Abstract
Mineralocorticoid-receptor antagonists (MRAs) have proven to be effective in some types of hypertension, especially in resistant hypertension (RHTN). In this phenotype of hypertension, the renin–angiotensin–aldosterone pathway plays an important role, with MRAs being especially effective in reducing blood pressure. In this review, we show the relevance of aldosterone in RHTN, as well as some clinical characteristics of this condition and the main concepts involving its pathophysiology and cardiovascular damage. We analyzed the mechanisms of action and clinical effects of two current MRAs – spironolactone and eplerenone – both of which are useful in RHTN, with special attention to the former. RHTN represents a significant minority (10%–15%) of hypertension cases. However, primary-care physicians, cardiologists, nephrologists, neurologists, and geriatricians face this health problem on a daily basis. MRAs are likely one of the best pharmacological options in RHTN patients; however, they are still underused.
Introduction
The association between arterial hypertension (HTN) and primary aldosteronism (PA) has been investigated for over 50 years. The first reported case was a patient with severe HTN and hypokalemia associated with elevated aldosterone secretion. In this patient, HTN was cured by adrenalectomy.Citation1 From this initial finding, researchers sought to establish a cause-and-effect association between HTN and elevated plasma aldosterone levels.Citation2 Although the PA that accompanies Conn’s syndrome is well defined as a cause of secondary HTN, many aspects related to the pathophysiology, prevalence, and relevance of this association in the absence of a tumor mass detectable by conventional diagnostic methods are being discussed in research centers worldwide.Citation3–Citation6 For decades, researchers have demonstrated varying degrees of association between HTN and PA,Citation7–Citation14 especially the clinical presentation in normokalemia, first described in 1965.Citation15 Subsequently, evidence suggested an important role of aldosterone in the pathophysiological mechanism of target-organ damage observed in hypertensive disease.Citation16,Citation17 Many authors have shown a relationship between aldosterone and left-ventricular hypertrophy (LVH), renal injury, vascular disease,Citation18–Citation25 atrial flutter and atrial fibrillation,Citation26 and structural and functional alterations of medium-caliber arteries,Citation27,Citation28 as well as microcirculation injuries and alterations in endothelial function.Citation29–Citation35
In 2002, Calhoun et alCitation36 found a PA prevalence of 20% in resistant hypertensive subjects submitted to high sodium intake (>200 mEq/24 hours) with renin dosage >12 μg/24 hours or under renin suppression (<1 ng·mL/hour). Gallay et al reported a PA prevalence of 17% in resistant hypertensive patients in Seattle (WA, USA) based on a high aldosterone:renin ratio (ARR).Citation37 Likewise, researchers from the Czech Republic showed that 19% of patients referred for treatment for moderate–severe HTN presented PA.Citation38 Researchers in Oslo found a prevalence of 23% of the same condition in resistant hypertensive subjects,Citation39 thereby confirming higher PA prevalence in individuals with resistant HTN (RHTN) than in the general hypertensive population.
In recent systematic reviews and meta-regression analyses, authors have demonstrated a wide range of prevalence in primary-care centers (3.2%–12.7%) and HTN referral centers (1%–29.8%).Citation40 Moreover, data on patients with RHTN in five studies reported on the relationship between PA prevalence and HTN severity. Because of the heterogeneity of PA prevalence, further multicenter studies with standardized screening and a confirmation test are necessary. On the other hand, there is no robust evidence that mineralocorticoid-receptor antagonists (MRAs) prevent more cardiovascular events in RHTN patients than other antihypertensive drugs.
In 2008, the American Heart Association defined RHTN as “… blood pressure [BP] that remains above goal in spite of the concurrent use of three antihypertensive agents of different classes. Ideally, one of the three agents should be a diuretic and all agents should be prescribed at optimal dose amounts”.Citation41 In addition, patients, who require four or more medications to control BP are considered resistant to treatment. Almost a decade later, besides the required thiazide diuretic, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), and calcium-channel blockers probably constitute the “gold trio” in the treatment of RHTN.
The complex pathophysiology of RHTN involves multiple mechanisms, including hyperactivity of the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system, volume overload, endothelial dysfunction, and lifestyle factors. However, no specific fourth-line drug is recommended for RHTN treatment, although five antihypertensive drug classes (MRAs, α1-adrenergic antagonists, α2-adrenergic agonists, β-blockers, and peripheral vasodilators) are suggested as alternatives. In addition, the increased aldosterone and plasma volume in RHTN patients suggest that aldosterone antagonists should be the fourth drug for this condition. In light of the growing clinical benefits reported in RHTN, the mechanisms of action and effects of aldosterone antagonists deserve attention and are summarized herein.
