Abstract
Elevated plasma levels of homocysteine (Hcy), known as hyperhomocysteinemia (HHcy), are associated with osteoporosis. A decrease in bone blood flow is a potential cause of compromised bone mechanical properties. Therefore, we hypothesized that HHcy decreases bone blood flow and biomechanical properties. To test this hypothesis, male Sprague–Dawley rats were treated with Hcy (0.67 g/L) in drinking water for 8 weeks. Age-matched rats served as controls. At the end of the treatment period, the rats were anesthetized. Blood samples were collected from experimental or control rats. Biochemical turnover markers (body weight, Hcy, vitamin B12, and folate) were measured. Systolic blood pressure was measured from the right carotid artery. Tibia blood flow was measured by laser Doppler flow probe. The results indicated that Hcy levels were significantly higher in the Hcy-treated group than in control rats, whereas vitamin B12 levels were lower in the Hcy-treated group compared with control rats. There was no significant difference in folate concentration and blood pressure in Hcy-treated versus control rats. The tibial blood flow index of the control group was significantly higher (0.78 ± 0.09 flow unit) compared with the Hcy-treated group (0.51 ± 0.09). The tibial mass was 1.1 ± 0.1 g in the control group and 0.9 ± 0.1 in the Hcy-treated group. The tibia bone density was unchanged in Hcy-treated rats. These results suggest that Hcy causes a reduction in bone blood flow, which contributes to compromised bone biomechanical properties.
Keywords:
Introduction
Osteoporosis is a pathological condition whereby the skeleton undergoes remodeling and reduction in bone density (BD).Citation1,Citation2 Osteoporosis is a very prevalent disease that affects more than 75 million people in Europe, the US, and Japan.Citation1 It has a widespread social and economic impact in the global population, costing US$16.9 billion in the US alone.Citation3 Over 10 years, postmenopausal women in the US experienced 5.2 million fractures of the hip, spine, or forearm, which is equivalent to 2 million years with a disability.Citation4 Hence, identifying factors involved in this process, aside from low vitamin D and calcium intake,Citation5 becomes paramount in our investigation.
Homocysteine (Hcy) is a sulfur-containing, nonproteinogenic amino acid formed during the metabolism of methionine.Citation6 Elevated levels of total plasma Hcy are known as hyperhomocysteinemia (HHcy). Recently, increased plasma Hcy has been suggested to be an independent risk factor for osteoporosis and bone quality.Citation7,Citation8 In addition, HHcy is accompanied by disruption of biochemical bone turnover markers, osteocalcin, and collagen I C-terminal cross-laps.Citation9 However, the mechanistic role of Hcy in osteoporosis is still unknown.
HHcy-induced oxidative stress may occur as a consequence of either decreased expression or activity of key antioxidant enzymes or increased enzymatic generation of superoxide anions.Citation10 Superoxide anions may react with nitric oxide (NO) to produce peroxynitrite with reduced NO bioavailability,Citation11 which may impair the osteoblast–osteoclast balance, with unpredictable consequences on bone remodeling. An increase in oxidative stress, which leads to reduce NO bioavailability, decreases bone blood flow and hence leads to osteoporosis.Citation12
Blood flow is essential for normal bone growth and bone repair.Citation13 Therefore, inadequate blood flow to skeletal structures could impair normal development and/or the healing process of bone. The two most essential factors in bone healing are stabilization of the osseous fragments and blood flow. After a bone fracture, blood flow to the injury site changes to meet the metabolic needs of the healing tissue.Citation13
Several previous studies have shown that HHcy alters the ability of vasculature to dilate because it decreases NO bioavailability, which increases vascular resistance.Citation14 Considering the convincing studies in which HHcy resulted in increased vascular resistance, we proposed that HHcy rats would have decreased bone blood flow as a consequence of this, thereby altering biomechanical properties such as density and net weight.
Materials and methods
Induction of HHcy
Male Sprague–Dawley rats, 8 weeks of age (250–300 g), were kept in cages at room temperature under a day/night rhythm and fed a standard laboratory chow. The rats were divided into a wild-type group (n = 10) and an HHcy group (n = 12), both groups receiving an identical diet. In accordance with previous studies, to create a condition of mild HHcy, Hcy was supplemented (0.67 g dl-Hcy [Sigma H-4628; Sigma-Aldrich, St Louis, MO, USA]/L drinking water) for 8–12 weeks. The low Hcy diet was selected because studies on rats had illustrated that this dose-induced mild HHcy was well tolerated.Citation14 Control rats received Hcy-free water. The animals were killed with sodium pentobarbital (70 mg/kg) at the end of the experiments. All animal procedures were performed in accordance with National Institutes of Health guidelines for animal research and were reviewed and approved by the University of Louisville Animal Care and Use Committee.
