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guidelineJ Clin Endocrinol Metab20129792969298922962670
- JacobsonTAItoMKMakiKCNational Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 – executive summaryJ Clin Lipidol20148547348825234560
- StoneNJRobinsonJGLichtensteinAH2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesJ Am Coll Cardiol20146325 pt B2889293424239923
- BirjmohunRSHuttenBAKasteleinJJStroesESEfficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trialsJ Am Coll Cardiol200545218519715653014
- RobinsonJGWangSSmithBJJacobsonTAMeta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease riskJ Am Coll Cardiol200953431632219161879
- DavidsonMHBaysHESteinETRIMS InvestigatorsEffects of fenofibrate on atherogenic dyslipidemia in hypertriglyceridemic subjectsClin Cardiol200629626827316796078
- FarnierMUpdate on the clinical utility of fenofibrate in mixed dys-lipidemias: mechanisms of action and rational prescribingVasc Health Risk Manag200845991100019183747
- FrickMHEloOHaapaKHelsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart diseaseN Engl J Med198731720123712453313041
- RubinsHBRobinsSJCollinsDGemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study GroupN Engl J Med1999341641041810438259
- The BIP Study GroupSecondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. The Bezafibrate Infarction Prevention (BIP) studyCirculation20001021212710880410
- KeechASimesRJBarterPEffects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trialLancet200536695001849186116310551
- LeeMSaverJLTowfighiAChowJOvbiageleBEfficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: a meta-analysisAtherosclerosis2011217249249821592479
- DavidsonMHArmaniAMcKenneyJMJacobsonTASafety considerations with fibrate therapyAm J Cardiol2007993C18C
- ChangJTStaffaJAParksMGreenLRhabdomyolysis with HMG-CoA reductase inhibitors and gemfibrozil combination therapyPharmacoepidemiol Drug Saf200413741742615269925
- KyrklundCBackmanJTNeuvonenMNeuvonenPJGemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearanceClin Pharmacol Ther200373653854412811363
- GinsbergHNElamMBLovatoLCEffects of combination lipid therapy in type 2 diabetes mellitusN Engl J Med2010362171563157420228404
- BruckertELabreucheJAmarencoPMeta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosisAtherosclerosis2010210235336120079494
- LavignePMKarasRHThe current state of niacin in cardiovascular disease prevention: a systematic review and meta-regressionJ Am Coll Cardiol201361444044623265337
- CannerPLBergeKGWengerNKFifteen year mortality in coronary drug project patients: long-term benefit with niacinJ Am Coll Cardiol198686124512553782631
- HPS2-THRIVE Collaborative GroupHPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatmentEur Heart J201334171279129123444397
- BodenWEProbstfieldJLAndersonTNiacin in patients with low HDL cholesterol levels receiving intensive statin therapyN Engl J Med2011365242255226722085343
- GuytonJRSleeAEAndersonTRelationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (atherothrombosis intervention in metabolic syndrome with low HDL/high triglycerides and impact on global health outcomes)J Am Coll Cardiol201362171580158423916935
- GuytonJRBaysHESafety considerations with niacin therapyAm J Cardiol2007996A22C31C
- AstraZenecaEpanova Prescribing Information 2014 Available from: http://www.astrazeneca-us.com/pi/epanova.pdfAccessed August 26, 2015
- GlaxoSmithKline [webpage on the Internet]Lovaza Prescribing Information 2014 Available from: https://www.gsksource.com/gskprm/htdocs/documents/LOVAZA-PI-PIL.PDFAccessed August 26, 2015
- Trygg Pharma [webpage on the Internet]Omtryg Prescribing Information 2014 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204977s000lbl.pdfAccessed August 26, 2015
- Amarin Corporation [webpage on the Internet]Vascepa Prescibing Information 2014 Available from: www.vascepa.com/full-prescribing-information.pdfAccessed August 26, 2015
- HarrisWSn-3 fatty acids and serum lipoproteins: human studiesAm J Clin Nutr1997655 suppl1645S1654S9129504
- GISSI-Prevenzione InvestigatorsDietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardicoLancet1999354917744745510465168
- YokoyamaMOrigasaHMatsuzakiMEffects of eicosap-entaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysisLancet200736995671090109817398308
- KromhoutDGiltayEJGeleijnseJMAlpha Omega Trial Groupn-3 fatty acids and cardiovascular events after myocardial infarctionN Engl J Med2010363212015202620929341
- RauchBSchieleRSchneiderSOMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarctionCirculation2010122212152215921060071
