Abstract
Background
Men with coronary artery disease (CAD) have been shown to have enhanced arterial stiffness. Arterial function may change over time according to treatment, but the prognostic value of these changes has not been investigated.
Objectives
The aim of the present study was to assess whether an improvement in large artery rigidity in response to treatment, could predict a more favorable prognosis in a population of men with CAD.
Methods
A total of 161 men with CAD (mean age 56.8 ± 10.9 years) being treated with conventional therapy underwent brachial-ankle pulse wave velocity (PWVba) measurements at baseline and after six months. Follow-up period was 3.5 years. End-points were major adverse cardiac events (MACE): acute myocardial infarction, unstable angina, coronary intervention, or cardiac death.
Results
During the three-year follow-up period (since initial six-month follow-up), 30 patients experienced MACE. After six-month follow-up, PWVba had not improved (ΔPWVba ≥ 0%, relative to baseline) in 85 (52.8%) of 161 men (Group 1), whereas it had improved (ΔPWVba < 0%) in the remaining 76 men (47.2%) (Group 2). During follow-up, we noticed 24 cardiovascular events in Group 1 and six events in Group 2 (P < 0.001). Cox proportional hazards analyses demonstrated that independent of conventional risk factor changes, absence of PWVba decrease was a predictor of MACE (RR 3.99; 95% CI:1.81–8.78; P = 0.004). The sensitivity of ΔPWVba was 80% and its specificity was 54%.
Conclusions
This study demonstrates that an improvement in arterial stiffness may be obtained after six months of conventional therapy and clearly identifies patients who have a more favorable prognosis.
Introduction
Elevated arterial stiffness has been associated with progression of cardiovascular morbidity and mortality due to arterial hypertension, end-stage renal failure, diabetes mellitus, and other conditions.Citation1–Citation3 Data exist to show that decreased arterial elasticity is a predictor of future development of cardiovascular complications in patients with coronary artery disease (CAD).Citation4,Citation5 In order to consider increased arterial stiffness as something necessitating therapeutic focus, crucial data has been acquired showing that decreasing arterial rigidity leads directly to a decreased risk of major adverse cardiac events (MACE). Obtaining such confirmation was the purpose of this work.
Methods
Patients
After giving informed consent, ambulatory patients aged 35–75 years, with proven clinically stable coronary artery disease, were considered for the study. Eligible participants were recruited by the outpatient department of the Russian Cardiology Research Center in Moscow if they met at least one of the following inclusion criteria: 1) history of myocardial infarction; 2) angiographic evidence of at least 50% stenosis in at least one coronary artery; 3) evidence of exercise-induced ischemia on treadmill electrocardiogram or stress nuclear perfusion imaging; or 4) history of coronary revascularization.
The study began in September 2004 and recruitment was completed in September 2005. The mean ± SD for follow-up was 586 ± 461 days. Of the 224 consecutive patients initially screened, 161 remained on the study for our 6-month follow-up examination. All 161 patients were white males, with a 12% prevalence of diabetes mellitus (). The mean ± SD age of the cohort at inclusion was 56.8 ± 10.9 years. Major exclusion criteria included: acute myocardial infarction (MI) or stroke within the past three months; percutaneous coronary intervention (PCI), coronary-artery bypass grafting (CABG), implantation, or planned implantation of a cardioverter defibrillator or biventricular pacemaker within the past three months; clinically significant, uncorrected primary valvular heart; hypertrophic cardiomyopathy; systemic disease; acute or chronic liver disease; plasma creatinine level >2.5 mg/dL; current presence of clinical signs of heart failure, and ejection fraction below 40% as assessed locally by echocardiography or another method if echocardiography was technically impossible; diabetes mellitus (DM) requiring medication; any other condition that would substantially reduce life expectancy or limit compliance with the protocol.
Table 1 Characteristics of studied population (n = 161)
All patients received conventional therapy. Characteristics of the studied population are reported in .