Aldosterone in the cardiovascular system in RHTN
BP control
Many observational studies have linked aldosterone excess to lack of BP control in RHTN subjects. This is a clinical condition different from PA, which is a secondary cause of HTN. In RHTN, Pimenta et alCitation42 showed that patients with elevated 24-hour urinary aldosterone excretion presented sustained increases in 24-hour ambulatory BP, including nocturnal BP levels. Furthermore, these authors highlighted an interesting aldosterone–age interaction in relation to 24-hour systolic BP: as subjects with high ARR got older, systolic BP also increased by as much as 20 or 30 mmHg. Some authors have also indicated that a subpopulation of RHTN – uncontrolled subjects (currently referred to as refractory hypertensive patients)Citation43 – present increases in circulating levels of aldosterone,Citation44–Citation46 which supports the relationship between aldosteronism and poor BP control. However, previous studies suggested that this association might be driven by overactivity of the sympathetic nervous system,Citation47,Citation48 another common pathophysiology mechanism underlying RHTN.Citation49,Citation50 Finally, a phenomenon named “aldosterone escape” was described in patients treated with ACE inhibitors or ARBs.Citation51,Citation52 Aldosterone escape is a condition where after an initial decline, plasma aldosterone levels increase above those identified prior to treatment. This increase neutralizes the benefits of BP reductions and the cardiovascular and renal protection obtained with antihypertensive therapy provided by RAAS blockers,Citation53 which suggests a need for MRAsCitation54 for proper BP control.
Hypervolemia and cardiac effects
RHTN patients have concomitant intravascular volume expansion in addition to the ARR elevation, despite an extensive use of diuretics.Citation55 Similar findings supporting the involvement of intravascular volume expansion in the pathophysiology of RHTN have been reported by researchers at the Mayo Clinic using thoracic impedance.Citation56 However, increased intravascular volume is not limited to patients with measurable evidence of aldosterone excess, which suggests that other factors may contribute to fluid retention or that conventional evaluation of aldosterone levels may not accurately reflect its functional role in respect to blood volume. In fact, other important regulators, such as sympathetic outflow and plasma renin, participate in the balance of sodium and fluid in RHTN. However, aldosteronism contributes to heart-volume overload in RHTN individuals, despite the use of diuretics, leading to greater LVH,Citation57–Citation60 LV diastolic dysfunction,Citation52 and increased incidence of cardiovascular events,Citation61 including myocardial infarction, stroke, and arrhythmia.Citation62 Additionally, experimental studies have shown that excessive aldosterone in the presence of high salt intake in the diet induces LVH and fibrosis.Citation63,Citation64
Experimental studies and recent studies in humansCitation65,Citation66 support the occurrence of clinically relevant and bidirectional interactions between aldosterone and parathyroid hormone (PTH), with potential impact on the cardiovascular system. There is evidence that PTH increases the adrenal aldosterone secretion directly and indirectly by activating the RAAS. This stimulation of PTH hormones in the RAAS may potentiate risks in the development and progression of HTN as well as increase the risk of cardiovascular disease in patients with primary hyperparathyroidism.Citation54,Citation67–Citation69
Obstructive sleep-apnea syndrome (OSAS), autonomic dysfunction, and obesity
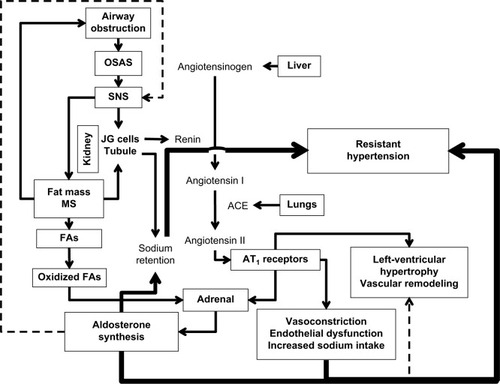
Goodfriend and CalhounCitation70 demonstrated an association between OSAS and aldosteronism in RHTN. Several mechanisms are involved in the pathophysiology of RHTN associated with OSAS, in particular hyperactivity of the sympathetic nervous system, manifested in excitement of chemoreceptors and dysfunction of baroreceptors leading to high BP.Citation71 Excessive aldosterone seems to contribute to OSAS by mechanisms involving fluid retention and edema of the nasopharynx, with consequent obstruction of the upper airways.Citation71,Citation72 Obesity also seems independently to stimulate the autonomic nervous system and thus contribute to the lack of BP control in RHTN individuals ().
Figure 1 Relationships among factors in resistant hypertension.
Abbreviations: SNS, sympathetic nervous system; OSAS, obstructive sleep-apnea syndrome; JG, juxtaglomerular; MS, metabolic syndrome; FAs, fatty acids.
Aldosterone receptors, aldosterone synthase, and polymorphisms
The effects of aldosterone are mediated through its binding to MRs,Citation73 which promotes extracellular volume retention by a genomic pathway and endothelial dysfunction, inflammation, and oxidative stress, among other cardiovascular and renal structural and functional abnormalities by nongenomic actions.Citation74 In addition to this, obesity – through the release of select adipokinesCitation75 – and overactivity of the sympathetic nervous system may stimulate aldosterone secretion.Citation70 In this context, and due to the complex condition of RHTN and its associated comorbidities, it is important to remember that aldosterone, with its elevated levels mediating several maladaptive changes, seems to be a complicating factor in subjects resistant to antihypertensive therapy.
