Abstract
Background
In this study we compared the outcomes of the everolimus-eluting stent (EES) versus the zotarolimus-eluting stent (ZES) in patients treated at a tertiary medical center, with up to one year of follow-up.
Methods
Unselected consecutive patients were retrospectively recruited following stenting with the ZES (n = 197) or EES (n = 190). The first 100 consecutive patients in each cohort underwent syntax scoring. The primary endpoint of the study was target vessel failure, defined as the combined endpoint of cardiac death, non-fatal myocardial infarction, or target vessel revascularization. Secondary endpoints included target lesion revascularization, target lesion failure, acute stent thrombosis, total death, cardiac death, and non-fatal myocardial infarction.
Results
The two groups were similar, including for Syntax scores (19.6 ± 12.8 versus 20.6 ± 13.6), number of stents per patient (2.9 ± 1.9 versus 2.9 ± 2.1), and cardiovascular risk factors. By one year, the primary outcome occurred in 20.8% EES versus 26.7% ZES (P = 0.19) patients. The secondary endpoints were as follows: target lesion revascularization (8.9% versus 20.6%, P = 0.003), target vessel revascularization (18.9% versus 25.6%, P = 0.142), definite and probable stent thrombosis (0% versus 2.5%), non-fatal myocardial infarction (2.7% versus 3.6%), and mortality (3.2% versus 5.1%) for the EES versus the ZES, respectively.
Conclusion
EES had similar target vessel failure to ZES, but superior target lesion revascularization and target lesion failure at one year of follow-up in an unselected cohort of patients.
Introduction
The zotarolimus-eluting stent (ZES), Endeavor® (Medtronic, Minneapolis, MN) and the everolimus-eluting stent (EES), Xience® or Promus™ (Abbott Laboratories, Abbott Park, IL) are second-generation drug-eluting stents with lower target lesion revascularization and adverse event rates than bare metal stents.Citation1–Citation4
The ZES and EES stents have been compared in randomized trials with the paclitaxel-eluting stent (Boston Scientific, San Diego, CA) and the sirolimus-eluting stent (Cordis Corporation, Bridgewater, NJ),Citation5–Citation7 but few comparative data exist between the Endeavor ZES and the EES. Herrador et alCitation8 compared the ZES and EES in coronary bifurcating lesions, and found higher 12-month adverse event and target lesion revascularization rates in the ZES group. Recently, a new ZES, the Resolute™ (Medtronic, Minneapolis, MN) was compared with the EES in a large randomized trial, with no differences in outcomes found between these two stents.Citation9 However, the Resolute ZES had prolonged elution of the drug compared with the Endeavor ZES. Compared with historic controls, the Resolute ZES stent outperformed the Endeavor ZES stent, with superior target lesion and vessel revascularization.Citation10 In this single-center study, we compared the Endeavor ZES and the EES for late outcomes at one year in an unselected consecutive group of patients.
Materials and methods
Unselected consecutive patients were retrospectively recruited from a single center following stenting with the ZES or the EES. Both de novo and restenotic lesions were included. Patients with bypass graft stenting or who received mixed stents were excluded. The first 100 consecutive patients in each cohort underwent Syntax scoring by an independent investigator blinded to patients’ outcomes. The investigator underwent basic training in Syntax scoring using the online tutorial on the Syntax score website (http://www.syntaxscore.com) followed by extensive training with an interventional cardiologist experienced in Syntax scoring and having performed over 50 cases with close supervision.
and show the demographic, clinical, and procedural variables collected by reviewing medical records. Angiographic variables are shown in , and were obtained by independent reviewing of the angiograms by an investigator blinded to patient outcomes. Ejection fraction was obtained from the procedural records as assessed qualitatively during the index procedure using left ventriculography.
Table 1 Demographic and clinical variables
Table 2 Indications for angiography and coronary artery distribution
Follow-up was limited to one year from the index procedure and was performed using medical records, phone calls, or both. Patients were initially mailed a brief letter describing the protocol, followed by a phone call to obtain verbal consent to be part of the study (using a standardized script approved by our institutional review board). All events reported by patients were verified by cross-reference to medical records. Patients who were deceased had their death certificate retrieved when possible to evaluate the cause of death.
The primary outcome of the study was target vessel failure (defined as cardiac death, non-fatal myocardial infarction, and target vessel revascularization). Secondary outcomes included target lesion revascularization, target vessel revascularization, target lesion failure (defined as cardiac death, non-fatal myocardial infarction, and target lesion revascularization), acute stent thrombosis as defined by the Academic Research Consortium,Citation11 non-fatal myocardial infarction, and cardiac death.
