Abstract
Central arterial structure and function comprise a primary determinant of vascular health, and are integral to the important concept of ventriculo-vascular coupling or interaction. Central aortic stiffening is a major influence on central blood pressure, and directly relates to coronary perfusion. The joint session of the International Society of Vascular Health (Eastern Region) and the Ukrainian Congress of Cardiology was held in Kiev, Ukraine, on September 23, 2011; it provided an expert forum to discuss arterial evaluations, clinical applications, and progress toward translating arterial protection into cardiovascular benefits. The conclusions of the expert panel were:
Aortic stiffness is not presently a treatment target but may be useful for substratifying cardiovascular risk in individuals in order to better target the intensity of conventional therapy, and it may be useful in assessing response to treatment.
Crosstalk between macro- and microcirculation in hypertension has important implications for pharmacological treatment. An antihypertensive regimen should abolish the vicious cycle between the increased resistance in the microcirculation and the increased stiffness of the larger arteries. Such treatment should be based on drugs with multiple actions on the vascular tree, or on drug combinations that target the various segments of the arterial system.
Several blood pressure-independent mechanisms of large artery stiffness exist. Future considerations for clinical understanding of large artery stiffness should involve new drugs and new evaluation methods – with a focus on vascular health, for the initiation of cardiovascular prevention, for newly designed studies for treatment evaluation, and for new studies of drug combinations.
Arterial stiffening is a sign of cardiovascular aging and is a major factor affecting the biomechanics of large arteries. Arterial stiffness is an attractive therapeutic target in terms of vascular aging. Healthy lifestyle, physical exercise, and smoking cessation are the most effective ways of preventing and treating early vascular aging. Long-term effects of cardiovascular drugs on arterial stiffness need to be further investigated.
The emerging clinical data on the cardio ankle vascular index (CAVI) technique of arterial health assessment is presented, showing that the CAVI is elevated in aging, coronary artery diseases, chronic kidney disease, hypertension, diabetes mellitus, smoking, and stress. The CAVI decreased with the administration of statins, angiotensin II receptor blocking agents, and calcium channel blockers. The CAVI is suggested as an important predictor of cardiovascular diseases.
Future development of a clinical understanding of large artery stiffness is important and should include consideration of new drugs and new evaluation methods, with a focus on vascular health aimed at cardiovascular prevention.
Introduction
The continuing development of applicable noninvasive technology and the work of many clinicians and scientists over the last few decades have clearly established the pathophysiological importance of the structure and function of the central arterial vasculature in maintaining vascular health and in influencing cardiovascular outcome.
A joint session of the International Society of Vascular Health (Eastern Region) and the Ukrainian Congress of Cardiology was held in Kiev, Ukraine, on September 23, 2011, to discuss these important contemporary topics in cardiology and cardiovascular medicine.
This meeting brought together experts from around the world to share their experience in the assessment of arterial mechanics and function and to discuss the current state-of-the-art clinical place and interpretation of these methodologies.
Under the chairmanship of Professors Yuri Sirenko and Roland Asmar, sessions were held dealing, respectively, with arterial evaluations and clinical applications, and with translating arterial protection into cardiovascular benefits. In keeping with the aims of the International Society of Vascular Health of advancing high levels of vascular health in individuals and in the community, and to catalyze clinical cooperation between health care professionals, international experts covered topics ranging from basic vascular physiology to large artery mechanics, from microvascular to macrovascular influences, and from basic measurement to novel assessment devices.
Evaluation of arterial hemodynamics
Vascular health has become a very important issue in different areas, such as in pathophysiology to better understand the disease mechanisms, in cardiovascular prevention to better identify patients at high risk, and in pharmacology to better evaluate drug effects.
Several arterial hemodynamic models have been proposed, each of them intended to evaluate one or several hemodynamic or structural parameters. Each of these methods has advantages but also limitations; among the most popular used in clinical practice are discussed next.Citation1
Systemic compliance or stiffness
Some systemic compliance, or stiffness, methods are based on a modified Windkessel model; others are using the “area method,” which requires measurement of aortic blood flow and associated tonometric pressure of the carotid artery.Citation1,Citation2 Another approximation of systemic compliance has been used in the past: the ratio between stroke volume and pulse pressure. The stroke volume/pulse pressure ratio has been investigated in comparison to invasive measurement using the two-element Windkessel modelCitation3–Citation6 and, noninvasively by use of echocardiography, predicted the risk of subsequent cardiovascular events in hypertensive patientsCitation7 and in a community-based sample of elderly men. However, these methods rely on numerous theoretical approximations, and hard end-point evidence from longitudinal studies is lacking.
