Abstract
Obesity is associated with an increased risk of developing cardiovascular disease (CVD), particularly heart failure (HF) and coronary heart disease (CHD). The mechanisms through which obesity increases CVD risk involve changes in body composition that can affect hemodynamics and alters heart structure. Pro-inflammatory cytokines produced by the adipose tissue itself which can induce cardiac dysfunction and can promote the formation of atherosclerotic plaques. When obesity and HF or CHD coexist, individuals with class I obesity present a more favorable prognosis compared to individuals who are normal or underweight. This phenomenon has been termed the “obesity paradox.” Obesity is defined as an excess fat mass (FM), but individuals with obesity typically also present with an increased amount of lean mass (LM). The increase in LM may explain part of the obesity paradox as it is associated with improved cardiorespiratory fitness (CRF), a major determinant of clinical outcomes in the general population, but particularly in those with CVD, including HF. While increased LM is a stronger prognosticator in HF compared to FM, in patients with CHD excess FM can exert protective effects particularly when not associated with increased systemic inflammation. In the present review, we discuss the mechanisms through which obesity may increase the risk for CVD, and how it may exert protective effects in the setting of established CVD, with a focus on body composition. We also highlight the importance of measuring or estimating CRF, including body composition-adjusted measures of CRF (ie, lean peak oxygen consumption) for an improved risk status stratification in patients with CVD and finally, we discuss the potential non-pharmacologic therapeutics, such as exercise training and dietary interventions, aimed at improving CRF and perhaps clinical outcomes.
Introduction
The prevalence of obesity has increased over the last few decades reaching epidemic proportions.Citation1 Obesity is defined as an excess fat mass (FM) that impairs health,Citation1 which is most commonly defined by determination of a body mass index (BMI) ≥30 kg/m2. Obesity is often subdivided into classes (Class I: BMI=30.0–34.9, Class II: BMI=35.0–39.9, Class III: BMI ≥40.0) to further stratify health risk.Citation2 Using BMI-based diagnostic criteria, 39.8% of the US population meets the definition of obesity with 7.7% having class III obesity or severe obesity, defined as a BMI≥40.0 kg/m2.Citation3 Furthermore, there exists race and ethnic disparities with regard to obesity as the prevalence of obesity is 47.0% of Hispanics and 46.8% of non-Hispanic blacks as opposed to only 37.9% and 12.7% of non-Hispanic whites and Asians.Citation4 The incidence of obesity is increasing and is alarming as the excess FM characteristic of obesity increases the risk of most cardiovascular (CV) diseases (CVD), metabolic diseases such as type-2 diabetes mellitus (T2DM), and their related risk factors (ie, metabolic syndrome).Citation5,Citation6 The financial burden and healthcare utilization from morbidity and mortality resulting from complications of obesity and CVD is also increasing at an alarmingly rate.Citation7
Obesity is a strong independent predictor of CVD even in the absence of other risk factors, however, interestingly after onset of CVD the relationship between higher BMI and clinical outcomes is not linear. Obesity increases the risk for CVD in primary prevention,Citation8 and as such clinicians and researchers have historically assumed that excess body mass would also be detrimental in secondary prevention settings. Contrary to this line of thinking, this assumption is not necessarily correct as several retrospective and prospective epidemiologic studies have demonstrated a potentially protective effect of obesity when it coexists with CVD; a phenomenon termed the “obesity paradox.”Citation9–Citation11 The obesity paradox has been investigated the most in patients with heart failure (HF) and coronary heart disease (CHD), however, more recent data implicates the obesity paradox also in other CVD, such as hypertension,Citation12,Citation13 atrial fibrillation,Citation14,Citation15 pulmonary arterial hypertension,Citation16 and congenital heart disease.Citation17
In this review article, we will discuss the role of obesity and particularly of body composition compartments (ie, fat mass, fat-free mass, lean mass), on the CV system. We will also review the evidence suggesting the presence of an obesity paradox in patients with established CHD and HF and discuss the potential mechanisms through which obesity may exert such protective effects, with a focus on the role of body composition compartments. We will also discuss the data regarding cardiorespiratory fitness (CRF) as a measure to improve risk stratification in obesity and CVD, and conclude by discussing the role of lifestyle measures (eg, exercise training, dietary interventions and weight loss) in affecting prognosis and outcomes.
Nutritional status assessment in obesity: BMI and body composition
The World Health Organization (WHO) defines obesity as an excess FM that negatively affects health. Due to the lack of population-specific cut-offs to define excess FM, the WHO proposes the use of BMI for an initial nutritional status assessment.Citation1 However, the WHO cautions against absolute reliance on BMI as a measure of FM and recommends its use as an imprecise measure to guide nutritional assessment since it may misclassify severity of FM.Citation1 Body mass index does not take into consideration whether excess body weight results from different body composition compartments,Citation18,Citation19 and such limitation is extremely relevant in those conditions in which body weight changes may reflect changes in different body composition compartments and distribution. Nevertheless, particularly in those individuals without CVD, increased BMI is highly correlated with an increase in FM, paralleled by an increase in fat-free mass (FFM),Citation20,Citation21 and it maintains a strong prognostic role.Citation22 Of note, the term FFM is often used in the literature to define lean mass (LM) and skeletal muscle mass (SMM), however, they all define different body composition compartments.Citation23 FFM accounts for most of the total body mass as it includes total body water (intra- and extra-cellular water), bone, and SMM. The FFM without bone defines LM, which is perhaps the most commonly used body composition compartment to estimate SMM in individuals with and without CVD.