Statistical analysis
Descriptive analysis was performed on all variables. The t-test was used for continuous variables and Chi-square testing for dichotomous variables. Univariate analysis compared the demographic, clinical, angiographic, and outcome variables between the two groups. Survival analysis (Kaplan-Meier) was performed for target vessel failure over one year of follow-up. SPSS (IBM, New York, NY) software was used to conduct the analysis.
Results
A total of 235 ZES and 208 EES patients met the inclusion criteria and were recruited to the study. Patients were excluded if they refused to give the verbal consent required by the institutional review board (n = 25 ZES, n = 10 EES) or were lost to follow-up (n = 13 ZES, n = 8 EES). A total of 197 ZES patients (270 vessels, 403 segments) and 190 EES patients (306 vessels, 479 segments) were included in the final analysis.
Descriptive analysis for all patients is shown in . There was a high proportion of patients who had had prior percutaneous coronary intervention and prior bypass surgery in both cohorts. Approximately two thirds of the patients were current or prior smokers and 36.5% were diabetics. There were no statistical differences for any of the clinical and demographic variables between the EES and ZES stents, except for a higher incidence of prior myocardial infarction in the EES group (38.9% versus 28.9%; P = 0.041) and a higher incidence of cerebrovascular disease in the ZES group (16.2% versus 4.7%; P = 0.001). Also, there was no bias regarding utilization of particular stent types at the medical center between the 11 interventionalists, who used the ZES and EES at the same statistical frequency.
Indications for the procedure were similar between the two groups, with about half the patients treated for an acute coronary syndrome. The distribution of disease was also similar between the cohorts, with 6%–10% of patients having had their left main stem treated (). Angiographic and procedural variables are shown in . There was a relatively high number of stents placed per patient (2.8–2.9 stents), and approximately one third of patients had restenotic index lesions. Patients in the EES group had a lower ejection fraction than those in the ZES group (51.5% versus 59.7%, P = 0.001); however, ejection fractions were in the normal range in both groups. Long lesion lengths were noted, but were similar in both groups. The Syntax scores for the first 100 consecutive patients, which reflect angiographic complexity, were statistically nearly identical between the EES and ZES (19.6 ± 12.8 versus 20.6 ± 13.6).
Table 3 Angiographic and procedural variables
At one-year follow-up, target vessel failure was 26.7% for the ZES versus 20.8% for the EES (P = 0.19, , ). The secondary endpoint of target lesion revascularization (20.6% versus 8.9%, P = 0.003) was superior for the ZES versus the EES. Target vessel revascularization (26.7% versus 20.8%), cardiac death (2.0% versus 1.6%), non-cardiac death (2.1% versus 1.1%), and definite and probable stent thrombosis (2.5% versus 0%) trended in favor of the EES, but no statistical difference was seen.
Figure 1 Kaplan-Meier curve showing target vessel failure survival for the Endeavor zotarolimus stent (solid line) versus the Everolimus stent (dashed line).
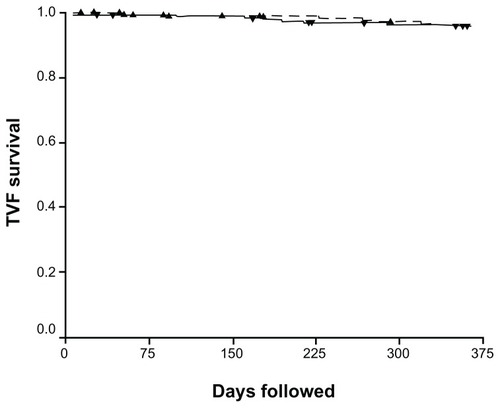
Table 4 Patient outcomes
Clinical history of patients with definite or probable acute stent thrombosis
Five patients in the ZES cohort had definite or probable stent thrombosis. The first patient was a 92-year-old female with a prior myocardial infarction and a history of hypertension. Stent thrombosis occurred 7 days after the index procedure. She was on clopidogrel and aspirin. She underwent target lesion revascularization but later died during the same hospital stay. The second patient was a 49-year-old male with a prior myocardial infarction, and hyperlipidemia. Stent thrombosis occurred 8 days following the index procedure. He was on clopidogrel and aspirin. He underwent target lesion revascularization successfully. The third patient was a 71-year-old male with history of hyperlipidemia, diabetes mellitus, and prior myocardial infarction. Stent thrombosis occurred 100 days following the index procedure. He was on aspirin and clopidogrel. He underwent target lesion revascularization but later died during the same hospital stay. The fourth patient was a 68-year-old male with a history of hyperlipidemia, prior tobacco use, and diabetes. He was on clopidogrel and aspirin. Stent thrombosis occurred 78 days after the index procedure. He underwent successful target lesion revascularization. The fifth patient was a 65-year-old female with hypertension, hyperlipidemia, diabetes mellitus, and a prior history of smoking. Stent thrombosis occurred at 192 days after the index procedure. She was on clopidogrel and aspirin. She underwent target lesion revascularization successfully.