Local stiffness
Local stiffness can be determined using ultrasound devices or cine magnetic resonance imaging. A major advantage of this approach is that local arterial stiffness is determined directly from the change in pressure driving changes in volume. This is the only method for noninvasively determining the elastic properties of the arterial wall material. Limitations of these methods are the high degree of technical expertise required, the long duration of the examination procedures, and finally, because it is local, its evaluation of only one local point of the arterial tree.
Intima-media thickness
Several methods are used to measure the carotid intima-media thickness based on either the video signal or the radio-frequency signal assessments, the latter being the better method in terms of reproducibility and sensitivity. Advantages of this method lie in the widespread use of ultrasound devices and the facility of the examination procedures. Major limitations of this method are its evaluation of only one local point of the arterial tree (eg, carotid) and that the given information is related only to the geometry (thickness) of the arterial wall; there is no information on the structure or function of the arterial wall.Citation1,Citation8
Regional stiffness
Several techniques are now available to measure regional stiffness. Most of them use pulse-wave velocity (PWV) measurements using mechanical transducers, tonometers, or Doppler signals. For regional stiffness, the aorta is a major vessel of interest because it makes the largest contribution to the buffering function and it is the major location of atherosclerosis. Carotid-femoral (aortic) PWV is generally accepted as the most simple, noninvasive, and reproducible method for determining regional arterial stiffness. It is considered the gold standard measurement of arterial stiffness. Some limitations on PWV measurement should be mentioned: the femoral wave may be difficult to record; in the presence of aortic or iliac-femoral stenosis, the pressure wave may be attenuated and delayed; obesity may cause overestimates of the travel distance; and its being a global estimation of arterial stiffness, no direct information about arterial diameter or thickness are given.Citation1,Citation9,Citation10
Pulse-wave analysis
Pulse-wave analysis allows evaluation or measurement of central and peripheral blood pressure, the augmentation index, and other parameters related to arterial stiffness. Most of these methods use the tonometric method mainly to evaluate central blood pressure (BP), which represents the true pressure load imposed on the left ventricle.Citation11
The central pressure waveform can be estimated either from the radial artery waveform, using a transfer function (calculation), or from the common carotid waveform (measurement). The most widely used approach is to perform radial artery tonometry and then apply a transfer function to calculate the aortic pressure waveform from the radial waveform. Several questions have been raised about the accuracy of the transfer function. Carotid tonometry requires a higher degree of technical expertise, but a transfer function is not necessary, since the arterial site is very close to the aorta; therefore, when possible, it is preferred to use the direct carotid measurement. Central pressure and the Augmentation Index (AIx) provide additional information concerning wave reflections.
Cardio-ankle vascular index
The cardio-ankle vascular index (CAVI) has recently been developed by measuring PWV and BP. The CAVI is a new stiffness index of the systemic arteries from the origin of the aorta to the ankle and has been described as measuring arterial stiffness independently of BP.Citation12 This index is originated from stiffness parameter β, which is applied to a segment of the elastic artery. The most conspicuous feature of the CAVI is the independence of blood pressure at measuring time. The CAVI reflects a severity of athero- or arteriosclerosis, and also reflects the vascular tone, by which the blood from the heart with a pulsatile flow is transmitted to the peripheral artery with a steady flow.Citation12 The latter is just a Windkessel function. The clinical data on the CAVI have increased over recent years.Citation13 The CAVI is elevated in aging, coronary artery diseases, chronic kidney disease, patients undergoing hemodialysis therapy, hypertension, diabetes mellitus, smoking, and stress. The CAVI decreases with the administration of statins, angiotensin II receptor blocking agents, and calcium channel blockers (CCBs). Furthermore, the relationships between the CAVI and cardiac functions are important. The CAVI is an important predictor of cardiovascular diseases.
Other methods to evaluate arterial stiffness using ambulatory BP monitoring over 24 hours have also been described. One of them is based on the QKD interval, the time delay between the Q wave of the ECG and the diastolic Korotkoff sound.Citation14 Others are using the slope between diastolic and systolic BP to calculate the ambulatory arterial stiffness index,Citation15 or are using specific algorithms derived from the oscillometric BP signal. More data are needed in order to better and more fully evaluate these recent methods.
Arterial stiffness in cardiovascular protection
Stiffness is a fundamental physical property of any material, and the functional and pathophysiological importance of the stiffness of the proximal elastic aorta on central hemodynamics and cardiovascular outcome is now well recognized.