Due to its multifactorial condition, HTN may be influenced by a genetic factor, which may also explain the current interindividual variability in plasma aldosterone levels. Aldosterone synthase (CYP11B2) is an interesting target that catalyzes the final step of aldosterone synthesis in the adrenal cortex. The differences in aldosterone biosynthesis between individuals may be attributed to genetic polymorphisms of the CYP11B2 gene, as demonstrated by the community-based Framingham Heart Study.Citation76 Indeed, the –344 C/T polymorphism has been widely investigated in cardiovascular conditions. Recently, a cross-sectional study including RHTN subjects revealed that individuals with the TT polymorphism presented higher plasma aldosterone concentrations than those with the CT and CC polymorphisms, even with the use of spironolactone.Citation77 A meta-analysis demonstrated that homozygous individuals (CC) for this polymorphism were at 17% lower risk of HTN compared to TT subjects.Citation78 The presence of the T allele was also associated with higher BPCitation79 and urinary aldosterone excretion.Citation80 Furthermore, genetic polymorphisms of the MR gene (NC3C2) have also been explored. Ritter et al showed that subjects with RHTN carrying the G allele for the I180V polymorphism presented higher aldosterone levels, systolic ambulatory BP, and LVH, despite a higher proportion of ACE inhibitors and β-blocker use than homozygous AA individuals. Even with its cross-sectional design, this study suggests that this genetic variation might be a risk factor for resistance to antihypertensive therapy.Citation81
Finally, aldosterone function has been extensively discussed in recent years as a key piece in RHTN. Therefore, the addition of MRA to the usual antihypertensive treatment in this hard-to-treat condition is of great clinical importance, since it may provide additional and pronounced BP reductions.Citation82,Citation83
Spironolactone
Pharmacological aspects
MRAs being indicated for the treatment of RHTN is based on studies that have shown effectiveness, safety, and cardiovascular and renal protection.Citation82,Citation84–Citation88 Spironolactone is an unselective MRA that has a complex metabolism and a half-life exceeding 12 hours in healthy individuals, 24 hours in patients with heart failure, and up to 58 hours in cirrhotic patients with ascites. The most common side effects observed with spironolactone – gynecomastia, breast pains, erectile dysfunction, and menstrual irregularities – result from the binding of the drug to the androgen receptor, preventing its interaction with dihydrotestosterone. The incidence of these adverse effects is not high (approximately 2%–9% of patients) and reversible after discontinuation of treatment.Citation82,Citation85
Spironolactone in RHTN
In 2003, Nishizaka et alCitation89 highlighted the importance of adding a low dose of spironolactone to the therapeutic scheme of patients with RHTN, with the aim of obtaining an additional reduction in BP in both black and Caucasian populations, regardless of ARR. Sartori et alCitation90 conducted the first prospective study involving difficult-to-control hypertensive patients with high ARR, and showed the importance of this ratio in the pathophysiology of RHTN, even in the absence of clinical manifestations, thus reinforcing the inclusion of aldosterone antagonists in the therapy of these patients. Lane et alCitation91 evaluated resistant hypertensive patients, adding spironolactone (25–50 mg/day) to standard triple therapy. These authors observed an additional antihypertensive effect in this group of subjects, suggesting that the addition of spironolactone may be useful, even in the absence of an elevated ARR in RHTN. Other studiesCitation84,Citation92–Citation95 substantiated the importance of the addition of spironolactone in antihypertensive therapy of RHTN patients. However, the high incidence of gynecomastia and breast pain among patients taking this drug was significant.
Eplerenone
A multicenter, double-blinded, placebo-controlled trial demonstrated that eplerenone was effective in reducing BP in subjects with mild–moderate HTN compared to a placebo. In addition, no clinically relevant safety issues were observed in eplerenone-treated subjects.Citation96 Selective aldosterone blockade with eplerenone was also useful as an add-on therapy in hypertensive patients who were inadequately controlled on either ACE inhibitors or ARBs alone.Citation97 Either alone or in combination with enalapril, eplerenone also proved to be effective in regression of target-organ damage, such as LVH in hypertensive subjectsCitation98 and albuminuria in type 2 diabetic patients,Citation99 but was found to be even better when combined with an ACE inhibitor. Moreover, eplerenone reduces arterial stiffness, the collagen:elastin ratio, and circulating inflammatory mediators.Citation100 All these findings in HTN favor the use of eplerenone as the fourth drug to treat RHTN.
