Abstract
Introduction
Dipeptidyl peptidase-4 (DPP-4) inhibitors reduce hyperglycemia in patients with type 2 diabetes mellitus (T2DM) by enhancing insulin and suppressing glucagon secretion. Since T2DM is associated with progressive loss of β-cell function, we hypothesized that the DPP-4 inhibitor action to improve β-cell function would be attenuated with longer duration of T2DM.
Methods
Data from six randomized, placebo-controlled trials of 24 weeks duration, where β-cell response to vildagliptin 50 mg twice daily was assessed, were pooled. In each study, the insulin secretory rate relative to glucose (ISR/G 0–2h) during glucose load (standard meal or oral glucose tolerance test) was assessed at baseline and end of study. The mean placebo-subtracted difference (PSD) in the change in ISR/G 0–2h from baseline for each study was evaluated as a function of age, duration of T2DM, baseline ISR/G 0–2h, glycated hemoglobin (HbA1c), fasting plasma glucose, body mass index, and mean PSD in the change in HbA1c from baseline, using univariate model.
Results
There was a strong negative association between the PSD in the change from baseline in ISR/G 0–2h and duration of T2DM (r= −0.89, p<0.02). However, there was no association between the PSD in the change from baseline in ISR/G 0–2h and the PSD in the change from baseline in HbA1c (r=0.33, p=0.52). None of the other characteristics were significantly associated with mean PSD change in ISR/G 0–2h.
Conclusion
These findings indicate that the response of the β-cell, but not the HbA1c reduction, with vildagliptin is dependent on duration of T2DM. Further, it can be speculated that glucagon suppression may become the predominant mechanism via which glycemic control is improved when treatment with a DPP-4 inhibitor, such as vildagliptin, is initiated late in the natural course of T2DM.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Progressive deterioration of pancreatic β-cell function contributes to the worsening of hyperglycemia in patients with type 2 diabetes mellitus (T2DM). In the UKPDSCitation1 and ADOPTCitation2 studies, increasing hyperglycemia with time was associated with decrease in β-cell function despite therapy with a sulfonylurea and/or metformin. Dipeptidyl peptidase-4 (DPP-4) inhibitors are a new class of oral antidiabetic drugs for treatment of T2DM.Citation3 Physiologically, DPP-4 inhibitors increase the availability of active glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) in plasma, which in turn, improves the sensitivity of pancreatic β- and α-cells to glucose.Citation3,Citation4
Although DPP-4 inhibitors have been used extensively in a variety of clinical settings, the optimal stage for their use in T2DM treatment is still not well understood. It has been previously shown that there was no association between duration of diabetes and reduction in glycated hemoglobin (HbA1c) with DPP-4 inhibitor treatment.Citation5 However, the effect of DPP-4 inhibitors on β-cell response during the treatment journey of patients with T2DM is unclear. Therefore, we evaluated the effect of vildagliptin on β-cell response (assessed as insulin secretory rate relative to glucose [ISR/G 0–2h]) and its association with factors such as disease duration, baseline ISR/G, HbA1c, age, and placebo-subtracted difference (PSD) in HbA1c change from baseline to end of study.
Materials and methods
Patients and study design
Data were pooled from six previously published, double-blind, randomized, placebo-controlled trials with 24 weeks study duration wherein β-cell response (ISR/G) to vildagliptin 50 mg twice daily or placebo was assessed at baseline and end of study (n=615).Citation6–Citation11
Assessments
In all the studies, β-cell response (ISR/G 0–2h) following a standard solid meal (breakfast) containing 500 kcal (60% carbohydrate, 30% fat and 10% protein), or oral glucose tolerance test (in one study),Citation11 was assessed at baseline and at week 24.
Data analysis
The mean PSD (vildagliptin−placebo) in ISR/G 0–2h change from baseline in each study was evaluated as a function of mean age, disease duration, ISR/G 0–2h, HbA1c, fasting plasma glucose (FPG), body mass index (BMI) at baseline, and mean PSD (vildagliptin−placebo) in HbA1c change from baseline, using a univariate model.
Ethics and good clinical practice
All study participants provided written informed consent to participate in the respective clinical trials included in this pooled analysis. All protocols were approved by independent ethics committees/institutional review boards; individual study results have been reported as required by protocol and duly referenced in this article. All studies were conducted as per Good Clinical Practice and in accordance with the Declaration of Helsinki.
Results
The demographic and clinical characteristics of the participants by study and treatment group are presented in . The mean age, HbA1c, and T2DM duration of the study participants ranged from 50.2 to 59.3 years, 8.3% to 8.8%, and 1.6 to 13.2 years, respectively.
Table 1 Demographic and clinical characteristics of the participants by study and treatment group
There was a strong negative association between the mean PSD in ISR/G 0–2h change from baseline and mean baseline disease duration (r= −0.89, p<0.02) (). However, no association was observed between the mean PSD in HbA1c change from baseline and mean baseline disease duration (r= −0.23, p=0.66) ().
Figure 1 Association between mean difference (vilda–pbo) in ISR/G change from baseline and mean difference (vilda–pbo) in HbA1c change from baseline vs mean duration of diabetes at baseline.
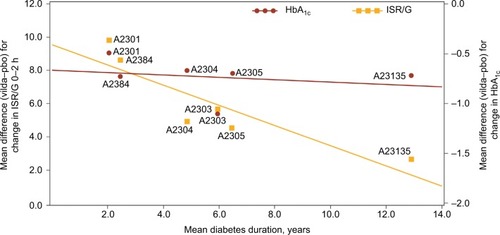
In addition, there was no association between the mean PSD in ISR/G 0–2h change from baseline and the mean PSD in HbA1c change from baseline (r=0.33, p=0.52), age (r= −0.81, p=0.05), baseline HbA1c (r= −0.59, p=0.11), ISR/G 0–2h (r=0.43, p=0.39), FPG (r=0.34, p=0.51), or BMI (r=0.70, p=0.12).