Aortic stiffness may contribute to cardiovascular risk through influences on left ventricular afterload and hypertrophy (via decreased aortic compliance) or by determining central PWV and, thereby, the magnitude and timing of any reflected pressure wave. A number of parameters associated with aortic stiffness have been widely investigated as potential predictors of an individual’s cardiovascular risk. PWV, the central augmentation index, and systemic arterial compliance have all been proposed and have been shown in specific populations to be associated with arterial disease burden and to be predictive of subsequent risk.Citation16
Most clinical application has been in groups already established as being at high cardiovascular (CV) risk on the basis of classical risk factors, eg, those with past history of CV disease, renal impairment, or smoking.Citation16 Recently, consensus evidence has supported PWV as the current gold standard in the clinical assessment of arterial stiffness, partly based on it having been shown to be an independent predictor of CV risk and also related to its ease of application in the clinic, along with the lack of excessive underlying assumptions or confounders (eg, compared to transfer function or model-based approaches).Citation10,Citation17 It is now well known by vascular clinicians and cardiologists that aortic PWV is inversely proportional to aortic distensibility and directly proportional to aortic stiffness (),Citation1 associations manifest in the accepted observation that increased PWV indicates relative stiffening of the aorta and a more deleterious arterial state, usually associated with inappropriate central blood pressure and blood flow to the organs.Citation18
Equation 1 The mathematical relationship between PWV and vessel distensibility and wall stiffness.

Although increased PWV is established as being associated with increased cardiovascular risk,Citation19 the place of assessment of central arterial stiffness in either primary or secondary prevention of CV disease over and above standard, classical risk identification and stratification is not established. A fundamental issue in the use of aortic stiffness, assessed as PWV, as an indicator of vascular health or disease, is the inherent nonlinear pressure dependence of aortic stiffness on blood pressure: in the normal aorta, increased operating blood pressure (best assessed as mean BP) results in an increase in measured PWV, independent of any underlying change in the aortic wall, with a consequent difficulty in separating any underlying CV risk due to a BP effect from the risk related directly to aortic stiffening.Citation20
In relation to cardiovascular protection, aortic stiffness could, in general terms, be useful as:
A predictor of individual future risk: Increased aortic stiffness has been established as an independent predictor of cardiovascular events.Citation19 The European Network for Noninvasive Investigation of Large Arteries has provided a classification of clinical conditions associated with increased arterial stiffness that includes aging, presence of classical cardiovascular risk factors, and underlying CV disease, including inflammatory conditions such as rheumatoid arthritis and systemic lupus.Citation10
A potential therapeutic target: The 2007 guidelines for the management of arterial hypertension report a carotid-femoral PWV of >12 m/sec as a relatively cost-effective biomarker typifying target organ damage and influencing prognosis.Citation21 On this basis, it is reasonable to surmise that direct intervention for lowering PWV would be beneficial.
Providing an insight into the mechanisms of central hemodynamics: In spite of a significant amount of work and literature – indicating the potential clinical significance of the issue, we are left with an important unresolved issue, namely, whether aortic stiffness is merely a marker of a systemic underlying condition (eg, aging, inflammation, or atherosclerosis) or if it is a major key component of a fundamental derangement of the mechanisms determining central blood pressure and blood flow to organs such as the brain, heart, and kidneys.Citation22
The central hemodynamic profile is determined by the interaction of the stroke volume, the forward and reflected pressure-wave components associated with PWV, and the distances to reflecting sites in the distal vasculature.Citation23 Remaining to be determined is the net contribution and effect on CV and other organ damage resulting from the alteration from a “healthy” phenotype of impedance changes along the aorta to an “unhealthy/pathological” impedance mismatch that causes increased transmitted pressure – possibly associated with renal impairment and disease – and disturbance of systolic/diastolic pressure levels in the proximal aorta,Citation24 with increased cerebral and retinal pulse pressure and with increased afterload and decreased coronary perfusion (), all of which are associated with proximal aortic stiffening.
Figure 1 The cyclical interrelation of aortic stiffness, myocardial performance, and functional capacity.
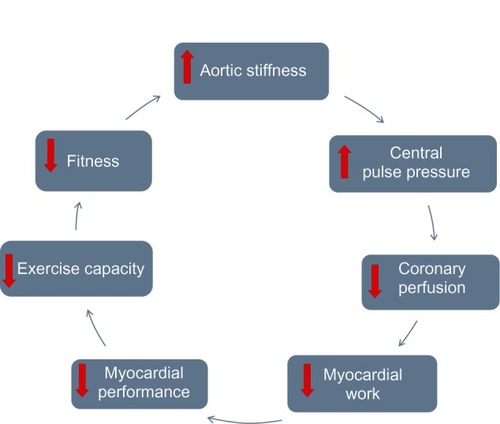
For the above reasons, it is appealing to consider influencing the onset or progression of these age- and disease-associated changes through direct therapeutic action on the aorta; however, to date, there is no specific therapy targeted at aortic stiffness or impedance. There is some evidence that angiotensin-converting enzyme inhibitors (ACEIs) and/or CCBs have an additional benefit for aortic function over and above their effects on blood pressure-lowering effects,Citation25,Citation26 but the only intervention established to have an apparently direct effect through increasing aortic compliance is aerobic exercise.Citation27
Current data have enabled the definition of normal PWV ranges stratified by both BP grade and decade of age,Citation21 and this goes some way toward defining an individual’s state of aortic function and holds the potential to assess response to treatment or to further substratify individual CV risk over and above classical risk calculation. It remains true, however, that given current knowledge, management of cardiovascular risk remains based on traditional assessment using traditional therapies.