The distribution of FM has diverse effects on the CV system and metabolism,Citation24 thus determining its location is a crucial step as it helps to identify individuals with similar BMI and FM, but with different CVD risk profiles. Accumulation of visceral FM has been recognized as a major cardiometabolic risk factor,Citation25,Citation26 which favors the production of pro-inflammatory cytokines and adipokines with cardiodepressant and pro-atherosclerotic properties.Citation27–Citation29 In contrast, the association between increased subcutaneous FM and cardiometabolic risk is not necessarily as linear as for visceral FM.Citation30 Due to the important prognostic role of visceral FM, its clinical assessment is typically performed indirectly (eg, waist circumference [WC])Citation31 and should be encouraged in routine clinical and research settings. The cut-offs for WC recommended for men and women are 102 cm and 88 cm respectively, suggesting that those individuals with greater values have a substantially increased cardiometabolic risk, independent of their BMI and total FM.Citation31 Furthermore, an additional assessment of hip circumference allows us to further stratify the risk, by calculating the waist-to-hip ratio (WHR) with cut-offs for men and women of ≥0.90 and ≥0.85, respectively.Citation31 Importantly, the proposed cut-off for WC may vary depending on the race and ethnicity of the population investigated because despite similar WC and WHR, the cardiometabolic risk of individuals can differ.Citation31
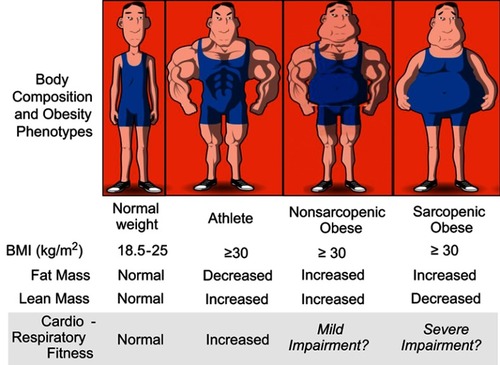
The BMI also does not quantify LM, much like it is unable to quantitate the severity and distribution of FM. While the classical definition of obesity focused solely on excess FM, emerging evidence acknowledges that the classical obese phenotype is associated with a parallel increase in both FM and LM, including the SMM compartment.Citation21 In populations with limited physical activity, obesity may also be associated with reduction in the amount and/or functionality of LM. A reduction of the amount and/or functionality of LM alone is defined as sarcopeniaCitation32 and when excess FM (ie, obesity) accompanies sarcopenia, it is defined as sarcopenic obesity ().Citation9,Citation33–Citation35 Sarcopenia and sarcopenic obesity are associated with worse prognosis and functional capacity in several chronic diseases, particularly in cancer and more recently also in HF.Citation9,Citation32,Citation35–Citation37
Figure 1 Obesity phenotypes, cardiac function and cardiorespiratory fitness. The figure highlights the proposed major role of lean mass in the development of cardiac dysfunction and cardiorespiratory fitness, suggesting that individuals with similar body mass index (BMI) can present a different body composition, resulting in different cardiac function and cardiorespiratory fitness. Reprinted from Mayo Clin Proc., 92(2), Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox, 266–279, Copyright (2017), with permission from Elsevier.Citation9
Obesity increases HF risk
Data resulting from the Framingham study initially suggested that overweight and obesity increase the risk of developing HF.Citation38 While obesity remains a strong risk factor for all forms of HF, recent data suggest that obesity specifically increases the risk of a specific form of HF, termed heart failure with preserved ejection fraction (HFpEF) due to HF signs/symptoms in the presence of a normal left ventricular ejection fraction (LVEF) ().Citation39 HFpEF accounts for nearly half of all HF diagnoses and with limited therapeutic options. It has been recently proposed that because overweight/obesity are so prevalent in this population,Citation40 targeted therapeutics for patients with concomitant obesity and HFpEF should be developed to improve outcomes.Citation41–Citation43
Figure 2 Obesity and risk for heart failure. Obesity increases the risk of heart failure (HF), particularly of HF with preserved ejection fraction (HFpEF) (top panel) compared to HF with reduced ejection fraction (HFrEF) (bottom panel). Reprinted from J Am Coll Cardiol, 69(9), Pandey A, LaMonte M, Klein L, et al, Relationship between physical activity, body mass index, and risk of heart failure, :1129–1142, Copyright (2017), with permission from Elsevier.Citation39

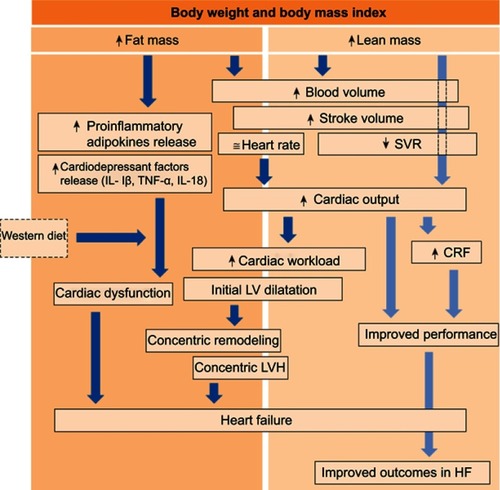
The exact mechanisms of obesity-induced HF are incompletely understood, however, the excess FM and FFM, resulting from both increased SSM and body water (ie, LM) play a central role ().Citation9 As mentioned, increased FM, particularly visceral FM, can induce the synthesis of several adipokines and pro-inflammatory cytokines, responsible for the characteristic low-grade systemic inflammation seen in patients with obesity.Citation29 Many of the products of adipose tissue, namely interleukin (IL)-1β and IL-18, have cardio-depressant properties.Citation29 In animal models, these cytokines induce a reversible cardiac dysfunction when administered to healthy mice,Citation44–Citation46 which have prompted investigation of therapeutics targeting these cytokines in clinical trials in patients with HF.Citation47,Citation48 Furthermore, obesity is typically associated with a Westernized diet rich in saturated fats and sugars,Citation49 which can further contribute to the