Measurement of plasma Hcy
Blood samples were collected after an overnight fast. The samples were stored at −80°C until the day of analysis. Plasma was used to detect the concentrations of Hcy by high-pressure liquid chromatography, vitamin B12, and folate as described previously.Citation9,Citation15
In vivo bone blood flow measurement
Bone blood flow was measured as described previously.Citation13 On the day of the bone blood flow measurement experiment, the rats were anesthetized. Body temperature was maintained between 37°C and 39°C using a heating pad. The left carotid artery was cannulated to measure arterial blood pressure. The left tibia was exposed at a midshaft position by surgical incision, and 5 mM of soft tissue and periosteum were removed to expose the cortex. A laser Doppler flow meter was used to assess bone blood flow. The flow probe was positioned along the anteromedial surface of the tibia. Estimates of flow were obtained every 3–4 sec over a period of 4–6 min. At the conclusion of the experiment, the tibia was excised and weighed.
Measurement of BD
The apparent BD was determined by the Archimedes principle to decide whether there was a change in bone mechanical properties.
Other measures
Height and weight were measured by scale, and tibia mass index was calculated as weight in kilograms divided by the square of height in meters.
Statistical analysis
Values are reported as mean ± standard error of the mean in all four groups. Differences between groups were tested by two-way analysis of variance. Tukey’s multiple comparison test was used to compare group means, and results were considered significant if P < 0.05.
Results
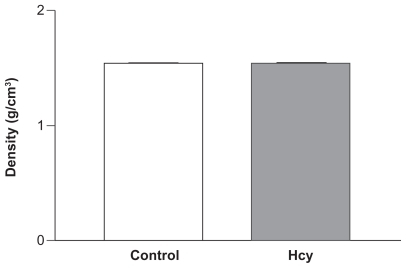
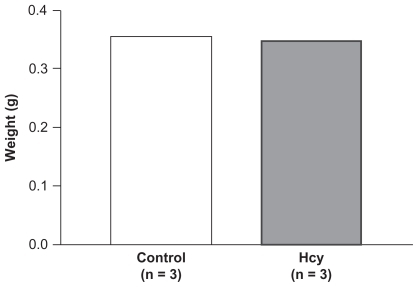
There was no significant difference in initial body weights of control (208 ± 0.01) and Hcy-treated (238.1 ± 0.03) rats (). As expected, plasma Hcy concentrations were significantly increased in the Hcy-treated group (17.2 ± 9 μmol/L) compared with the control group (3.0 ± 5 μmol/L). Serum folate concentration was similar in both groups (35.5 ± 4.2 nmol/L in the HHcy group and 32.5 ± 4.7 nmol/L in the control group), whereas vitamin BCitation12 was significantly lower in the HHcy group (338 ± 49 pmol/L) compared with the control group (450 ± 85 pmol/L). The tibial mass was 1.1 ± 0.1 g in the control group and 0.9 ± 0.1 g in the Hcy- treated group.
Table 1 Biochemical findings in animals at the end of the treatment period
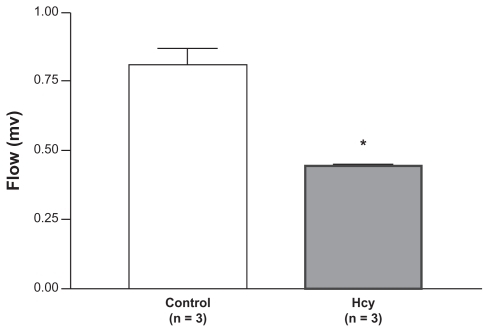
The bone blood flow index was reduced in Hcy-treated rats. Blood flow to the tibia of control rats (0.78 ± 0.09 units, n = 5) was significantly higher than the blood flow to the tibia of Hcy-treated rats (0.51 ± 0.09 units, n = 5) ().
Figure 1 Flow index was reduced in Hcy-treated rats. Blood flow to the tibia of control rats (0.78 ± 0.09 units) was significantly higher than blood flow to the Hcy-treated rats (0.51 ± 0.09 units).
Note: *P < 0.05 compared with control.
There was no difference in right tibia wet weight in Hcy-treated rats (0.3 g, n = 3). There was no difference in the wet tibia weight for control and Hcy-treated rats (n = 5) ().
Figure 2 No difference was observed in right tibia wet weight in Hcy-treated rats. There was no difference in the tibia wet weight between control and Hcy-treated rats.