The selective aldosterone antagonist eplerenone has also been explored in RHTN. This drug proved to be effective and well tolerated, with modest changes in serum potassium in this high-risk population. At the end of a 12-week active-treatment period added to the complex medication regimen of RHTN subjects, the change from baseline in 24-hour mean BP was −12.2/−6 mmHg (P<0.0001).Citation82 Moreover, the addition of eplerenone enabled 39% of patients to achieve 24-hour average ambulatory BP levels <135/85 mmHg and a 63.5% success rate in achieving office systolic BP <140 mmHg. Despite this, aldosterone and renin activity could not predict BP responses to eplerenone in this study population.Citation82 In addition, a randomized, open-label, parallel-controlled trial demonstrated that endothelial function – assessed by flow-mediated vasodilation – improved after 12 weeks of eplerenone, which seems to be a BP-independent effect.Citation101 Taken together, these findings suggest that eplerenone treatment not only reduces BP levels but also limits end-organ damage.
The use of aldosterone antagonists in RHTN: current insights and perspectives
Currently, MRAs are not indicated to be used as first-, second-, or even third-line drugs in RHTN. On the other hand, due to the high prevalence of PA in RHTN, studies have shown relevant antihypertensive benefits of adding an MRA to the existing multidrug regimen.Citation85,Citation102 Williams et alCitation83 performed the first randomized controlled trial (the PATHWAY-2 study) comparing different antihypertensive treatments in a rigorous assessment of subjects with RHTN. In this study, spironolactone (25–50 mg/day) was clearly considered the most effective drug added to the three recommended drugs – an ACE inhibitor/angiotensin II-receptor blocker, plus a calcium-channel blocker, plus a thiazide-like diuretic – in the treatment of RHTN.Citation49 This trial is considered a milestone, since it overcame the limitations of previous observational and interventional studies, mainly because PATHWAY-2 was designed to use active comparators (widely used antihypertensive drugs), instead of just a placebo.Citation103 Moreover, a recent systematic review and meta-analysis has supported it by comparing MRAs with other fourth-line antihypertensive agents in patients with RHTN. MRAs reduced BP more effectively than the other fourth-line agents in randomized and nonrandomized studies.Citation104
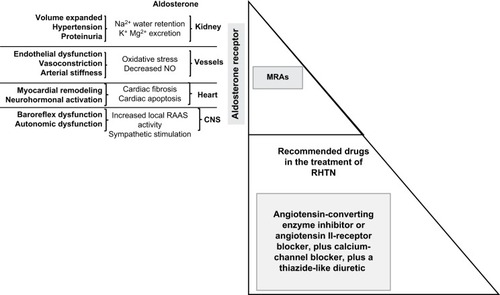
It is well known that the effect of MRAs on BP reduction includes renal and extrarenal pathways. Treatment with MRAs has been shown to attenuate end-organ damage, mainly because of prevention of nongenomic effects of aldosterone, which lead to increased arterial stiffness and oxidative stress. For instance, reduced cardiac hypertrophy was seen at 3- and 6-month follow-ups in RHTN subjects (with or without PA) taking spironolactone (added to the ongoing antihypertensive regimen at 25 mg/day and force-titrated to 50 mg/day at 4 weeks).Citation105 The BP-lowering effect and BP-independent effect contribute to place MRAs as very attractive drugs to treat the complex and multifactorial condition of RHTN ().
Figure 2 Fourth-line antihypertensive agents in patients with RHTN.
Abbreviations: RHTN, resistant hypertension; MRAs, mineralocorticoid-receptor agonists; RAAS, renin-angiotensin-aldosterone system; CNS, central nervous system.
The most concerning adverse effect of MRA treatment is hyperkalemia.Citation106,Citation107 Therefore, periodic monitoring of serum potassium and renal function are mandatory, especially in patients at high risk of developing this disorder (elderly patients with renal impairment or diabetes).Citation108 Taking concurrent pharmacotherapies associated with hyperkalemia, such as potassium supplements, other potassium-sparing diuretics, and nonsteroidal anti-inflammatory drugs, should be avoided. Furthermore, the concomitant use of an MRA with ACE inhibitors or ARBs requires special attention.Citation109,Citation110 Because of this, experts have recommended MRAs for subjects with RHTN only with careful monitoring of serum potassium levels. Finally, it has been demonstrated that the combined use of spironolactone with a thiazide diuretic, such as chlorthalidone, at optimal doses not only provides greater efficacy but also reduces the risk of spironolactone-induced hyperkalemia.Citation111
Eplerenone in the treatment of RHTN still lacks data, but substantial efficacy and good tolerability have been demonstrated with modest changes in plasma potassium.Citation82 The most recently developed MRA, finerenone, has not been studied in RHTN, although its pharmacokinetic profile is promising.Citation112 Other agents similarly affecting aldosterone effects may be alternatives in patients with RHTN. A recent highly selective and potent aldosterone-synthase inhibitor – RO6836191 – has the potential of being used in the clinical setting.Citation113 Another aldosterone-synthase inhibitor – CYP11B2 (LC1699) – reduces both office and ABPM pressures in PA, but its development was discontinued because of cortisol response to adrenocorticotropic hormone stimulation was significantly suppressed in 20% of patients.Citation114 These findings suggest that new compounds will be identified for future use to treat RHTN.Citation115 Finally, thiazolidinedione agonists, which act on PPARγ receptors and are widely used in the treatment of type 2 diabetes, in addition to their known antiproliferative effects, can also suppress aldosterone production.Citation116 Therefore, they may potentially be useful in combination with conventional antihypertensive therapy for patients with RHTN who also have insulin resistance and type 2 diabetes.