pro-inflammatory state of patients, particularly since these macronutrients can activate pro-inflammatory pathways similar to those described above.Citation50,Citation51 In addition to the detrimental effects of excess FM, the excess amount of LM in patients with obesity can further increase the risk for cardiac dysfunction and ultimately HF. Due to its high blood-flow requirements, LM is responsible for the typical increase in plasma volume in individuals with obesity,Citation52 causing an increase in preload and stroke volume. Although an increased stroke volume can initially be considered potentially beneficial as it increases cardiac output, when such increase persists over time, the cardiac workload may result in an initial LV dilation followed by a compensatory concentric remodeling and concentric LV hypertrophy, ultimately leading to an increased risk for HF.Citation42,Citation53–Citation55 These cardiac structure and hemodynamic abnormalities can cause cardiac impairments, most typically diastolic in the presence of a preserved LVEF (ie, HFpEF). Obesity is also associated with increased LV end-diastolic pressure as well as right atrial pressure and pulmonary wedge pressure.Citation56–Citation58 Finally, obesity increases several risk factors for HF.Citation8 Weight gain in particular is associated with an increase in blood pressure leading to arterial hypertension,Citation59,Citation60 which is a leading cause of HF.Citation61
Figure 3 Body composition and heart failure. Proposed mechanisms driving obesity to heart failure (HF) and to the obesity paradox once HF is diagnosed. The dark blue arrows indicate the potential detrimental effects of body composition components (fat mass and lean mass) on cardiac function and eventually HF development. The light blue arrows indicate the potential mechanisms by which body composition improves cardiorespiratory fitness (CRF). Reprinted from Mayo Clin Proc., 92(2), Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox, 266–279, Copyright (2017), with permission from Elsevier.Citation9
The obesity paradox in HF
Although it is undeniable that obesity poses a major risk for HF and HF-related risk factors, after HF diagnosis, obesity exerts some protective effects. This paradoxical relationship is particularly evident in individuals with class I obesity. The obesity paradox in HF was initially observed in patients with advanced disease where coexistence of overweight or obesity was associated with improved prognosis compared to normal weight or underweight patients.Citation62 Subsequent studies have confirmed the obesity paradox in both HF with reduced LVEF (HFrEF) and HFpEF.Citation63 The mechanisms through which obesity improves prognosis in patients with HF are incompletely understood, however several hypotheses have been proposed.Citation9,Citation10,Citation64 Particularly, the increased LM in obese individuals may play a crucial role as it has been associated with improved long-term outcomes. Once HF has been established, excess LM may allow a higher CRF (),Citation9,Citation34,Citation35 which is associated with improved prognosis in HF and several other chronic non-communicable diseases.
In patients with a peak oxygen consumption (VO2) >14 mL•kg−1•min−1 or 4 Metabolic Equivalents of Task (MET; 1 MET equals 3.5 mLO2•kg−1•min−1), the obesity paradox has not been reported,Citation65–Citation67 positioning obesity as a protective factor only in those with low CRF. Peak VO2 relative to body weight (mL•kg−1•min−1) as it is most commonly expressed may underestimate CRF levels in obese patients.Citation68,Citation69 To overcome this limitation, the FFM-adjusted peak VO2 (ie, lean peak VO2) has been proposed in the literature and the value of 19 mL•kglean mass−1•min−1 has been found to be superior to the previously defined 14 mL•kg−1•min.−1Citation70 Of note, this cut-off was established decades ago when patients with HF and a peak VO2>14 mL•kg−1•min−1 presented a survival that was similar to those who underwent heart transplantation due to advanced HF.Citation71 More recent evidence has also confirmed that peak VO2 is more strongly dependent of lean mass and not body mass, even in individuals without HF.Citation72 The exact mechanisms through which increased LM may improve CRF and possibly prognosis are still largely unclear. Because the O2 pathway utilized to calculate peak oxygen consumption (VO2) (ie, CRF) highly relies on muscle diffusion capacity and mitochondrial respiration capacity at the skeletal muscle level, increased LM may result in increasing both of these variables.Citation73 In addition, increased LM has been associated with greater skeletal muscle strength, a strong predictor for adverse outcomes even when assessed in adolescents several years before the occurrence of CVD-related deaths.Citation74 Even in patients with HF the assessment of muscular strengths provides crucial information for improved risk status stratification, and it has been proposed to be even superior than peak VO2, at least in patients with HFrEF,Citation75 which could represent another mechanism through which increased LM may exert beneficial effects. In HFpEF lower LM, particularly appendicular LM has been associated with lower muscle strength,Citation76 however, its role in predicting clinical outcomes requires further study.