Data collection
Information compiled from the questionnaire filled out at inclusion included: personal and family histories; smoking habits; and prior history of cardiovascular disease, including cardiac insufficiency, peripheral vascular disease, and cerebrovascular disease. Twice—at baseline and after six months—blood pressure (BP), fasting blood glucose (FBG), serum total cholesterol (TC), triglycerides (TG), body mass index (BMI), arterial stiffness, and other risk factors were assessed. BP was measured in the right arm after five minutes of recumbence using a mercury sphygmomanometer and cuff of appropriate size. Phases I and V of the Korotkoff sounds were taken as the systolic BP (SBP) and diastolic BP (DBP) values respectively. The mean BP (MBP) was calculated as follows: MBP = DBP + [(SBP – DBP)/3]. Three measurements were made at two-minute intervals; the last two measurements were averaged and considered to be representative. The patient’s heart rate was determined from the three-lead orthogonal ECG. Arterial stiffness was assessed using the brachial-ankle pulse wave velocity (PWVba) with a VaSera VS-1000 device (Fukuda Denshi, Tokyo, Japan).Citation6,Citation7 Measurements were made according to the recommendations for user procedures.Citation8 The PWVba was measured at inclusion and again after six months. The change in PWVba (ΔPWVba), measured in meters per second (m/s) and used as a prognostic variable, was quantified as follows: ΔPWVba = (PWVba after six months) – (PWVba at inclusion).
Follow-up
The patients were followed every six months. Upon follow-up, patients were asked to fill out a questionnaire regarding hospitalizations and outpatient clinic visits in the preceding six months. ACE of interest for this study were acute myocardial infarction, unstable angina, coronary intervention, or cardiac death. When a possible event was recorded by the participant, hospital discharge letters, results of laboratories, and other relevant examinations were collected.
Analyses
The primary analysis concerned the survival curves and Cox proportional hazards model. Survival was estimated by the Kaplan–Meier product-limit method and compared using the Mantel (log-rank) test. Factors prognostic of survival were identified with use of the Cox proportional hazards regression model. The assumption of proportional hazards over time was verified before the analyses and was met by all covariates. The assumption concerning linearity of continuous covariates was also verified before analysis. Multivariate Cox modeling was the primary statistical analysis used to determine the independent relationship of PWV changes and other baseline characteristics with survival. Variables were considered to be prognostic when they were found to be statistically significant in the Cox proportional hazards regression model (P < 0.05, adjusted for all variables retained in the final model). Adjusted hazard rate ratios (RR) were calculated as the antilogarithm of the ß coefficient of the Cox proportional hazards regression of the outcome events, with different variables entered in the models. The 95% confidence intervals (CI) for the adjusted RR estimates were obtained using the following formula: antilogarithm (ß ± 1.96 SE), where SE is the standard error of ß.
To assess ΔPWV as a prognostic variable test with the use of receiver operating characteristic (ROC) curves, we calculated sensitivities, specificities, positive predictive values, and negative predictive values to predict mortality at different cutoff values. Optimal PWV cutoff values were defined as the maximization of the sum of sensitivity and specificity.
Data are expressed as mean ± SD for normally distributed variables or median (lowest quartile; highest quartile) for not normally distributed variables. ANOVA was used for comparison of normally distributed variables and in the alternative situation, the non parametric equivalent Kruskal–Willis test was used. Differences in frequency were tested by χ2 analysis. All tests were two-sided, and analyses were performed using SPSS (v. 14.0;SPSS, Chicago, IL) and STATISTICA (v. 6.0;Statsoft, Tulsa. OK) software. A value of P < 0.05 was considered significant.
Results
In the studied cohort, from six months until the end of the study, we recorded 30 cardiovascular complications: six MI, 11 PCI and CABG, 10 hospitalizations due to unstable angina, and three cardiac deaths. Patients who experienced MACE in the first six months of observation were not included in our analyses. All investigating parameter changes, from inclusion until six months time, are reported in . For the first six months, only diastolic BP and MAP showed a small (non significant) decrease, while all other parameters remained unchanged.
Table 2 Changes in investigating parameters from inclusion until six months of follow-up in entire cohort
The ΔPWV was correlated with changes in MAP (r = 0.36; P < 0.001), SBP (r = 0.32; P < 0.001), DBP (r = 0.32; P < 0.001), but not to ΔFGB, ΔTC, or ΔTG.
Patients were separated into two groups based on the direction of change in the stiffness of their arterial walls. The ROC curve showed that the cutoff value for ΔPWV was 0.05 m/s (ie, ≅ 0). Area under the curve was 0.62 ± 0.05 (P = 0.032). In six months, PWVba decreased in 76 patients (Group 1) and in the other 85 patients, either an increase or no change was observed (Group 2). In patients who were observed to have improved mechanical characteristics of their large arteries in the first six months, there were six MACE, while the others had 24 MACE. In our analyses, an increased or unchanged PWVba was defined as “positive” and a decrease in PWVba was considered “negative”. Characteristics of patients in each group are provided in .