The tibia BD was unchanged in Hcy-treated rats. The densities of the tibias from control and Hcy-treated rats suggested no difference (1.6 g/cm3, n = 5) ().
Discussion
The present study clearly demonstrated that there was a significant decrease in bone blood flow in Hcy-treated rat tibia versus control rat tibia (). This result was consistent with our plethora of evidence that indicated Hcy had some role in modulating those systems involved in vasodilation both in vivo and in vitro. A study in humans showed that high plasma Hcy level was independently associated with greater brachial-ankle pulse wave velocity.Citation16
Kumar et al showed decreased carotid artery blood flow in heterozygote cystathionine β-synthase mice (CBS+/−, genetically HHcy mice).Citation17 Elevated plasma levels of Hcy in these mice (CBS+/−) caused an increase in arterial remodeling, in part due to an increase in the collagen/elastin ratio, resulting in amplified vascular resistance, and led to a measured decrease in carotid artery blood flow.Citation17 One possible mechanism for this was that Hcy caused oxidative stress that led to the activation of matrix metalloproteinase in the extracellular matrix.
In concordance with those studies, another study showed that HHcy impaired angiogenesis in a murine model of limb ischemia. This could have resulted in greater vascular resistance and decreased bone blood flow.Citation18 Riza Erbay et al showed that patients with slow coronary flow had high level of plasma Hcy compared with patients with normal coronary flow; this study suggested a correlation between high Hcy and coronary flow and a possible role in the pathogenesis of impaired flow.Citation19
Hence, we clearly see a role of Hcy in modulating bone blood flow in a variety of contexts and experimental models. Our results have provided yet another context and model by which Hcy alters bone blood flow. The pathological consequences of this remain to be elucidated.
We proposed that there would be a decrease in BD in the Hcy-treated group of rats. However, we did not note any changes in density or dry or wet weight of tibia among the Hcy group versus control rats ( and ). However, BD is not the only clinically important value in determining susceptibility to fracture; in fact, many studies have noted bone quality as a major risk factor for fracture, with differences in the following measures: geometry, bone mass distribution, trabecular bone architecture, microdamage, remodeling activity, bone mineral, and matrix tissue properties.Citation20–Citation22 Therefore, although BD did not decrease under our experimental conditions, there could possibly have been a change in bone quality. However, we did not perform these measures, and this is warranted for future study.
Both folate and vitamin B12 are cofactors of the methionine synthase reaction and play an important role in Hcy metabolism.Citation6 The consumption of folate and vitamin B12 is high in the presence of high Hcy concentrations in plasma. In the present study, serum vitamin B12 concentration was significantly lower in the Hcy-treated group compared with control rats; however, there was no significant change in folate concentration. As vitamin B12 and folate are directly involved in the regulation of Hcy concentrations in blood, the question arises whether a reduction in concentrations of these vitamins has any effect on biochemical markers of bone. Carmel et al demonstrated reductions in biochemical markers (osteocalcin) in vitamin B12-deficient individuals.Citation23
Mice treated with folate antagonist showed reduced bone growth,Citation24 and this was further supported by data suggesting that folate may have significant effects on bone that are independent of Hcy.Citation25 In contrast, recently, other studies have shown that folate supplementation does not affect biochemical markers of bone turnover, although it decreases Hcy concentrations in healthy subjects.Citation26
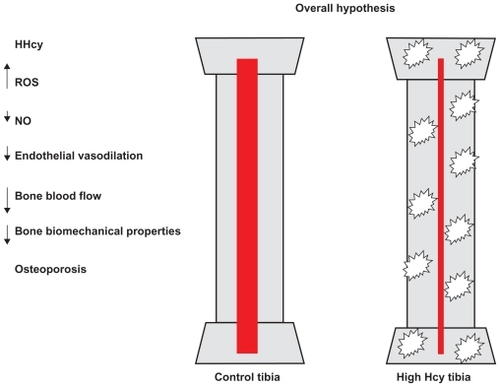
We found no difference in weight and density, so there may be some compensatory mechanism. Further studies are required. A longer exposure to Hcy or a higher plasma level could further reduce bone blood flow and potentially compromise bone mechanical properties, as suggested in human studies. Previous evidence from our laboratory suggested that Hcy increased oxidative stress and decreased the bioavailability of NO, an important dilator of the bone vasculature, and hence supports the hypothesis presented in . In summary, the present study provides strong evidence that HHcy is a major cause of weakening of the bone. Future studies are required to clarify the mechanisms of the HHcy effect.