Obesity increases CHD risk
Obesity increases the risk for CHD, by increasing the load of the atherosclerotic plaques, characterized by greater macrophage infiltration and plaque instability.Citation77,Citation78 The chronic low-grade systemic pro-inflammatory status in patients with obesity seems to be responsible, at least in part, for the increased CHD risk. Systemic inflammation (eg, high-sensitivity C-reactive protein [hsCRP]) has long been implicated in the pathophysiology of atherosclerosis. Recently, an IL-1β targeted anti-inflammatory therapy was proven to be effective in reducing major adverse CVD events in patients with elevated systemic inflammation and established atherosclerotic CVD.Citation79 However, non-targeted anti-inflammatory therapies may not be efficacious and perhaps could be even detrimental.Citation80 In addition to the inflammatory hypothesis which may drive obesity to CHD, obesity is also associated with several major risk factors for CHD, like T2DM and dyslipidemia, which can, in turn, increase the risk for CHD further.Citation8
The obesity paradox in CHD
Although obesity is a major risk factor for CHD, patients with established CHD and a higher BMI have a more favorable prognosis, forming an obesity paradox similar to that seen in HF.Citation81–Citation83 Although the mechanisms through which obesity may be protective in patients with established CHD are not completely understood, epidemiologic studies suggest that the changes in body composition compartments in obesity may mediate some of the reported benefits.
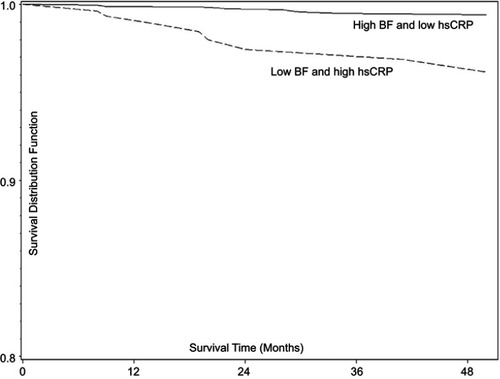
Similar to that described in patients with HF, increased LM in obese individuals seems to exert protective effects. However, in addition to the increased LM, the excess adiposity may also be protective in patients with established CHD as greater amounts of LM and concomitant increases in FM present a more favorable prognosis when compared to individuals with high LM and low FM.Citation84 This finding is extremely interesting as it suggests that excess adiposity when accompanied by increased LM may, in fact, be protective in the setting of CHD. The increased adiposity seems to be particularly beneficial in those individuals with low systemic inflammation defined as hsCRP <3 mg/L ().Citation85 Although the exact trigger responsible for the increased hsCRP in obese individuals is unknown, the adipose tissue itself may propagate production of pro-inflammatory cytokines and increase in systemic low-grade systemic inflammation. This is supported in the literature, showing that patients with CHD that have high FM and low hsCRP have the most favorable prognosis suggesting that excess total adiposity may not be as detrimental when it is not accompanied by increased systemic inflammation. As such, one could speculate that therapeutics targeting systemic inflammation may induce an even more profound beneficial effect in obese individuals, independent of changes in body mass and body composition.
Figure 4 Body fat and systemic inflammation in coronary heart disease. Survival estimates (Kaplan–Meier) by body fat (BF) and high-sensitivity C-reactive protein (hsCRP) strata: low hsCRP = CRP<3 mg/L; high hsCRP = CRP ≥3 mg/L; low BF = BF<25%; high BF ≥25%. Copyright ©2016. Reproduced from De Schutter A, Kachur S, Lavie CJ, Boddepalli RS, Patel DA, Milani RV. The impact of inflammation on the obesity paradox in coronary heart disease. Int J Obes (Lond). 2016;40(11):1730–1735.Citation85
As previously described for HF, assessment of CRF is extremely important in defining the CVD risk of different individuals, as BMI and even body composition assessment alone may not be sufficient.Citation86 A high CRF is an independent predictor for better clinical outcomes in patients with established CHD, independent of BMI, total FM or visceral adiposity (assessed via measurement of waist circumference).Citation86 This suggests that improving CRF should be a priority in patients with CHD, even without apparent changes in BMI or other anthropometric and body composition parameters.
CRF in CVD: a new clinical vital sign
The importance of assessing CRF has been recently highlighted in a scientific statement released by the American Heart Association.Citation87 The strength of CRF as a robust predictor of health outcomes should compel clinicians to include its assessment as a standard of care. The direct measurement of peak VO2 with a maximal cardiopulmonary exercise test (CPX) provides the most precise assessment of CRF, accurately objectifies the relative patient effort, and offers insight into the pathophysiology of exercise intolerance. However, if CPX is not available estimation of CRF using the peak workload obtained during a treadmill or cycle ergometer exercise test provides useful information.Citation87 It is important to emphasize that indirect assessment of CRF (ie METs derived from treadmill speed/%grade) can lead to overestimation of exercise capacity in those with pathology causing impaired oxygen uptake kinetics or handrail use during treadmill exercise.Citation88 In addition to the objective methods listed above, prediction equations have been developed to estimate CRF,Citation87 particularly in those situations in which exercise test cannot be performed. However, because several different equations exist in the literature, it is important to select the most appropriate equation based on the population being investigated.