In conclusion, based on the many lines of evidence, such MRAs as spironolactone should be considered the fourth-line drug in the treatment of RHTN.Citation104,Citation117–Citation119 Unfortunately, a retrospective cohort study, which examined antihypertensive-medication trends from the introduction of the 2008 American Heart Association Scientific statement on RHTN to December 2014, highlighted the persistent infrequent use of recommended therapies, including spironolactone, in RHTN subjects. Spironolactone use increased by about 38% from 2008 to 2014, although with low overall prevalence: it was used in only one in ten cases of RHTN.Citation120 With the recent evidence, greater efforts are needed to increase the use of recommended antihypertensive drugs, including such MRAs as spironolactone, among patients with RHTN.
Disclosure
The authors report no conflicts of interest in this work.
References
- ConnJWPrimary aldosteronismJ Lab Clin Med195545466166414368032
- GordonRDFishmanLMLiddleGWPlasma renin activity and aldosterone secretion in a pregnant woman with primary aldosteronismJ Clin Endocrinol Metab19672733853885337160
- FishmanLMKüchelOLiddleGWMichelakisAMGordonRDChickWTIncidence of primary aldosteronism uncomplicated “essential” hypertension: a prospective study with elevated aldosterone secretion and suppressed plasma renin activity used as diagnostic criteriaJAMA196820574975025695306
- MarsenBDissmannTOelkersWLohmannFWMolzahnMGotzenREndocrinologic and circulatory findings in a case of primary aldosteronismDtsch Med Wochenschr19719622951954 German5578596
- RossiCAEcheverriaHPrimary aldosteronismPrensa Med Argent19715715777782 Spanish5094920
- LaraghJHHormonal profiling in the diagnosis and treatment of essential hypertensionDel Med J19744631251334361408
- McCaaCSLangfordHGCushmanWCMcCaaREResponse of arterial blood pressure, plasma renin activity and plasma aldosterone concentration to long-term administration of captopril in patients with severe, treatment-resistant malignant hypertensionClin Sci (Lond)197957Suppl 5371s373s396084
- LaraghJHLetcherRLPickeringTGRenin profiling for diagnosis and treatment of hypertensionJAMA1979241215115631492
- LimRCJrNakayamaDKBiglieriEGSchambelanMHuntTKPrimary aldosteronism: changing concepts in diagnosis and managementAm J Surg198615211161213728804
- GriffingGTWilsonTEMelbyJCAlterations in aldosterone secretion and metabolism in low renin hypertensionJ Clin Endocrinol Metab1990716145414602229301
- GordonRDStowasserMTunnyTJKlemmSAFinnWLKrekALClinical and pathological diversity of primary aldosteronism, including a new familial varietyClin Exp Pharmacol Physiol19911852832862065471
- GordonRDStowasserMTunnyTJKlemmSARutherfordJCHigh incidence of primary aldosteronism in 199 patients referred with hypertensionClin Exp Pharmacol Physiol19942143153187923898
- LimPOYoungWFMacDonaldTMA review of the medical treatment of primary aldosteronismJ Hypertens200119335336111288803
- LimPOMacDonaldTMPrimary aldosteronism, diagnosed by the aldosterone to renin ratio, is a common cause of hypertensionClin Endocrinol (Oxf)200359442743014510903
- ConnJWCohenELRovnerDRNesbitRMNormokalemic primary aldosteronism: a detectable cause of curable “essential” hypertensionJAMA1965193320020614310325
- PessinaACSacchettoARossiGPLeft ventricular anatomy and function in primary aldosteronism and renovascular hypertensionZanchettiADevereuxRBHanssonLGoriniSHypertension and the HeartHeidelbergSpringer19976369
- MackenzieSMConnellJHypertension and the expanding role of aldosteroneCurr Hypertens Rep20068325526117147925
- DuprezDABauwensFRDe BuyzereMLInfluence of arterial blood pressure and aldosterone on left ventricular hypertrophy in moderate essential hypertensionAm J Cardiol199371317A20A
- BlacherJAmahGGirerdXAssociation between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertensionAm J Hypertens19971012 Pt 1132613349443767
- RochaRStierCTJrKiforIAldosterone: a mediator of myocardial necrosis and renal arteriopathyEndocrinology2000141103871387811014244
- El-GharbawyAHNadigVSKotchenJMArterial pressure, left ventricular mass, and aldosterone in essential hypertensionHypertension200137384585011244006
- RochaRFunderJWThe pathophysiology of aldosterone in the cardiovascular systemAnn N Y Acad Sci20029708910012381544
- BlasiERRochaRRudolphAEBlommeEAPollyMLMcMahonEGAldosterone/salt induces renal inflammation and fibrosis in hypertensive ratsKidney Int20036351791180012675855
- RossiGPCesariMPessinaACLeft ventricular changes in primary aldosteronismAm J Hypertens2003161969812517694
- DartschTFischerRGapelyukAAldosterone induces electrical remodeling independent of hypertensionInt J Cardiol2013164217017821764470