Discussion
The UKPDS demonstrated that glycemic control is progressively lost over time and that this loss is associated with a progressive loss of β-cell function.Citation1 The ADOPT study showed that treatment with sulfonylurea, metformin, and pioglitazone demonstrates varying degrees of sustained durability, but eventually glycemic control is lost over time,Citation12 and this loss is associated with a reduction in β-cell function.Citation2 We hypothesized that this would translate into a reduction in β-cell response over time following vildagliptin therapy. The present analysis demonstrates that β-cell response (ISR/G) to vildagliptin treatment was indeed attenuated in patients with longer duration of diabetes.
The ISR/G depends on the sensitivity of β-cells to glucose and their maximum secretory capacity. β-cell function diminishes early in the disease progression due to decrease in the sensitivity of the β-cells to glucose and later due to a reduction in the maximum insulin-secretion capacity. DPP-4 inhibitors are known to improve the sensitivity of the β-cells to glucose, but they do not have a pronounced effect on the maximum secretory capacity which is largely determined by β-cell mass.Citation13 We assume that early in the disease, vildagliptin mostly corrects the sensitivity defect, and continues to do so even later, while the overall effect on ISR/G is diminished by the progressive loss of the maximum capacity of insulin secretion.
We have previously shown with a larger pooled analysis of vildagliptin data that baseline-adjusted reduction in HbA1c is the same in drug-naïve patients with a short duration of diabetes and in patients with a long duration of diabetes on insulin therapy.Citation5 In the current pooled analysis, which is a subset of the original pool, wherein β-cell function was assessed, we replicated this finding. This suggests that some element of vildagliptin action is progressively increasing to compensate for the reduced β-cell response in patients with T2DM.
Vildagliptin prolongs the meal induced increase in GLP-1 and GIP, which increases the sensitivity of the β-cells to glucose to increase insulin secretion during meals. The GLP-1 also increases the sensitivity of the α-cells to glucose to decrease glucagon secretion during meals. Physiologically, the reduction in glucagon secretion may be as important as the increase in insulin secretion in reducing hepatic glucose production.Citation4 As the β-cell capacity declines, there is no known corresponding change in α-cell capacity. However, the diminished β-cell capacity could result in a reduced insulin paracrine inhibition of glucagon secretion, which is not mediated via GLP-1.Citation14 The attenuated paracrine inhibition could lead to further increase in the already-increased glucagon level, which can, however, be suppressed by GLP-1 and its associated increase in α-cell sensitivity following DPP-4 inhibition. Thus, we speculate that glucagon suppression resulting from the enhanced GLP-1 levels following DPP-4 inhibition may be compensating the reduced β-cell function, leading to a similar baseline adjusted reduction in HbA1c.
Conclusion
The results indicate that the response of the β-cell, but not the HbA1c reduction, with vildagliptin is dependent on the duration of T2DM, and it can be speculated that glucagon suppression may become the predominant mechanism via which glycemic control is improved when treatment with a DPP-4 inhibitor such as vildagliptin is initiated late in the natural course of T2DM.
Author contributions
All authors participated in the design, data review, data interpretation, and critically revised the manuscript. PK and JEF wrote the manuscript, and VB carried out the statistical analyses. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
The authors acknowledge the patients, investigators, and staff at participating sites of all the studies. The authors also thank Sashi Kiran Goteti Novartis Healthcare Pvt. Ltd., Hyderabad, India, for editorial assistance.
Disclosure
PK and JEF are employed by and own stocks in Novartis. VB is employed by Novartis. The study was funded by Novartis Pharma AG. Part of this data was presented as a poster (1093-P) at the 76th Scientific Sessions of the American Diabetes Association, June 10–14, 2016, New Orleans, LA, USA. The authors report no other conflicts of interest in this work.
References
- No authors listedU.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study GroupDiabetes19954411124912587589820
- KahnSELachinJMZinmanBEffects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPTDiabetes20116051552156021415383
- AhrénBSchweizerADejagerSVillhauerEBDunningBEFoleyJEMechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humansDiabetes Obes Metab201113977578321507182
- AhrénBFoleyJEImproved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolismDiabetologia201659590791726894277
- SchweizerADejagerSFoleyJEImpact of insulin resistance, body mass index, disease duration, and duration of metformin use on the efficacy of vildagliptinDiabetes Ther201231822736406
- DejagerSRazacSFoleyJESchweizerAVildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose studyHorm Metab Res200739321822317373638
- Pi-SunyerFXSchweizerAMillsDDejagerSEfficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetesDiabetes Res Clin Pract200776113213817223217
- BosiECamisascaRPColloberCRochotteEGarberAJEffects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metforminDiabetes Care200730489089517277036
- GarberAJSchweizerABaronMARochotteEDejagerSVildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled studyDiabetes Obes Metab20079216617417300592
- GarberAJFoleyJEBanerjiMAEffects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulfonylureaDiabetes Obes Metab200810111047105618284434
- KothnyWFoleyJKozlovskiPShaoQGallwitzBLukashevichVImproved glycemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitusDiabetes Obes Metab201315325225723039321
- KahnSEHaffnerSMHeiseMAGlycemic durability of rosiglitazone, metformin, or glyburide monotherapyN Engl J Med2006355232427244317145742
- FoleyJEBunckMCPoelmaMBeta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomized controlled trialDiabetologia20115481985199121547496
- FoleyJELiqueros-SaylanMHeYLEffect of vildagliptin on glucagon secretion during meals in patients with type 1 diabetesHorm Metab Res2008401072773018597213