Improvements in CRF are associated with reduced mortality in the general populationCitation89 and this association is more pronounced in those with established CVD or with CVD risk factors.Citation90,Citation91 As such, a major effort by clinicians and researchers is required to develop and implement therapeutic strategies aimed at improving CRF, using non-pharmacologic and pharmacologic interventions, individually or combined.Citation92 The obesity paradox has not been reported in patients with greater CRF,Citation65–Citation67 suggesting that improving CRF levels should be investigated further as a key clinical outcome, particularly in those patients with HF and CHD. Exercise training (both aerobic training and resistance training) perhaps as part of a cardiac rehabilitation (CR) program, is currently the best modality for improving CRF clinically.Citation93,Citation94 However, due to limited financial coverage and reimbursement for CR and structured exercise training programs, implementation of those strategies aimed at improving CRF, particularly in patients with HFpEF, remains challenging.Citation95 Although the ideal amount and intensity of exercise required to improve clinical outcomes in individuals with obesity and established CVD is unclear, the beneficial effects of exercise training on CRF have been confirmed in several studies. In patients with obesity and concomitant HFpEF, a 1-hour supervised aerobic exercise (ie, walking intensity based on individual CRF measured at baseline) 3 times per week for an average of 49 mins per session for 20 weeks results in improved CRF (+1.2 mL•kg−1•min−1). Such improvements were further increased in those undergoing concomitant caloric restriction (+2.5 mL•kg−1•min−1), supporting the beneficial effects of exercise associated with dietary intervention. Similarly, another study in older adults individuals with obesity but without HF, undergoing caloric restriction, both aerobic exercise and resistance training (60 mins per session, 3 times per week) resulted in greater CRF (+3.3 mL•kg−1•min−1 and +1.3 mL•kg−1•min−1) after 6 months compared to the control group that did not undergo caloric restriction nor exercise training.Citation96 In the absence of structured exercise programs, non-structured exercise in the form of increased daily physical activity and a reduction in sedentary behaviorCitation97,Citation98 is also an effective strategy to increase CRF, although less efficacious than exercise training.Citation99
In patients with obesity and established HF, one of the major focuses of this review article, exercise can improve CRF independent of changes in body weight.Citation93 This suggests that improvements in both CV and non-CV factors are responsible for an increase in CRF. Notably, caloric restriction-induced weight loss has also demonstrated improvements in both CRF and quality of life in patients with obesity and HF, particularly HFpEF.Citation100,Citation101 This demonstrates that improved CRF can also be achieved through mechanisms that seem to be at least partially independent from changes in cardiac function. This data proposes that improvements in non-cardiac factors such as reduction in the intramuscular fat/skeletal muscle mass ratio and improved skeletal muscle functionality may be responsible for the improvements seen in patients undergoing weight loss.Citation32,Citation102–Citation104 Pharmacologic strategies approved for the treatment of obesity have been rarely investigated in a rigorous manner in patients with established CVD as most clinical trials do not enroll this population. We believe that there is a potential for beneficial effects of these therapies even in patients with CVD,Citation105 however, further investigation is needed at this time before being able to recommend and implement such strategies in a broad spectrum of individuals in clinical practice.
A different consideration should be made in those patients in which weight loss is unintentional. In both HF and CHD, unintentional weight loss has been associated with worse clinical outcomes and may be linked to other medical contributing factors.Citation106–Citation108 Although the driver for worsening outcomes is not completely understood, unintentional weight loss is typically associated with a reduction in FM but more importantly of LM, leading to an increased risk for cachexia and sarcopenia, even in individuals with obesity.Citation109 For such reasons, unintentional weight loss should always be investigated further, independent of the initial body weight and BMI.
Conclusion
Overweight and obesity are strong risk factors for the development of CVD, particularly HF and CHD. Although the exact mechanisms connecting obesity and the development of these conditions are not completely understood, the ability of the adipose tissue to expand and produce pro-inflammatory cytokines that can directly impair cardiac systolic and diastolic function as well as the formation of atherosclerotic plaques plays a major role. Similarly, other body composition changes typical of obesity can also lead to initial hemodynamic and structural changes of the heart. However, when obesity and HF or CHD coexist, the prognosis in patients with obesity seems to be more favorable as compared to those who are normal weight or underweight, particularly in the setting of reduced CRF. Of note, the presence of an obesity paradox should not be seen as a promotion of obesity in the general population or in those individuals without established CVD. In fact, if obesity were prevented, they may not have developed that specific CVD in the first place, resulting in a longer and healthier life free of CVD.Citation110
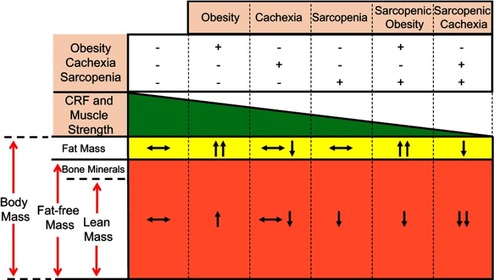
The increased amount of LM reported in individuals with the typical obesity phenotype is a major driver for increased CRF. Greater CRF is associated with improved survival and may partially explain the beneficial effects of obesity. Individuals with reduced amounts of LM (ie, sarcopenia), even in the setting of obesity (ie, sarcopenic obesity) present with a worse CRF (). Such results suggest that strategies that can increase LM, such as exercise training and dietary interventions may represent effective therapeutic strategies. Clearly, long-term studies investigating the effects of such interventions on clinical outcomes are required to implement them in clinical practice and engage providers in facilitating and referring patients for such interventions.
Figure 5 Relationship between body composition phenotypes, cardiorespiratory fitness (CRF) and muscle strength in heart failure. Body composition compartments changes can directly affect CRF and muscle strength in patients with heart failure. Reproduced with permission from Ventura HO, Carbone S, Lavie CJ. Muscling up to improve heart failure prognosis. Eur J Heart Fail. 2018;20(11):1588–1590. © 2018 The Authors. European Journal of Heart Failure © 2018 European Society of Cardiology.Citation35
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health Organization. Obesity and overweight fact sheet. WHO Media Cent. 2016 Available from: https://www.who.int/newsroom/fact-sheets/detail/obesity-and-overweight. Accessed March 27, 2019.