Table 3 Characteristics of patients in groups with “negative” and “positive” PWVba dynamics for the first six months of observation (n = 161)
In patients with a demonstrated decrease in PWVba at six months (Group 1), it was shown that there was a higher baseline PWVba 14.19 ± 2.40 m/s, compared with 13.15 ± 2.16 m/s (P = 0.004) in Group 2.
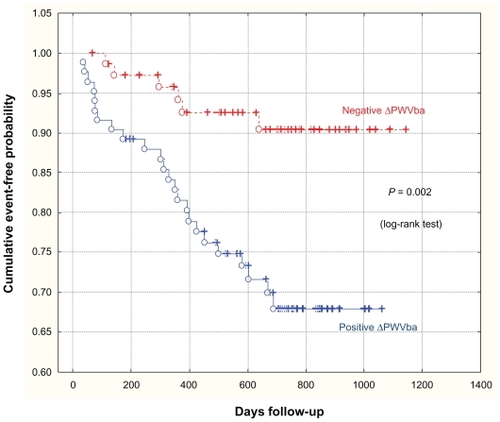
For 70 of 76 patients in Group 1, there were no MACE in the last three years of the study, while 24 out of 85 patients in Group 2 had complications. The sensitivity of ΔPWV was 80% and its specificity was 54%.
Thus, with the aid of univariate Cox proportional hazard regression analysis, we studied the influence of given parameters, at the six-month follow-up, on the occurrence of MACE in the next three years. Absence of arterial stiffness decrease was a predictor of MACE. Increase, or absence of change in PWVba (positive ΔPWVba), increased the risk of MACE in the following three years by nearly four times (RR 3.77; 95% CI:1.83–7.77; t = 3.02, P = 0.003).
The role of factors such as smoking, BMI, previous MI, and DM were not found to be significant. Only 12% of patients were found to have DM and all of these were treated with dietary adjustments. Age at baseline was not correlated with prognosis in this cohort (RR 0.99; 95% CI:0.96–1.03, P = 0.787). Six-month change in SBP (RR 0.99; 95% CI:0.67–1.01; P = 0.270), DBP (RR 0.97; 95% CI:0.94–1.01; P = 0.159), PP (RR 0.99; 95% CI:0.97–1.02; P = 0.600), MAP (RR 0.98–1.01; 95% CI:0.95–1.01; P = 0.171), and blood chemistry were not significant. Also, the type of medication was not found to be predictive of outcome.
To assess the influence of changes in PWVba on occurrence of MACE when considering the contribution of other factors, three models of the Cox proportional hazards regression, with a different set of dependent variables, have been created (). ΔPWVba was not found to influence prognosis, when age at baseline, and changes in MAP at six-month follow-up here applied to the first model (Model 1). In Model 2, we included ΔPWVba, ΔTC, and ΔSBP at six months, as well as age and whether the patient was a smoker at the moment of inclusion. After applying adjustments for other studied factors (Model 3), PWVba was still found to have an influence on prognosis ().
Table 4 Multivariate Cox proportional hazard regression analyses of MACE (n = 161)
Kaplan–Meier curves show the probabilities of event-free survival of patients with negative ΔPWV or positive ΔPWV ().
Discussion
Carotid-femoral PWV method is considered to be the “gold standard” for measurement of arterial rigidity. This method has been used in the majority of epidemiological studies demonstrating the predictive value of aortic stiffness for cardiovascular events. In their studies preceding our investigation, Japanese researchers focused on the value of brachial-ankle pulse wave velocity (PWVba) testing and showed the aortic PWV as the primary independent correlate of PWVba.Citation9 We employed the PWVba method after considering that it is simple to use, inexpensive, well reproduced, and its informative value has been demonstrated in multiple studies.Citation5–Citation7,Citation10 Additionally, we used the PWVba method because it is more comfortable for the average patient, since it allows him/her to remain fully dressed during the procedure.
Our own previous publications, as well as those by other investigators, have shown that increased arterial rigidity in patients with CAD is associated with increased risk of MACE.Citation5,Citation4,Citation11 The expert consensus on arterial stiffness states that clinical trials need to be conducted in order to determine whether a reduction in arterial stiffness is a desirable therapeutic goal, in terms of hard clinical endpoints such as morbidity and mortality.Citation12 In the current study, we aimed to answer the question of whether a decrease in arterial stiffness leads to improved prognosis in male patients suffering from CAD.