A specific benefit of PWV measurement may be related to the possibility of assessing the maintenance of the normal pressure-dependent change in aortic PWV. It may be that those in whom an appropriate decrease in PWV is observed upon instigation of BP management are relatively better off than those who show no pressure-dependent response, even at similar brachial BP reductions.Citation28 While appealing and plausible, this hypothesis requires formal clinical trial-testing to support it. It has also been suggested that assessment of the change in PWV with traditional risk-factor treatment may encourage both the physician and patient to strive for better compliance with available medication.
In summary, to fully judge the potential place of assessment of arterial stiffness in cardiovascular protection would require:
An effective directly acting “de-stiffening” agent
Evidence that therapeutic lowering of aortic stiffness is equivalent in terms of risk to a natively less-stiff aorta
Properly designed clinical trials employing appropriate assessment criteria.
It is therefore appropriate to conclude that aortic stiffness is not presently a treatment target in its own right but may be clinically useful to substratify, particularly in intermediate cardiovascular-risk individuals, and to better target the intensity of conventional therapy. It may also be useful in assessing response to treatment, and, in the future, emerging evidence may link improved aortic function to group effects of specific classes of BP treatments.
Crosstalk between large and small arteries in hypertension
Hypertension is associated with a number of structural and functional changes in the vascular tree. At the level of the large arteries, there is a gradual stiffening and loss of endothelial function. This leads to an augmented pulse pressure. There is now good evidence that these changes already occur in early stages of hypertension and that they are predictors of later risk for cardiovascular morbidity and mortality, independent of blood pressure.Citation9,Citation29
At the level of small arteries, there is also a loss of endothelial function in hypertension, contributing to an augmented vasopressor sensitivity. This enhanced contractility is reinforced by structural changes in small arteries characterized by an increased wall-to-lumen ratio. Taken together, these changes at the small artery level cause an enhanced peripheral resistance and reduced tissue perfusion at the level of different target organs of hypertensive disease, such as the brain, heart, and kidney. The smallest level of the vascular tree is the microcirculation consisting of arterioles, capillaries, and venules. Again, both structural and functional changes take place at this level during the development of hypertension. The most important of these are arteriolar narrowing and rarefaction of the number of arterioles and capillaries.
There are several important forms of crosstalk between the macro- and microcirculation in hypertension.Citation8 The first is the effect of an enhanced pulse pressure on small arteries and – in certain organs – the microcirculation. Recent evidence from both epidemiological and experimental studies shows that an enhanced pulse pressure contributes to target-organ damage at the level of the brain, thus causing vascular dementia; at the level of the eye, thus causing macular degeneration; and at the level of the kidney, thus contributing to albuminuria and other forms of renal damage.Citation23 It remains to be determined whether increased pulse pressure also contributes to damage to the heart in the form of myocardial ischemia.
A second important form of crosstalk between the macro- and microcirculation is the role of wave reflections in causing an enhanced pulse pressure. Although the exact site of wave reflections remains enigmatic, there is now convincing evidence that the wave reflections from sites within the distal arterial tree alter the shape and height of the central arterial pressure wave.Citation24 This phenomenon plays an important role in the altered wave form during aging, but also in conditions like hypertension and diabetes.
The crosstalk between the macro- and microcirculation in hypertension has important implications for the pharmacological treatment of hypertension. Ideally, an antihypertensive regimen should abolish the vicious cycle between the increased resistance in the microcirculation and the increased stiffness of the larger arteries. Such a treatment should be based on drugs with multiple actions on the vascular tree or based on drug combinations that target the various segments of the arterial system.