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253.
- Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US Youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–1725. doi:10.1001/jama.2018.306029570750
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity among Adults and Youth: United States, 2015–2016.
- Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018. doi:10.1001/jamacardio.2018.0022
- Iliodromiti S, Celis-Morales CA, Lyall DM, et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J. 2018;39:1514–1520. doi:10.1093/eurheartj/ehy05729718151
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009;28(5):w822–31. doi:10.1377/hlthaff.28.5.w82219635784
- Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol. 2017;15(1):45–56. doi:10.1038/nrcardio.2017.10828748957
- Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92(2):266–279. doi:10.1016/j.mayocp.2016.11.00128109619
- Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61(2):142–150. doi:10.1016/j.pcad.2018.07.00329981771
- Lavie CJ, De Schutter A, Parto P, et al. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. doi:10.1016/j.pcad.2016.01.00826826295
- Stamler R, Ford CE, Stamler J. Why do lean hypertensives have higher mortality rates than other hypertensives? Findings of the hypertension detection and follow-up program. Hypertens (Dallas, Tex 1979). 1991;17(4):553–564. doi:10.1161/01.HYP.17.4.553
- Jayedi A, Shab-Bidar S. Nonlinear dose–response association between body mass index and risk of all-cause and cardiovascular mortality in patients with hypertension: A meta-analysis. Obes Res Clin Pract. 2018;12(1):16–28. doi:10.1016/j.orcp.2018.01.002
- Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123(7):646–651. doi:10.1016/j.amjmed.2009.11.02620609687
- Sandhu RK, Ezekowitz J, Andersson U, et al. The “obesity paradox” in atrial fibrillation: observations from the ARISTOTLE (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. 2016;37(38):2869–2878. doi:10.1093/eurheartj/ehw12427071819
- Agarwal M, Agrawal S, Garg L, Lavie CJ. Relation between obesity and survival in patients hospitalized for pulmonary arterial hypertension (from a nationwide inpatient sample database 2003 to 2011). Am J Cardiol. 2017;120:489–493. doi:10.1016/j.amjcard.2017.04.05128601194
- Brida M, Dimopoulos K, Kempny A, et al. Body mass index in adult congenital heart disease. Heart. 2017;103(16):1250–1257. doi:10.1136/heartjnl-2016-31057128237971
- Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond). 2016;40(5):761–767. doi:10.1038/ijo.2015.24326620887
- Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care. 2015;18:535–551. doi:10.1097/MCO.000000000000021626335310
- Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–239.8561156
- Forbes GB, Welle SL. Lean body mass in obesity. Int J Obes. 1983;7(2):99–107.6862762
- Ortega FB, Sui X, Lavie CJ, Blair SN. Body mass index, the most widely used but also widely criticized index: would a criterion standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin Proc. 2016;91(4):443–455. doi:10.1016/j.mayocp.2016.01.00826948431
- Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38(8):940–953. doi:10.1177/014860711455018925239112
- Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. doi:10.1161/CIRCULATIONAHA.111.06726422949540
- Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi:10.1152/physrev.00033.201123303913
- Carbone S, Elagizi A, Lavie CJ. Obesity and mortality risk in heart failure: when adipose tissue distribution matters. Eur J Heart Fail. 2018;20(9):1278–1280. doi:10.1002/ejhf.127929999227
- Carbone S, Shah KB, Van Tassell BW, et al. Obesity and diastolic heart failure: is inflammation the link? Transl Med. 2013;3:e124. doi:10.4172/2161-1025.1000e124
- Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27(8):875–888. doi:10.1038/sj.ijo.080232612861227
- Ballak DB, Stienstra R, Tack CJ, Dinarello CA, Van Diepen JA, van Diepen JA. Cytokine IL-1 family members in the pathogenesis and treatment of metabolic disease : focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75(2):280–290. doi:10.1016/j.cyto.2015.05.00526194067
- Piché M-E, Poirier P, Lemieux I, Després J-P. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61(2):103–113. doi:10.1016/j.pcad.2018.06.00429964067
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008. World Health Organization; 2011.