Based on the data from the group as a whole, we did not note distinctions in the levels of PWVba, BP, TC, TG, and FBG throughout the term of the study. Regression analysis demonstrated that ΔPWVba was significantly correlated with ΔBP. The closest correlation we found was between ΔPWVba and delta MAP (r = 0.36; P < 0.001). In many studies executed on hypertensive and normotensive patients, there is a parallel decrease in BP and PWV as a result of therapy.Citation13,Citation14 However, it is important to remember that in and of itself, BP is one of the most meaningful factors of cardiovascular risk and a decrease in BP results in improved prognosis.Citation15 The next question is whether reduction of morbidity and mortality in patients with cardiovascular pathology is only due to the influence of therapy on BP, or if some medications possess their own additional influence on the patient’s prognosis. In the last few years, there have been a few published studies focusing on some medications which have a “BP-independent” positive influence on stiffness of large arteries and central hemodynamics.Citation16–Citation18
The vast majority of patients received massive vasoactive therapy (angiotensin-converting-enzyme inhibitor or angiotensin II receptor blockers about 60%; β-blockers > 70%; calcium channel blockers and nitrates > 20%), both before inclusion and throughout the course of our study. One of the goals of the study was to find out whether variations in arterial stiffness have an influence on the three-year prognosis of patients with CAD, irrespective of change in BP.
As it stands today, only Guerin et al have shown the influence of aortic stiffness attenuation on survival of patients (a study of end-stage renal failure patients).Citation19 In their study, all patients received antihypertensive therapy at inclusion. From entry until the end of follow-up, the changes in aortic PWV in response to decreased BP were measured using ultrasound. At target BP, patients were separated into two groups based on the direction of change in the PWV. PWV, like in our study, correlated with ΔSBP (r = 0.54; P < 0.0001) and was not tied to variations in biochemical parameters or type of applied therapy. Analysis at the end of the study revealed that in the group with ΔPWVba ≥ 0, there was a 70% mortality rate, while in the other group, this rate was only 26%.
Following the method of Guerin et alCitation19 we too separated patients into groups according to the direction of variation in their PWVba in the first six months. For 85 patients, arterial stiffness either remained unchanged, or increased (ΔPWVba ≥ 0), while it decreased for the other 76 patients (ΔPWVba < 0). Thus, 80% of all MACEs occurred in the group with positive PWVba, as compared with 20% in the negative PWVba group. Based on our data, the sensitivity of PWV was 80% and its specificity was 54%. For Guerin et al these parameters were 56% and 84%, respectively.Citation19
Univariate Cox analysis showed that the absence of PWVba decrease in the first six months carried a 3.8 times MACE risk increase for these patients. Changes in other risk factors were not associated with improved prognosis. Adjustment for age and ΔMAP (Model 1), age and smoking profile at inclusion, and change in BP and TC at six-month follow-up (Model 2), and other factors (Model 3), PWVba was found to have maintained its prognostic predictive value.
With the exception of statins, there were no differences in medication between groups with “positive” and “negative” ΔPWVba. In the group with “positive” ΔPWVba there was a 12% higher rate of statin use than in the “negative” ΔPWVba group (P = 0.023).
It should be noted that patients who showed a decreased PWVba in six months had, on average, higher values of this parameter at inclusion in the study than patients with an increase in PWVba. In the “negative” ΔPWVba group, at inclusion, there were higher values for BP and TC (P < 0.05). Taking these facts into account, at baseline, patients showing a decrease in arterial stiffness seem to be at higher risk of MACE. Also, patients who in the first six months of therapy were found to have an increased or unchanged PWVba, likely had more advanced vascular lesions which were either irreversible or required a more prolonged period of treatment.
Many authors, when discussing pharmacological influence on arterial rigidity, separate “acute” effects from “chronic” effects. Acute effects are to a greater degree connected with reduced vascular tone, while chronic effects are mediated through structural modifications of the arterial wall.Citation20,Citation21 In various studies, positive vascular remodeling was evaluated after 6–9 months or more, and was not dependent upon other risk factors.Citation16,Citation18,Citation22 These and other works have allowed for the 2006 Expert Consensus document on arterial stiffness to conclude that arterial stiffness attenuation may reflect the true reduction of arterial wall damage, whereas BP, glycemia, and lipids can be normalized in a few weeks by using antihypertensive, anti diabetic, and lipid-lowering drugs, leading to a strong reduction in CV risk scores, but without any improvement yet of atherosclerotic lesions and arterial stiffness, which requires a long-lasting correction of biochemical abnormalities.Citation12 Therefore, it can be supposed that a therapeutic strategy, having the goal of reducing arterial stiffness, can lead to more significant improvement of prognosis than correction of individual risk factors.