- RossiGPSecciaTMGallinaVProspective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY study): rationale and study designJ Hum Hypertens201327315816322718050
- RossiGPBolognesiMRizzoniDVascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patientsHypertension20085151366137118347224
- Martinez-AguayoACarvajalCACampinoCPrimary aldosteronism and its impact on the generation of arterial hypertension, endothelial injury and oxidative stressJ Pediatr Endocrinol Metab201023432333020583536
- FarquharsonCAStruthersADSpironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failureCirculation2000101659459710673249
- RochaRStierCTJrPathophysiological effects of aldosterone in cardiovascular tissuesTrends Endocrinol Metab200112730831411504670
- RochaRRudolphAEFrierdichGEAldosterone induces a vascular inflammatory phenotype in the rat heartAm J Physiol Heart Circ Physiol20022835H1802H181012384457
- TakedaYRole of cardiovascular aldosterone in hypertensionCurr Med Chem Cardiovasc Hematol Agents20053326126615974890
- Yugar-ToledoJCBonalume-TacitoLHFerreira-MeloSELow-renin (volume dependent) mild-hypertensive patients have impaired flow-mediated and glyceryl-trinitrate stimulated vascular reactivityCirc J200569111380138516247215
- SchiffrinELEffects of aldosterone on the vasculatureHypertension200647331231816432039
- RuilopeLMAldosterone, hypertension, and cardiovascular disease: an endless storyHypertension200852220720818559719
- CalhounDANishizakaMKZamanMAThakkarRBWeissmannPHyperaldosteronism among black and white subjects with resistant hypertensionHypertension200240689289612468575
- GallayBJAhmadSXuLToivolaBDavidsonRCScreening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratioAm J Kidney Dis200137469970511273868
- StrauchBZelinkaTHampfMBernhardtRWidimskyJJrPrevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe regionJ Hum Hypertens200317534935212756408
- EideIKTorjesenPADrolsumABabovicALilledahlNPLow-renin status in therapy-resistant hypertension: a clue to efficient treatmentJ Hypertens200422112217222615480108
- KayserSCDekkersTGroenewoudHJStudy heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysisJ Clin Endocrinol Metab201610172826283527172433
- CalhounDAJonesDTextorSResistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure ResearchCirculation200811725e510e52618574054
- PimentaEGaddamKKPratt-UbunamaMNAldosterone excess and resistance to 24-h blood pressure controlJ Hypertens200725102131213717885558
- SiddiquiMCalhounDARefractory versus resistant hypertensionCurr Opin Nephrol Hypertens2017261141927798457
- MartinsLCFigueiredoVNQuinagliaTCharacteristics of resistant hypertension: ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffnessJ Hum Hypertens201125953253820927128
- de FariaAPDemacqCFigueiredoVNHypoadiponectinemia and aldosterone excess are associated with lack of blood pressure control in subjects with resistant hypertensionHypertens Res201336121067107223966059
- SabbatiniARFariaAPBarbaroNRDeregulation of adipokines related to target organ damage on resistant hypertensionJ Hum Hypertens201428638839224284384
- MenonDVArbiqueDWangZAdams-HuetBAuchusRJVongpa-tanasinWDifferential effects of chlorthalidone versus spironolactone on muscle sympathetic nerve activity in hypertensive patientsJ Clin Endocrinol Metab20099441361136619158191
- ModoloRde FariaAPAlmeidaAMorenoHResistant or refractory hypertension: are they different?Curr Hypertens Rep2014161048525139782
- AcelajadoMCPisoniRDudenbostelTRefractory hypertension: definition, prevalence, and patient characteristicsJ Clin Hypertens (Greenwich)201214171222235818
- BrambillaGBombelliMSeravalleGPrevalence and clinical characteristics of patients with true resistant hypertension in central and Eastern Europe: data from the BP-CARE studyJ Hypertens201331102018202423838657
- StaessenJLijnenPFagardRVerschuerenLJAmeryARise in plasma concentration of aldosterone during long-term angiotensin II suppressionJ Endocrinol19819134574657035596
- Ubaid-GirioliSde SouzaLAYugar-ToledoJCAldosterone excess or escape: treating resistant hypertensionJ Clin Hypertens (Greenwich)200911524525219534021
- SchjoedtKJAndersenSRossingPTarnowLParvingHHAldo-sterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rateDiabetologia200447111936193915551047
- TomaschitzARitzEPieskeBAldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular diseaseMetabolism2014631203124095631
- GaddamKKNishizakaMKPratt-UbunamaMNCharacterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansionArch Intern Med2008168111159116418541823
- TalerSJTextorSCAugustineJEResistant hypertension: comparing hemodynamic management to specialist careHypertension200239598298812019280
- RossiGPSacchettoAVisentinPChanges in left ventricular anatomy and function in hypertension and primary aldosteronismHypertension1996275103910458621194