- Carbone S, Billingsley HE, Rodriguez-Miguelez P, et al. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity and Cachexia. Curr Probl Cardiol In press 2019. doi:10.1016/j.cpcardiol.2019.03.006
- Prado CMM, Wells JCK, Smith SR, Stephan BCM, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. doi:10.1016/j.clnu.2012.06.01022809635
- Carbone S, Popovic D, Lavie CJ, Arena R. Future Cardiol. Epub 2017 Aug 10. doi:10.2217/fca-2017-0023
- Ventura HO, Carbone S, Lavie CJ. Muscling up to improve heart failure prognosis. Eur J Heart Fail. 2018;20(11):1588–1590. doi:10.1002/ejhf.131430225950
- Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Hear Fail Rep. 2015;12:3. doi:10.1007/s11897-015-0257-5
- Emami A, Saitoh M, Valentova M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20(11):1580–1587. doi:10.1002/ejhf.130430160804
- Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi:10.1056/NEJMoa02024512151467
- Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69(9):1129–1142. doi:10.1016/j.jacc.2016.11.08128254175
- Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68(2):200–203. doi:10.1016/j.jacc.2016.05.01927386774
- Carbone S, Pandey A, Lavie CJ. Editorial commentary: obesity and heart failure with preserved ejection fraction: A single disease or two co-existing conditions? Trends Cardiovasc Med. 2017. doi:10.1016/j.tcm.2017.12.007
- Obokata M, Reddy YN, Pislaru S, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19. doi:10.1161/CIRCULATIONAHA.116.02680728381470
- Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73–90. doi:10.1161/CIRCULATIONAHA.116.02188427358439
- Toldo S, Mezzaroma E, Mauro AG, Salloum F, Van Tassell BW, Abbate A. The inflammasome in myocardial injury and cardiac remodeling. Antioxid Redox Signal. 2015;22(13):1146–1161. doi:10.1089/ars.2014.598925330141
- Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128(17):1910–1923. doi:10.1161/CIRCULATIONAHA.113.00319924146121
- Toldo S, Mezzaroma E, O’Brien L, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Hear Circ Physiol. 2014;306(7):1025–1031. doi:10.1152/ajpheart.00795.2013
- Van Tassell BW, Canada J, Carbone S, et al. Interleukin-1 blockade in recently decompensated systolic heart failure. Circ Hear Fail. 2017;10:11. doi:10.1161/CIRCHEARTFAILURE.117.004373
- Van Tassell BW, Trankle CR, Canada JM, et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Hear Fail. 2018;11:8. doi:10.1161/CIRCHEARTFAILURE.118.005036
- Carbone S, Mauro AG, Mezzaroma E, et al. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol. 2015;198:66–69. doi:10.1016/j.ijcard.2015.06.13626151718
- Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KHG, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol Nutr Food Res. 2012;56(8):1212–1222. doi:10.1002/mnfr.20120005822700321
- Li X, Du N, Zhang Q, et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5(10):e1479. doi:10.1038/cddis.2014.43025341033
- Alpert MA, Alexander JK, editors. Hemodynamics Alterations with Obesity in Man. The Heart and Lung in Obesity. New York: John Wiley & Sons; 1998.
- Packer M. The conundrum of patients with obesity, exercise intolerance, elevated ventricular filling pressures and a measured ejection fraction in the normal range. Eur J Heart Fail. 2018. doi:10.1002/ejhf.1377
- Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull. 1953;1:39–44.
- Alpert MA, Terry BE, Mulekar M, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80(6):736–740.9315579
- Kasper EK, Hruban RH, Baughman KL. Cardiomyopathy of obesity: a clinicopathologic evaluation of 43 obese patients with heart failure. Am J Cardiol. 1992;70(9):921–924.1529947
- Kaw R. Obesity and pulmonary hypertension. What’s the link? Bjmp. 2009;2(2):4–5.
- Friedman SE, Andrus BW. Obesity and pulmonary hypertension : a review of pathophysiologic mechanisms. J Obes. 2012;2012:505274. doi:10.1155/2012/50527422988490
- Hall JE, Do Carmo JM, Da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi:10.1161/CIRCRESAHA.116.30569725767285
- Kawarazaki W, Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens. 2016;29(4):415–423. doi:10.1093/ajh/hpw00326927805
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the american heart association. Circulation. 2018;137(12):e67–e492. doi:10.1161/CIR.000000000000055829386200
- Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38(3):789–795.11527635
- Padwal R, Mcalister FA, McMurray JJV, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction : a meta-analysis of individual patient data. Int J Obes (Lond). 2013;38(8):1110–1114. doi:10.1038/ijo.2013.20324173404
- Parto P, Lavie CJ, Arena R, Bond S, Popovic D, Ventura HO. Body habitus in heart failure: understanding the mechanisms and clinical significance of the obesity paradox. Future Cardiol. 2016;12:639–653 In press. doi:10.2217/fca-2016-0029.27762638
- Lavie CJ, Cahalin LP, Chase P, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88(3):251–258. doi:10.1016/j.mayocp.2012.11.02023489451
- Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. Am J Cardiol. 2015;115(2):209–213. doi:10.1016/j.amjcard.2014.10.02325465933
- McAuley PA, Keteyian SJ, Brawner CA, et al. Exercise capacity and the obesity paradox in heart failure: the FIT (henry ford exercise testing) project. Mayo Clin Proc. 2018;93(6):701–708. doi:10.1016/j.mayocp.2018.01.02629731178
- Hothi SS, Tan DK, Partridge G, Tan LB. Is low VO2 max/kg in obese heart failure patients indicative of cardiac dysfunction? Int J Cardiol. 2015;184:755–762. doi:10.1016/j.ijcard.2015.02.01825827937
- Krachler B, Savonen K, Komulainen P, Hassinen M, Lakka TA, Rauramaa R. VO2max/kg is expected to be lower in obese individuals! Int J Cardiol. 2015;189:234. doi:10.1016/j.ijcard.2015.04.10025912289
- Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol. 2000;36(7):2126–2131.11127451
- Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786.1999029