Arterial biomechanic properties in cardiovascular diseases, and response to treatment
The arterial system is heterogenous in its structure, according to the major components of the media. Arteries nearer the heart are elastic, whereas the smaller peripheral arteries are muscular, with a high number of smooth muscle fibers in their walls. This heterogenous structure of the arterial wall is responsible for the difference in the biomechanics and function of arteries throughout the body, and for the progressive increase in stiffness from the ascending aorta to the peripheral muscular conduit arteries.Citation10,Citation20 Arterial hypertension is associated with a remodeling of arteries. Aortic stiffness is the most prognostically significant type of this remodeling.Citation30
Arteries deliver an adequate blood supply from the heart to peripheral tissues, as dictated by metabolic activity. Atherosclerosis, characterized by the presence of plaques and arterial narrowing, is the most common vascular disease that disturbs the conduit function.
The second role of arteries is their “buffering” function, which allows dampening of pressure oscillations and ensures a steady flow in the peripheral tissues and organs.
The compliant aorta stores this energy during ejection and releases it during diastole so that flow into the peripheral arteries continues throughout the cardiac cycle. Due to the peripheral resistance, only part of the stroke volume is forwarded directly to the peripheral tissues. About 50% of stroke volume is momentarily stored in the aorta and large elastic arteries, thereby stretching the arterial walls and raising BP. Under normal conditions, ~10% of the energy produced by the heart is diverted for the distension of arteries, and is “accumulated” in the vessel walls. During diastole, the accumulated energy recoils the aorta, squeezing the stored blood forward into the peripheral tissues, ensuring a continuous flow.Citation20
In regard to the buffering function, it is essential that the energy necessary for arterial distension and recoil be as low as possible; in other words, for a given stroke volume, the pulse pressure should be as low as possible. The efficiency of this function depends on the elastic properties of the arterial wall. Stiffening of the aorta leads to loss of buffering efficiency and higher pulse pressure for the same stroke volume. In the presence of a rigid aorta, the entire stroke volume will flow through the arterial system and peripheral tissues only during systole.Citation19,Citation20
It is widely recognized that arterial hypertension, dyslipidemia, diabetes mellitus, and smoking may cause advanced vascular aging. Aortic stiffness can be considered a measure, on the arterial tree, of the cumulative influence of CV risk factors for aging. Indeed, arterial stiffness reflects the true arterial wall damage, whereas snapshots of BP, glycemia, or lipids may not. Arterial stiffness integrates the long-lasting effects of all identified and unidentified cardiovascular risk factors.Citation12 Arterial stiffness contributes to central systolic and pulse pressure elevation, which directly damage target organs.Citation23,Citation24
Thus, the measurement of arterial stiffness is a useful tool for risk stratification. It can be noninvasively estimated mainly by three principal methodologies: (1) PWV measurement, (2) analysis of the arterial pressure-wave contour with the evaluation of central systolic and pulse pressures and an augmentation index, and (3) measurement of diameter or arterial luminal cross-sectional area changes together with the distending pressure.Citation1,Citation10
Aortic PWV is a strong predictor of future cardiovascular events and all-cause mortality. Carotid-femoral PWV measurement is recommended by current guidelines on arterial hypertension for target-organ damage evaluation.Citation17 PWV measurement may reclassify about 14.5% of patients into a higher CV risk category (Kotovskaya, unpublished data, 2012). Thus, the measurement of arterial stiffness may avoid patients being mistakenly classified as being at low or moderate risk when they actually have an abnormally high arterial stiffness. PWV is a BP-dependent measure of arterial stiffness whose interpretation may be limited by the action of antihypertensive drugs.
The CAVI is a relatively new estimate of arterial stiffness and atherosclerosis (see below). It is important that the CAVI calculation algorithm include PWV and minimize the BP influence on its value.Citation13 From a practical point of view, measurement of the CAVI is easy and has been applied at the University of Russia in different clinical settings and pharmacological trials. Our results showed significant correlation between the CAVI and age, and that the age gradient of the CAVI was found even in subjects aged over 65 years.Citation31 There is strong and independent positive correlation between the CAVI and cholesterol, fasting glucose, uric acid, and serum creatinine, and a negative correlation with GFR. In middle-aged hypertensives with metabolic syndrome, higher CAVI values were associated with subclinical inflammation, oxidative stress, left ventricular hypertrophy, and diastolic dysfunction. In heart failure, negative correlation between the CAVI and left ventricular ejection fraction was observed.Citation31 The Russian National Cardiology Center evaluated the utility of brachial-ankle PWV and the CAVI obtained >75% by VaSera® (Fukuda Denshi Co. Ltd. Bunkyo-ku, Tokyo, Japan) device for the detection of patients with coronary artery stenosis. ROC curves demonstrated a statistically significant discriminant value for both parameters.
Our group conducted an open label study in very elderly hypertensive patients. Arterial stiffness indices were evaluated using the VaSera device. A 12-week treatment with an indapamide slow-release formulation resulted in well-tolerated systolic and pulse pressure reduction. Hypokalemia or orthostatic hypotension was not observed. Indapamide treatment led to reduction of arterial stiffness, as assessed by PWV. The CAVI changes were not significant, confirming the BP-independent nature of the index as well as the difficult-to-reverse arterial rigidity in the very elderly.