- NishimuraMUzuTFujiiTCardiovascular complications in patients with primary aldosteronismAm J Kidney Dis199933226126610023636
- SchlaichMPSchobelHPHilgersKSchmiederREImpact of aldosterone on left ventricular structure and function in young normotensive and mildly hypertensive subjectsAm J Cardiol200085101199120610802001
- MatsumuraKFujiiKOnikiHOkaMIidaMRole of aldosterone in left ventricular hypertrophy in hypertensionAm J Hypertens2006191131816461184
- CatenaCColussiGNadaliniECardiovascular outcomes in patients with primary aldosteronism after treatmentArch Intern Med20081681808518195199
- CatenaCColussiGMarzanoLSechiLAAldosterone and the heart: from basic research to clinical evidenceHorm Metab Res201244318118722095099
- BrillaCGWeberKTMineralocorticoid excess, dietary sodium, and myocardial fibrosisJ Lab Clin Med199212068939011453111
- YoungMHeadGFunderJDeterminants of cardiac fibrosis in experimental hypermineralocorticoid statesAm J Physiol19952694 Pt 1E657E6627485478
- ChhokarVSSunYBhattacharyaSKHyperparathyroidism and the calcium paradox of aldosteronismCirculation2005111787187815710759
- LawPHSunYBhattacharyaSKChhokarVSWeberKTDiuretics and bone loss in rats with aldosteronismJ Am Coll Cardiol200546114214615992648
- ManieroCFassinaASecciaTMMild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronismJ Hypertens201230239039522179087
- TomaschitzARitzEPieskeBAldosterone and parathyroid hormone: a precarious couple for cardiovascular diseaseCardiovasc Res2012941101922334595
- ZhangYFengBAssociation of serum parathyrine and calcium levels with primary aldosteronism: a meta-analysisInt J Clin Exp Med201589146251463326628945
- GoodfriendTLCalhounDAResistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapyHypertension200443351852414732721
- PackAIGislasonTObstructive sleep apnea and cardiovascular disease: a perspective and future directionsProg Cardiovasc Dis200951543445119249449
- GonzagaCCGaddamKKAhmedMISeverity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertensionJ Clin Sleep Med20106436336820726285
- WehlingMEffects of aldosterone and mineralocorticoid receptor blockade on intracellular electrolytesHeart Fail Rev2005101394615947890
- SowersJRWhaley-ConnellAEpsteinMNarrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertensionAnn Intern Med20091501177678319487712
- Ehrhart-BornsteinMLamounier-ZepterVSchravenAHuman adipocytes secrete mineralocorticoid-releasing factorsProc Natl Acad Sci U S A200310024142111421614614137
- KathiresanSLarsonMGBenjaminEJClinical and genetic correlates of serum aldosterone in the community: the Framingham Heart StudyAm J Hypertens2005185 Pt 165766515882548
- FontanaVde FariaAPBarbaroNRModulation of aldosterone levels by −344 C/T CYP11B2 polymorphism and spironolactone use in resistant hypertensionJ Am Soc Hypertens20148314615124388430
- SookoianSGianottiTFGonzalezCDPirolaCJAssociation of the C-344T aldosterone synthase gene variant with essential hypertension: a meta-analysisJ Hypertens200725151317143166
- TakeuchiFYamamotoKKatsuyaTReevaluation of the association of seven candidate genes with blood pressure and hypertension: a replication study and meta-analysis with a larger sample sizeHypertens Res201235882583122456346
- DaviesEHollowayCDIngramMCAldosterone excretion rate and blood pressure in essential hypertension are related to polymorphic differences in the aldosterone synthase gene CYP11B2Hypertension199933270370710024332
- RitterAMFontanaVFariaAPAssociation of mineralocorticoid receptor polymorphism I180V with left ventricular hypertrophy in resistant hypertensionAm J Hypertens201629224525026049084
- CalhounDAWhiteWBEffectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertensionJ Am Soc Hypertens20082646246820409927
- WilliamsBMacDonaldTMMorantSSpironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trialLancet2015386100082059206826414968
- CalhounDALow-dose aldosterone blockade as a new treatment paradigm for controlling resistant hypertensionJ Clin Hypertens (Greenwich)200791 Suppl 1192417215651
- ChapmanNDobsonJWilsonSEffect of spironolactone on blood pressure in subjects with resistant hypertensionHypertension200749483984517309946
- PimentaECalhounDATreatment of resistant hypertensionJ Hypertens201028112194219520948392
- ColussiGCatenaCSechiLASpironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertensionJ Hypertens201331131523011526
- CatenaCColussiGSechiLAMineralocorticoid receptor antagonists and renal involvement in primary aldosteronism: opening of a new eraEur J Endocrinol20131681C1C523082005
- NishizakaMKZamanMACalhounDAEfficacy of low-dose spironolactone in subjects with resistant hypertensionAm J Hypertens20031611 Pt 192593014573330