Discussion
In this study, the primary outcome occurred in 20.8% of the EES versus 26.7% of the ZES (P = 0.19) patients, with no differences in the secondary outcome of target vessel revascularization (18.9% versus 25.6%, P = 0.142). However, the EES showed superior target lesion revascularization (8.9% versus 20.6%, P = 0.003) and target lesion failure (11.7 versus 22.6%, P = 0.003) when compared with the ZES. Both the ZES and EES have been shown to have lower target lesion revascularization and adverse event rates than bare metal stents.Citation1–Citation4 However, not all drug-eluting stents have similar outcomes. In this study, the EES had a superior one-year outcome than the ZES, with less target lesion revascularization and target lesion failure at the one-year follow-up. However, interestingly, target vessel revascularization was the same in both cohorts, resulting in statistically similar target vessel failure. In this retrospective study, there were no predefined endpoints as to when target lesion revascularization could be performed. It is possible that this biased the data in favor of the EES, considering that the ZES is known to have higher late lumen loss than the EES.Citation12
The ZES has been compared with the sirolimus-eluting stent in randomized trials.Citation5 Insegment binary angiographic restenosis was higher in the ZES cohort (11.7% versus 4.3%, P = 0.04). At 9 months, total (clinically and non-clinically driven) target lesion revascularization rates were 9.8% and 3.5% for the ZES and sirolimus-eluting stent groups, respectively (P = 0.04). However, clinically driven target lesion revascularization and target vessel failure did not differ significantly between the two stents. Further, the Endeavour ZES has been compared with the paclitaxel-eluting stent in the randomized ENDEAVOR IV trial. This trial showed that the ZES was noninferior to the paclitaxel-eluting stent at the 12-month follow-up, with rates of target vessel failure being 6.6% versus 7.1%, respectively (P ≤ 0.001). In Endeavor IV, there were no significant differences between the ZES and the paclitaxel-eluting stent for rates of cardiac death, myocardial infarction, target vessel revascularization, or stent thrombosis.Citation13 At 3-year follow-up, compared with the paclitaxel-eluting stent, the ZES showed reduced target vessel failure (12.3% versus 15.9%, P = 0.049) and myocardial infarction rates (2.1% versus 4.9%, P = 0.005), with similar ischemia-driven target lesion revascularization (6.5% versus 6.1%, P = 0.662).Citation14
The EES was also compared with the paclitaxel-eluting stent in the SPIRIT III trial. At the 2-year follow-up, compared with the paclitaxel-eluting stent, patients treated with the EES had a significant 32% reduction in target vessel failure (10.7% versus 15.4%, P = 0.04) and a 45% reduction in major adverse cardiac events (cardiac death, myocardial infarction, or target lesion revascularization; 7.3% versus 12.8%, P = 0.004).Citation7 In the elderly cohort (those >65 years of age) of SPIRIT III, the EES-treated patients had lower rates of binary insegment restenosis (3.4% versus 15.5%, P = 0.004) at 8 months, and a 48% lower incidence of 3-year target vessel failure (10.8% versus 20.8%, P = 0.009).Citation15
However, limited comparative data exist for the Endeavor ZES and the EES. Herrador et alCitation8 compared the ZES with the EES in coronary bifurcating lesions, and found a higher 12-month adverse events rate (23.1% versus 4.5%, P < 0.001) and higher target lesion revascularization (17.5% versus 3.2%, P < 0.001) in the ZES group. Our study included consecutive patients who received EES and ZES from the same medical center. EES outperformed the Endeavor ZES at the one-year follow-up, with less target lesion failure, driven mostly by less target lesion revascularization. However, target vessel failure was similar between the two stents at one year. Recently the new Resolute ZES (Medtronic) was compared with the EES in a large randomized trial and no differences in outcome were found between these two stents.Citation9 However, the Resolute ZES showed prolonged elution of the drug compared with the Endeavor ZES. Furthermore, compared with historic controls, the Resolute ZES stent outperformed the Endeavor stent in data presented recently at the American College of Cardiology scientific sessions.Citation10 The longer drug elution time of zotarolimus seems to be a key factor in reducing target lesion revascularization and target vessel failure in patients treated with ZES stents. The Resolute ZES stent has recently entered the US market and is likely to replace the Endeavor ZES.