A meta-analysis of individual data in 294 patients confirmed that antihypertensive treatment leads to reduction in arterial stiffness beyond BP lowering. Patients were treated by placebo, ACEIs, CCBs, beta blockers (BBs), or diuretics. Active treatment was independently related to the changes in PWV and explained 5% and 4% of the variance in the short-term and long-term trials, respectively. In the short-term trials, ACEIs were more effective than calcium antagonists and placebo in improving arterial stiffness. In the long-term trials, ACEIs, calcium antagonists, BBs, and diuretics significantly reduced PWV, compared to placebo. An interesting new finding was that changes in PWV during this long-term trial were not dependent on changes in mean BP. The authors concluded that there was a decoupling of PWV and BP, when control of BP was extended for a long period.Citation31
The DAPHNET (Diabetes Artery Perindopril Hypertension Normalization Excess sTiffness)Citation7 trial in hypertensive diabetic patients elegantly demonstrated the efficacy of a high dose of ACEI to improve carotid artery distensibility. Subjects who responded to perindopril 4 mg after a 1-month treatment were randomized, to continue the same or doubled dose of perindopril. After 6 months of follow up, a higher dose of perindopril improved carotid artery distensibility and reduced carotid pulse pressure significantly better than did 4 mg, despite a similar reduction in office and ambulatory blood pressure.Citation7
The Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) study showed beneficial effects of statin therapy on carotid artery stiffness, as assessed by an augmentation index.Citation33 This beneficial effect of statin was observed only in patients receiving perindopril- or amlodipine-based antihypertensive therapy.
Arterial stiffening is a sign of normal and early cardiovascular aging, and is a major factor affecting the biomechanics of large arteries. Arterial stiffness indices can be measured noninvasively in clinical practice and used for risk stratification. Long-term effects of cardiovascular drugs on arterial stiffness need to be further investigated.
From arterial protection to cardiovascular events prevention: therapeutic considerations
The list of clinical conditions associated with increased arterial stiffness includes genetic background, aging, menopausal status, cardiovascular risk factors, established cardiovascular diseases, and other primarily non-CV diseases (for example renal disease and diabetes) that have large artery involvement. In all these clinical conditions, we have different approaches for treatment, and the majority of them involve the so-called vascular approach. There are several markers of arterial stiffness that can be used as additional and independent markers of cardiovascular risk.Citation10,Citation17 Several studies have shown that some can also be used for monitoring disease progression after the initiation of treatment.Citation34,Citation35 The current and future initiatives raise the question, can we use these markers for the evaluation of treatment efficacy? The answer will relate to the eventual results from ongoing clinical trials.
The simplest way to improve large arterial stiffness is to lower BP. This leads to decreasing arterial wall tension and modifying both PWV and central aortic BP. Considering blood-pressure lowering as the goal of “vascular” treatment may also involve modification of arterial stiffness independently of BP, through a change in vasomotor tone, a change in arterial structure, or a combination of both.Citation25,Citation36 An active decrease in arterial stiffness may be obtained independently of the decrease in BP, with drugs specifically relaxing the smooth muscles in the wall of the large arteries. Another possible mechanism is the slowing and reduction of pulse-wave reflections through vasodilatation and decreased PWV. Accumulation of aortic collagen may be reduced by blockade of the renin-angiotensin system in association with diuretics and vasopeptidase inhibitors may also have this effect. Additional mechanisms do not directly affect aortic stiffness but can move pressure wave reflection points to a more distal part of the vascular bed. This change in reflection site may affect the augmentation index and central BP. It has been proven that lowering brachial BP reduces aortic BP, but not always similarly. The next important question is whether can we differentially affect aortic vs brachial BP with different treatment strategies. However, it has not been convincingly proven that we can affect aortic stiffness beyond having an impact on BP. And there is no positive answer for the most important question: can these interventions decrease cardiovascular morbidity and mortality? The data from available studies will be invaluable in establishing whether central pressure estimation is useful in routine clinical practice. However, the real paradigm shift will come only if and when studies demonstrate that selective reduction in central pressure reduces cardiovascular events.
outlines modern approaches that can improve large artery stiffness parameters. Lifestyle changes may also decrease arterial stiffness. In some selected studies, the degree of lifestyle change was comparable to that reported in drugs studies. Current evidence indicates that antihypertensive drugs affect PWV and aortic BP differently. It is evident that differences detected between classes of antihypertensive drug can be explained by their effects on large artery stiffness parameters due to their different modes of action, shifting wave reflections, and effects on arterial wall remodeling. The recently published meta-analysis by Ong et alCitation32 showed that in short-term studies (with a duration of over 4 weeks), only ACEIs reduced the PWV beyond the BP effect, whereas in studies of 4 weeks or more, all the drug classes studied were effective. Vasodilation may at least in part account for the effect beyond BP reduction by vasoactive drugs such as ACEIs, CCBs, and BBs with vasodilating properties, as well as angiotensin receptor blockers.