- Clinicaltrials.gov. [webpage on the Internet]A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High Risk Patients with Hypertriglyceridemia and on Statin. The Primary Objective is to Evaluate the Effect of 4 g/Day AMR101 for Preventing the Occurrence of a First Major Cardiovascular Event. (REDUCE-IT)Clinicaltrials.gov2014 Available from: https://clinicaltrials.gov/ct2/show/NCT01492361Accessed August 20, 2015
- Clinicaltrials.gov. [webpage on the Internet]Outcomes Study to Assess Statin Residual Risk Reduction with Epanova in High CV Risk Patients with Hypertriglyceridemia (STRENGTH)2015 Available from: https://clinicaltrials.gov/ct2/show/NCT02104817Accessed August 20, 2015
- KasteleinJJMakiKCSusekovAOmega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the Epanova for lowering very high triglycerides (EVOLVE) trialJ Clin Lipidol2014819410624528690
- WangCHarrisWSChungMn-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic reviewAm J Clin Nutr200684151716825676
- BaysHEBallantyneCMKasteleinJJIsaacsohnJLBraeckmanRASoniPNEicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the multi-center, placebo-controlled, randomized, double-blind, 12-week study with an open-label extension [MARINE] trial)Am J Cardiol2011108568269021683321
- BallantyneCMBaysHEKasteleinJJEfficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study)Am J Cardiol2012110798499222819432
- FilionKBEl KhouryFBielinskiMSchillerIDendukuriNBrophyJMOmega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trialsBMC Cardiovasc Disord2010102420525225
- HarrisWSGinsbergHNArunakulNSafety and efficacy of Omacor in severe hypertriglyceridemiaJ Cardiovasc Risk199745–63853919865671
- KostapanosMSMilionisHJElisafMSRosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemiaAm J Cardiovasc Drugs2010101112820104931
- GaudetDAlexanderVJDianeBAn antisense inhibitor of apolipoprotein C-III substantially decreases fasting apolipoprotein C-III and triglyceride levels in LPL deficiencyJ Clin Lipidol201483353354
- HuffMWHegeleRAApolipoprotein C-III: going back to the future for a lipid drug targetCirc Res2013112111405140823704213
- GaudetDMethotJKasteleinJGene therapy for lipoprotein lipase deficiencyCurr Opin Lipidol201223431032022691709
- TothPPEmerging LDL therapies: mipomersen-antisense oligonucleotide therapy in the management of hypercholesterolemiaJ Clin Lipidol201373 supplS6S1023642326
- TothPPShahPKWilkinsonMJDavidsonMHMcCulloughPAUse of microsomal triglyceride transfer protein inhibitors in patients with homozygous familial hypercholesterolemia: translating clinical trial experience into clinical practiceRev Cardiovasc Med201415111024762461
- National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III)Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final reportCirculation2002106253143342112485966
- FriedewaldWTLevyRIFredricksonDSEstimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem19721864995024337382
- HermansMPAhnSARousseauMFNovel unbiased equations to calculate triglyceride-rich lipoprotein cholesterol from routine non-fasting lipidsCardiovasc Diabetol2014135624612479
- MartinSSBlahaMJElshazlyMBFriedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implicationsJ Am Coll Cardiol201362873273923524048
- AnderssonCLyassAVasanRSMassaroJMD’AgostinoRBSrRobinsSJLong-term risk of cardiovascular events across a spectrum of adverse major plasma lipid combinations in the Framingham Heart StudyAm Heart J2014168687888325458651
- SharrettARBallantyneCMCoadySACoronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density sub-fractions: the Atherosclerosis Risk in Communities (ARIC) StudyCirculation2001104101108111311535564
- AssmannGSchulteHThe importance of triglycerides: results from the Prospective Cardiovascular Munster (PROCAM) StudyEur J Epidemiol19928suppl 1991031505660
- KamannaVSKashyapMLMechanism of action of niacinAm J Cardiol20081018A20B26B18157959
- BalkEMLichtensteinAHChungMKupelnickBChewPLauJEffects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic reviewAtherosclerosis20061891193016530201
- MortonAFurtadoJAmerineWKlingDDavidsonMThe effect of omega-3 carboxylic acids on apolipoprotein CIII containing lipoproteins in moderate to severe hypertriglyceridemiaCirculation2014130suppl 1A16864
- WeintraubHSOverview of prescription omega-3 fatty acid products for hypertriglyceridemiaPostgrad Med2014126771825387209
- WattsGFOoiEMChanDCDemystifying the management of hypertriglyceridaemiaNat Rev Cardiol2013101164866124060958