Overall, the rates of target lesion revascularization and target vessel failure with the ZES in our study are higher than that in the data published for the Endeavor ZES from the real-world prospective, multicenter E-Five registry.Citation1 The one-year outcome of the Endeavor ZES in the E-Five registry showed a target lesion revascularization rate of 4.5% and definite/probable stent thrombosis of 0.6%. Our data showed a higher target lesion revascularization and stent thrombosis rate than the E-Five registry, likely secondary to the more complex patients treated at our center. Compared with E-Five, our study had more bifurcating lesions (45.6% versus 18.9%), ostial lesions (8.3% versus 5.8%), instent restenosis (33.3% versus 4.8%), left main stenting (6.3% versus 2.4%), and longer total lesion length (54 ± 37.4 versus 18.51 ± 10.61). Furthermore, there were higher clinical risk features in our patients, with more prior percutaneous coronary interventions (67.5% versus 25.3%) and bypass surgeries (21.8% versus 7.5%). As disease complexity increases and major adverse event rates increase, it is more likely that small differences between drug-eluting stents would become more obvious and significant.
Study limitations
This study is limited by its retrospective nature. However, angiographic complexity was assessed using Syntax scoring by an investigator blinded to patient outcomes and showed no differences between the two cohorts. Interobserver and intraobserver variability was not determined in this study. However, we utilized a reliable training method recently shown to yield at least moderate agreement in Syntax calculation.Citation16 We also limited Syntax scoring to the first consecutive 100 patients in each cohort. However, the near identical results and similar angiographic variables between the two cohorts predict a low likelihood that differences will emerge in calculating syntax scores for the entire cohort.
It is unlikely that randomized trials will compare the Endeavor ZES with the EES because of the recent introduction of the Resolute ZES to the United States, which has proven superior results to the Endeavor ZES. However, these data continue to be of significance for patients who have already received the ZES and continue to receive the EES.
Disclosures
The Midwest Cardiovascular Research Foundation has received research and educational grants from Medtronic, Abbott, and Boston Scientific. This research was supported in part by the Nicolas and Gail Research Fund at the Midwest Cardiovascular Research Foundation, and was presented in part in abstract form at the Cardiovascular Research Technologies 2012 conference, February 4–7, 2012, Washington DC.
References
- MeredithIRothmanMErglisAE-Five InvestigatorsExtended follow-up safety and effectiveness of the Endeavor zotarolimus-eluting stent in real-world clinical practice: two-year follow-up from the E-Five RegistryCatheter Cardiovasc Interv201177993100020853351
- FajadetJWijnsWLaarmanGJLong-term follow-up of the randomised controlled trial to evaluate the safety and efficacy of the zotarolimus-eluting driver coronary stent in de novo native coronary artery lesions: five year outcomes in the ENDEAVOR II studyEuro Intervention2010656256721044908
- SerruysPOngAPiekJJA randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trialEuro Intervention20051586519758878
- TsuchidaKGarcia-GarciaHMOngATRevisiting late loss and neointimal volumetric measurements in a drug-eluting stent trial: analysis from the SPIRIT FIRST trialCatheter Cardiovasc Interv20066718819716400664
- KandzariDELeonMBPopmaJJComparison of zotarolimus eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trialJ Am Coll Cardiol2006482440244717174180
- LeonMBEndeavor clinical trial program Available from: http://www.crtonline.org/flash.aspx?PAGE_ID=4728Accessed October 16, 2007
- StoneGWMideiMNewmanWSPIRIT III InvestigatorsRandomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trialCirculation200911968068619171853
- HerradorJAFernandezJCGuzmanMComparison of zotarolimus-versus everolimus-eluting stents in the treatment of coronary bifurcation lesionsCatheter Cardiovasc Interv2011781086109221793165
- SerruysPWSilberSGargSComparison of zotarolimus eluting and everolimus-eluting coronary stentsN Engl J Med201036313614620554978
- LeonMLBCT III, Session 3014Presented at the American College of Cardiology 60th Annual Scientific SessionsApril 2–5, 2011New Orleans, LA
- Food and Drug AdministrationCirculatory System Devices Panel Meeting Available from: http://www.fda.gov/ohrms/dockets/ac/cdrh06.html#circulatoryAccessed February 9, 2007
- BrenerSJPrasadAJKhanZSacchiTJThe relationship between late lumen loss and restenosis among various drug-eluting stents: a systematic review and meta-regression analysis of randomized clinical trialsAtherosclerosis201121415816221122853
- LeonMBMauriLPopmaJJENDEAVOR IV InvestigatorsA randomized comparison of the ENDEAVOR zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trialJ Am Coll Cardiol20105554355420152559
- LeonMBNikolskyECutlipDEENDEAVOR IV InvestigatorsImproved late clinical safety with zotarolimus-eluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimus- and Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trialJACC Cardiovasc Interv201031043105020965463
- HermillerJBNikolskyELanskyAJClinical and angiographic outcomes of elderly patients treated with everolimus-eluting versus paclitaxel-eluting stents: three-year results from the SPIRIT III randomised trialEuro Intervention2011730731321729832
- GénéreuxPPalmeriniTCaixetaASYNTAX score reproducibility and variability between interventional cardiologists, core laboratory technicians, and quantitative coronary measurementsCirc Cardiovasc Interv2011455356122028472