Conclusion
Decreased PWVba on a six-month background of treatment is an independent predictor of favorable prognosis in the next three years for men with CAD. Our results suggest that for patients with CAD, on a long treatment background, reduction of rigidity in large arteries may appear to be a more reliable criterion of therapeutic efficacy than the dynamics of conventional cardiovascular risk factors.
Acknowledgment
This study was supported by Mr Alexander Shapeton of University of Massachusetts Medical School.
Disclosure
The authors report no conflicts of interest in this work.
References
- BlacherJGuerinAPPannierBMarchaisSJSafarMELondonGMImpact of aortic stiffness on survival in end-stage renal diseaseCirculation1999992434243910318666
- LaurentSBoutouyriePAsmarRAortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patientsHypertension2001371236124111358934
- CruickshankKRisteLAndersonSGWrightJSDunnGGoslingRGAortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function?Circulation20021062085209012379578
- StefanadisCDernellisJTsiamisEAortic stiffness as a risk factor for recurrent acute coronary events in patients with ischemic heart diseaseEur Heart J20002139039610666353
- TomiyamaHKojiYYambeMBrachial-ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndromeCirc J20056981582215988107
- YamashinaATomiyamaHTakedaKValidity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurementHypertens Res20022535936412135313
- LiuHYambeTZhangXComparison of brachial-ankle pulse wave velocity in Japanese and RussiansTohoku J Exp Med200520726327016272796
- van BortelLMDuprezDStarmans-KoolMJClinical applications of arterial stiffness, Task Force III: recommendations for user proceduresAm J Hypertens20021544545212022247
- SugawaraJHayashiKYokoiTBrachial-ankle pulse wave velocity: an index of central arterial stiffness?J Hum Hypertens20051940140615729378
- ImanishiRSetoSTodaGHigh brachial-ankle pulse wave velocity is an independent predictor of the presence of coronary artery disease in menHypertens Res200427717815005269
- OrlovaIaAKuz’minaAEMasenkoVPIarovaiaEBAgeevFTEffect of arterial stiffness on development of cardio-vascular complications in ischemic heart diseaseKardiologiia200949111720038275
- LaurentSCockcroftJvan BortelLfor European Network for Non-invasive Investigation of Large ArteriesExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J2006272588260517000623
- AsmarRTopouchianJPannierBBenetosASafarMfor Scientific, Quality Control, Coordination and Investigation Committees of the Complior StudyPulse wave velocity as endpoint in large-scale intervention trial. The Complior study. Scientific, Quality Control, Coordination and Investigation Committees of the Complior StudyJ Hypertens20011981381811330885
- KimKHJeongMHChoSHClinical effects of calcium channel blocker and Angiotensin converting enzyme inhibitor on endothelial function and arterial stiffness in patients with angina pectorisJ Korean Med Sci20092422323119399262
- StaessenJAWangJGThijsLCardiovascular protection and blood pressure reduction: a meta-analysisLancet20013581305131511684211
- TropeanoAIBoutouyriePPannierBBrachial pressure-independent reduction in carotid stiffness after long-term angiotensin-converting enzyme inhibition in diabetic hypertensivesHypertension200648808616702490
- KarallieddeJSmithAdeAngelisLValsartan improves arterial stiffness in type 2 diabetes independently of blood pressure loweringHypertension2008511717998477
- ProtogerouABlacherJStergiouGSAchimastosASafarMEBlood pressure response under chronic antihypertensive drug therapy: the role of aortic stiffness in the REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double-Blind) studyJ Am Coll Cardiol20095344545119179203
- GuerinAPBlacherJPannierBMarchaisSJSafarMELondonGMImpact of aortic stiffness attenuation on survival of patients in end-stage renal failureCirculation200110398799211181474
- LaurentSBoutouyriePLacolleyPStructural and genetic bases of arterial stiffnessHypertension2005451050105515851625
- LacolleyPGhodsiNGlazerEInfluence of graded changes in vasomotor tone on the carotid arterial mechanics in live spontaneously hypertensive ratsBr J Pharmacol1995115123512447582551
- BoutouyriePBussyCHayozDLocal pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatmentCirculation20001012601260610840011