Table 1 Summary of potential treatment strategies relating to arterial stiffness
The combination of an ACEI and a CCB has a synergistic effect on BP lowering, with a favorable effect on arterial function and structure. The ASCOT demonstrated superiority in cardiovascular outcomes over primary treatment of hypertension with CCBs (CCB + amlodipine), in comparison to BBs (atenolol) and thiazide. The results of the Conduit Artery Function Evaluation (CAFE) substudy of ASCOT provided one potential mechanism for these outcome differences, because central aortic pressure was substantially lower in the CCB compared to the BB treatment arm, despite only small differences in brachial BP. The difference between brachial and central aortic systolic BP and between treatment arms was greater at higher levels of brachial systolic BP. The superiority of ACEI and CCB combination over the BB and thiazide effect was more pronounced in patients with more severe hypertension.Citation37
New data demonstrated that BBs are not a homogeneous class, and that vasodilating BBs, such as celiprolol, carvedilol, and nebivolol, appear not to share some of the negative properties described for older compounds, especially atenolol. One randomized, double-blind study compared the effects of nebivolol and the beta-1 selective agent, metoprolol, on several hemodynamic parameters.Citation38 Both drugs reduced heart rate and brachial BP to the same extent, but there was a fall in brachial pulse pressure and central BP only in the nebivolol group.
A comprehensive review on the influence of antihypertensive drugs on large arteries was published in two parts by Protogerou et al,Citation39,Citation40 and highlighted the following:
There are important differences between the classes of antihypertensive drugs regarding their effects on markers of large arterial stiffness. These differences are based on the differential effects of drugs on arterial wall properties and the autonomic nervous system.
The newer antihypertensive drugs (ACEIs, angiotensin receptor blockers, and dihydropyridine CCBs) have a more beneficial effect on brachial-central blood pressure amplification than have the older drugs (diuretics and old nonselective β-blockers). The common features of these newer classes of drugs appear to be their arterial dilating capacity and their ability to reduce pressure-wave reflections, as expressed by the wave augmentation parameters.
There is convincing evidence regarding the negative effect of old BBs (mainly atenolol) on central BP. This is largely attributable to the lowering of heart rate, which leads to augmentation of aortic systolic BP, primarily due to the earlier timing of the reflected wave. The newer BBs with vasodilating properties, such as nebivolol, probably do not manifest these effects.
Interesting clinical results were obtained in trials with combinations of renin-angiotensin system blockers and CCBs (ASCOT, EXPLOR,Citation41 etc).
Use of the combination of antihypertensives with other cardiovascular drugs has revealed interesting results. Post hoc analysis of CAFE results showed that adding atorvastatin to amlodipine/perindopril combination led to a significant decrease in primary end-point development. In the atenolol/thiazide arm, the addition of atorvastatin did not change the results.Citation33 In this, a synergistic drug effect has been suggested.
Several other mechanisms of large artery stiffness exist and are independent of BP reduction, including the reduction of oxidative stress and inflammation, which lead to direct beneficial effects on the inflammation, endothelium, smooth muscle cells, collagen/elastin ratio, and extracellular matrix composition.
Conclusion
Future considerations for the development of a clinical understanding of large artery stiffness should include new evaluation methods and new drugs that focus on vascular health, understanding of early vascular changes, and detection for cardiovascular prevention. Newly designed studies for treatment evaluation are important, as well as new studies of drug combinations.
Acknowledgments
These proceedings from the 2011 Joint Session of the International Society of Vascular Health (East Region) and Ukrainian Society of Cardiology meeting are published on behalf of the Kiev writing group. As president of the International Society of Vascular Health I, James Cameron thanks the organizers of the Congress, especially Professor Sirenko, and all participants. We wish to acknowledge David Oldfield for his help in text editing and Catherine Dognon for her invaluable work as meeting coordinator.
Disclosure
The authors report no conflicts of interest in this work.