Figure 4 The hypothesis was that high levels of Hcy lead to increased ROS, decreased NO bioavailability and other factors, decreased endothelial vasodilation, and decreased bone blood flow, which could lead to decreased biomechanical properties. The hypothesis was that high levels of Hcy lead to increased vascular resistance from decreased vasodilation, which could lead to decreased biomechanical properties, including decreased BD.
Acknowledgments
We acknowledge Kathleen Hamilton for taking bone density measurements. A part of this study was supported by National Institutes of Health grants HL-71010 and NS-51568.
Disclosure
The authors report no conflicts of interest in this work.
References
- Who are candidates for prevention and treatment for osteoporosis?Osteoporos Int19977116
- LippunerKGolderMGreinerREpidemiology and direct medical costs of osteoporotic fractures in men and women in SwitzerlandOsteoporos Int200516Suppl 2S8S1715378232
- CummingsSRMeltonLJEpidemiology and outcomes of osteoporotic fracturesLancet200235993191761176712049882
- ChrischillesEShiremanTWallaceRCosts and health effects of osteoporotic fracturesBone19941543773867917575
- ChapuyMCArlotMEDuboeufFVitamin D3 and calcium to prevent hip fractures in the elderly womenN Engl J Med199232723163716421331788
- SelhubJHomocysteine metabolismAnnu Rev Nutr19991921724610448523
- McLeanRRJacquesPFSelhubJHomocysteine as a predictive factor for hip fracture in older personsN Engl J Med2004350202042204915141042
- Van MeursJBDhonukshe-RuttenRAPluijmSMHomocysteine levels and the risk of osteoporotic fractureN Engl J Med2004350202033204115141041
- OzdemSSamanciSTasatargilAExperimental hyperhomocysteinemia disturbs bone metabolism in ratsScand J Clin Lab Invest200767774875617852810
- LentzSRErgerRADayalSFolate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient miceAm J Physiol Heart Circ Physiol20002793H97097510993757
- BanfiGIorioELCorsiMMOxidative stress, free radicals and bone remodelingClin Chem Lab Med200846111550155518847368
- Sanchez-RodriguezMARuiz-RamosMCorrea-MunozEMendoza-NunezVMOxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymesBMC Musculoskelet Disord2007812418088440
- FlemingJTBaratiMTBeckDJBone blood flow and vascular reactivityCells Tissues Organs2001169327928411455124
- SteedMMTyagiNSenUSchuschkeDAJoshuaIGTyagiSCFunctional consequences of the collagen/elastin switch in vascular remodeling in hyperhomocysteinemic wild-type, eNOS−/−, and iNOS−/− miceAm J Physiol Lung Cell Mol Physiol20102993L30131120581102
- SenUTyagiNKumarMMoshalKSRodriguezWETyagiSCCystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cellsAm J Physiol Cell Physiol20072936C1779178717855772
- TayamaJMunakataMYoshinagaKToyotaTHigher plasma homocysteine concentration is associated with more advanced systemic arterial stiffness and greater blood pressure response to stress in hypertensive patientsHypertens Res200629640340916940702
- KumarMTyagiNMoshalKSHomocysteine decreases blood flow to the brain due to vascular resistance in carotid arteryNeurochem Int20085368214219
- Bosch-MarceMPolaRWeckerABHyperhomocyst(e)inemia impairs angiogenesis in a murine model of limb ischemiaVasc Med2005101152215920995
- Riza ErbayATurhanHYasarASElevated level of plasma homocysteine in patients with slow coronary flowInt J Cardiol2005102341942316004886
- ManolagasSCCorticosteroids and fractures: a close encounter of the third cell kindJ Bone Miner Res20001561001100510841168
- FratzlPGuptaHSPaschalisEPRoschgerPStructure and mechanical quality of the collagen–mineral nano-composite in boneJ Mater Chem20041421152123
- McCreadieBRGoldsteinSABiomechanics of fracture: is bone mineral density sufficient to assess risk?J Bone Miner Res200015122305230811127195
- CarmelRLauKHBaylinkDJSaxenaSSingerFRCobalamin and osteoblast-specific proteinsN Engl J Med1988319270753260008
- IqbalMPAhmedMUmerMMehboobaliNQureshiAAEffect of methotrexate and folinic acid on skeletal growth in miceActa Paediatr200392121438144414971796
- McFarlaneSIMuniyappaRShinJJBahtiyarGSowersJROsteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link?Endocrine200423111015034190
- HerrmannMStangerOPaulweberBHufnaglCHerrmannWFolate supplementation does not affect biochemical markers of bone turnoverClin Lab20065234131136