- SartoriMCaloLAMascagnaVAldosterone and refractory hypertension: a prospective cohort studyAm J Hypertens200619437338016580572
- LaneDAShahSBeeversDGLow-dose spironolactone in the management of resistant hypertension: a surveillance studyJ Hypertens200725489189417351384
- GaddamKKPratt-UbunamaMNCalhounDAAldosterone antagonists: effective add-on therapy for the treatment of resistant hypertensionExpert Rev Cardiovasc Ther20064335335916716096
- PimentaECalhounDAResistant hypertension and aldosteronismCurr Hypertens Rep20079535335918177580
- JansenPMDanserAHImholzBPvan den MeirackerAHAldosterone-receptor antagonism in hypertensionJ Hypertens200927468069119516169
- DasGDePAldosterone renin ratio in patients with resistant hypertensionQJM20101031189789920360029
- WeinbergerMHRonikerBKrauseSLWeissRJEplerenone, a selective aldosterone blocker, in mild-to-moderate hypertensionAm J Hypertens200215870971612160194
- KrumHNollyHWorkmanDEfficacy of eplerenone added to renin-angiotensin blockade in hypertensive patientsHypertension200240211712312154100
- PittBReichekNWillenbrockREffects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy studyCirculation2003108151831183814517164
- EpsteinMWilliamsGHWeinbergerMSelective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetesClin J Am Soc Nephrol20061594095117699311
- SavoiaCTouyzRMAmiriFSchiffrinELSelective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patientsHypertension200851243243918195160
- EguchiKKabutoyaTHoshideSIshikawaSKarioKAdd-on use of eplerenone is effective for lowering home and ambulatory blood pressure in drug-resistant hypertensionJ Clin Hypertens (Greenwich)201618121250125727296360
- OuzanJPeraultCLincoffAMCarreEMertesMThe role of spironolactone in the treatment of patients with refractory hypertensionAm J Hypertens2002154 Pt 133333911991219
- NarayanHWebbDJNew evidence supporting the use of mineralocorticoid receptor blockers in drug-resistant hypertensionCurr Hypertens Rep20161853427072827
- SinnottSJTomlinsonLARootAAComparative effectiveness of fourth-line anti-hypertensive agents in resistant hypertension: a systematic review and meta-analysisEur J Prev Cardiol201724322823827856806
- GaddamKCorrosCPimentaERapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical studyHypertension20105551137114220351345
- WithamMDGillespieNDStruthersADHyperkalemia after the publication of RALESN Engl J Med20043512324482450
- JuurlinkDNMamdaniMMLeeDSRates of hyperkalemia after publication of the Randomized Aldactone Evaluation StudyN Engl J Med2004351654355115295047
- McMurrayJJAdamopoulosSAnkerSDESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012Eur J Heart Fail201214880386922828712
- PittBBakrisGRuilopeLMDiCarloLMukherjeeRSerum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS)Circulation2008118161643165018824643
- JessupMAbrahamWTCaseyDE2009 Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice GuidelinesCirculation2009119141977201619324967
- EpsteinMCalhounDAAldosterone blockers (mineralocorticoid receptor antagonism) and potassium-sparing diureticsJ Clin Hypertens (Greenwich)201113964464821896143
- LentiniSHeinigRKimmeskamp-KirschbaumNWensingGPharmacokinetics, safety and tolerability of the novel, selective mineralocorticoid receptor antagonist finerenone: results from first-in-man and relative bioavailability studiesFundam Clin Pharmacol201630217218426604072
- BogmanKSchwabDDelporteMLPreclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2)Hypertension201769118919627872236
- CalhounDAWhiteWBKrumHEffects of a novel aldosterone synthase inhibitor for treatment of primary hypertension: results of a randomized, double-blind, placebo- and active-controlled phase 2 trialCirculation2011124181945195521986283
- ShibataHItohHMineralocorticoid receptor-associated hypertension and its organ damage: clinical relevance for resistant hypertensionAm J Hypertens201225551452322258336
- KashiwagiYMizunoYHaradaESuppression of primary aldosteronism and resistant hypertension by the peroxisome proliferator-activated receptor gamma agonist pioglitazoneAm J Med Sci2013345649750023313950
- LiuGZhengXXXuYLLuJHuiRTHuangXHEffect of aldosterone antagonists on blood pressure in patients with resistant hypertension: a meta-analysisJ Hum Hypertens201529315916625078487
- DahalKKunwarSRijalJThe effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studiesAm J Hypertens201528111376138525801902
- GuoHXiaoQClinical efficacy of spironolactone for resistant hypertension: a meta analysis from randomized controlled clinical trialsInt J Clin Exp Med2015857270727826221266
- HwangAYDaveCSmithSMTrends in antihypertensive medication use among US patients with resistant hypertension, 2008 to 2014Hypertension20166861349135427777360