- Krachler B, Savonen K, Komulainen P, Hassinen M, Lakka TA, Rauramaa R. Cardiopulmonary fitness is a function of lean mass, not total body weight: the DR’s EXTRA study. Eur J Prev Cardiol. 2015;22(9):1171–1179. doi:10.1177/204748731455796225381337
- Houstis NE, Eisman AS, Pappagianopoulos PP, et al. Exercise intolerance in heart failure with preserved ejection fraction. Circulation. 2018;137(2):148–161. doi:10.1161/CIRCULATIONAHA.117.02905828993402
- Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ. 2012;345(nov203):e7279. doi:10.1136/bmj.e727923169869
- Hülsmann M, Quittan M, Berger R, et al. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6(1):101–107. doi:10.1016/j.ejheart.2003.07.00815012925
- Bekfani T, Pellicori P, Morris DA, et al. Sarcopenia in patients with heart failure with preserved ejection fraction : impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 2016;222:41–46. doi:10.1016/j.ijcard.2016.07.13527454614
- De Rosa R, Vasa-Nicotera M, Leistner DM, et al. Coronary atherosclerotic plaque characteristics and cardiovascular risk factors - insights from an optical coherence tomography study. Circ J. 2017;81(8):1165–1173. doi:10.1253/circj.CJ-17-005428420816
- Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31(2):177–183. doi:10.1016/j.cjca.2014.11.03125661552
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa170791428845751
- Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2018:NEJMoa1809798 Doi:10.1056/NEJMoa1809798
- De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease-the obesity paradox. Prog Cardiovasc Dis. 2014;56:401–408. doi:10.1016/j.pcad.2013.08.00324438731
- Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi:10.1016/S0140-6736(06)69251-916920472
- Lavie CJ, Carbone S, Agarwal MA. An obesity paradox with myocardial infarction in the elderly. Nutrition. 2018;46:122–123. doi:10.1016/j.nut.2017.08.00329029870
- Lavie C, De Schutter A, Patel D, Artham S, Milani R. Body composition and survival in stable coronary heart disease. J Am Coll Cardiol. 2012;60(15):1374–1380. doi:10.1016/j.jacc.2012.05.03722958953
- De Schutter A, Kachur S, Lavie CJ, Boddepalli RS, Patel DA, Milani RV. The impact of inflammation on the obesity paradox in coronary heart disease. Int J Obes (Lond). 2016;40(11):1730–1735. doi:10.1038/ijo.2016.12527453423
- McAuley PA, Artero EG, Sui X, et al. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87(5):443–451. doi:10.1016/j.mayocp.2012.01.01322503065
- Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi:10.1161/CIR.000000000000046127881567
- Manfre MJ, Yu GH, Varmá AA, Mallis GI, Kearney K, Karageorgis MA. The effect of limited handrail support on total treadmill time and the prediction of VO2 max. Clin Cardiol. 1994;17(8):445–450.7955592
- Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi:10.1001/jama.2009.68119454641
- Oktay AA, Lavie CJ, Kokkinos PF, Parto P, Pandey A, Ventura HO. The interaction of cardiorespiratory fitness with obesity and the obesity paradox in cardiovascular disease. Prog Cardiovasc Dis. 2017;60(1):30–44. doi:10.1016/j.pcad.2017.05.00528502849
- Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. doi:10.1161/CIRCULATIONAHA.110.00323621422392
- Wedell-Neergaard A-S, Lang Lehrskov L, Christensen RH, et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2018. doi:10.1016/j.cmet.2018.12.007
- Lavie CJ, Arena R, Swift DL, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117(2):207–219. doi:10.1161/CIRCRESAHA.117.30520526139859
- De Schutter A, Kachur S, Lavie CJ, et al. Cardiac rehabilitation fitness changes and subsequent survival. Eur Hear J Qual Care Clin Outcomes. 2018;4(3):173–179. doi:10.1093/ehjqcco/qcy018
- Lavie CJ, Ozemek C, Arena R. Bringing cardiac rehabilitation and exercise training to a higher level in heart failure. J Am Coll Cardiol. 2019;73(12):1444–1446. doi:10.1016/j.jacc.2018.12.073
- Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943–1955. doi:10.1056/NEJMoa161633828514618
- Moholdt T, Lavie CJ, Nauman J. Sustained physical activity, not weight loss, associated with improved survival in coronary heart disease. J Am Coll Cardiol. 2018;71(10):1094–1101. doi:10.1016/j.jacc.2018.01.01129519349
- Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72(14):1622–1639. doi:10.1016/j.jacc.2018.08.214130261965
- Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise and cardiovascular health. Circ Res. 2019;124(5):799–815. doi:10.1161/CIRCRESAHA.118.312669
- Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315(1):36. doi:10.1001/jama.2015.1734626746456
- Carbone S, Canada JM, Buckley LF, et al. Obesity contributes to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68(22):2487–2488. doi:10.1016/j.jacc.2016.08.07227908355
- Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 2015;119(6):739–744. doi:10.1152/japplphysiol.00049.201525911681
- Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58(3):265–274. doi:10.1016/j.jacc.2011.02.05521737017
- Molina AJA, Bharadwaj MS, Van Horn C, et al. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. J AmColl Cardiol HF. 2016;4(8):636–645. doi:10.1016/j.jchf.2016.03.011
- Carbone S, Dixon DL. Selecting appropriate weight loss pharmacotherapies in older adults to reduce cardiovascular risk. Expert Opin Pharmacother. 2018;19(13):1399–1402. doi:10.1080/14656566.2018.151170430113233
- Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23(6):603–611.10411233
- Sierra-Johnson J, Romero-Corral A, Somers VK, et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15(3):336–340. doi:10.1097/HJR.0b013e3282f4834818525390
- Anker SD, Negassa A, Coats AJS, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet (London, England). 2003;361(9363):1077–1083. doi:10.1016/S0140-6736(03)12892-9
- Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487–491. doi:10.1007/BF0298271018615231
- Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention. J Am Coll Cardiol. 2018;72(13):1506–1531. doi:10.1016/j.jacc.2018.08.103730236314