References
- CameronJDEstimation of arterial mechanics in clinical practice and as a research techniqueClin Exp Pharmacol and Physiol19992628529410225138
- DuprezDAJacobsDRJrLutseyPLAssociation of small artery elasticity with incident cardiovascular disease in older adults: The multi-ethnic study of atherosclerosisAm J Epidemiol201117452853621709134
- PeraltaCAAdeneyKLShlipackMGStructural and functional vascular alterations and incident hypertension in normotensive adults – the multi-ethnic study of atherosclerosisAm J Epidemiol2010171637119951938
- RandallOSWesterhofNvan den BosGCAlexanderBReliability of stroke volume to pulse pressure ratio for estimating anddetecting changes in arterial complianceJ Hypertens19864SupplS293S296
- ChemlaDHébertJLCoiraultCTotal arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humansAm J Physiol1998274H500H5059486253
- LindLAndrénBSundströmJThe stroke volume/pulse pressure ratio predicts coronary heart disease mortality in a population of elderly menJ Hypertens20042289990515097228
- TropeanoAIBoutouyriePPannierBBrachial Pressure-Independent Reduction in Carotid Stiffness After Long-Term Angiotensin-Converting Enzyme Inhibition in Diabetic HypertensivesHypertension200648808616702490
- SafarMEStruijker-BoudierHACross-talk between macro and microcirculation in hypertensionActa Physiol (Oxf)201019841743020050837
- SafarMEBlacherJJankowskiPArterial stiffness, pulse pressure, and cardiovascular disease – is it possible to break the vicious circle?Atherosclerosis201121826327121621778
- LaurentSCockcroftJVan BortelLEuropean Network for Non-invasive Investigation of Large ArteriesExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J2006272588260517000623
- AvolioAPButlinMWalshAArterial blood pressure measurement and pulse wave analysis – their role in enhancing cardiovascular assessmentPhysiol Meas2010311R1R4719940350
- ShiraiKHirutaNSongMCardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectivesJ Atheroscler Thromb2011181192493821628839
- ShiraiKSongMSuzukiJContradictory effects of β1- and α1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI) – the independency of CAVI from blood pressureJ Atheroscler Thromb201118495521071883
- CremerAButlinMCodjoLDetermination of central blood pressure by a noninvasive method (brachial BP and QKD interval)J Hypertens20123081533153922688261
- AznaouridisKVlachopoulosCProtogerouAStefanadisCAmbulatory systolic-diastolic pressure regression index as a predictor of clinical events: a meta-analysis of longitudinal studiesStroke201243373373922282885
- ZoungasSAsmarRPArterial stiffness and cardiovascular outcomeClin Exp Pharmacol Physiol200734764765117581224
- ManciaGDe BackerGDominiczakA2007 guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)Eur Heart J2007281462153617562668
- LeungMCMeredithITCameronJDAortic stiffness affects the coronary blood flow response to percutaneous coronary interventionAm J Physiol Heart Circ Physiol20062902H624H63016143654
- BoutouyriePTropeanoAIAsmarRAortic Stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal studyHypertension2002391101511799071
- NicholsWO’RourkeMVlachopoulosCMcDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles6th edLondonHodder Arnold2011
- The Reference Values for Arterial Stiffness CollaborationDeterminants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”Eur Heart J2010312338135020530030
- LiangY-LShielLMTeedeHEffects of blood pressure, smoking and their interaction on carotid artery structure and functionHypertension200137161111208749
- MitchellGFEffects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damageJ Appl Physiol200810551652166018772322
- VasanRSPathogenesis of elevated peripheral pulse pressure: some reflections and thinking forwardHypertension2008511333618071060
- MorganTLauriJBertramDAndersonAEffect of different anti-hypertensive drug classes on central aortic pressureAm J Hypertens20041711812314751652
- DearyAJSchumannALMurfetHHaydockSFooRSBrownMJInfluence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertensionClin Sci (Lond)2002103549349912401122
- CameronJDDartAMExercise training increases total systemic arterial compliance in humansAm J Physiol19942662 Pt 2H693H7018141370
- GuerinAPBlacherJPannierBMarchaisSJSafarMELondonGMImpact of aortic stiffness attenuation on survival of patients in end-stage renal failureCirculation2001103798799211181474
- MuiesanMLSalvettiMRizzoniDPulsatile hemodynamics and microcirculation. Evidence for a close relationship in hypertensive patientsHypertension20136113013623150518
- NilssonPMBoutouyriePLaurentSVascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and preventionHypertension20095431019487587
- KotovskayaYKobalavaZVialovSVialovIPeripheral arteries properties in hypertensive elderly patientsAm J Hypertens2005185 Pt 216A
- OngKDelermeSPannierBAortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patientsJ Hyperten20112910341042
- WilliamsBLacyPSCruickshankJKImpact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation–Lipid-Lowering Arm (CAFE-LLA) StudyCirculation2009119536119103995
- AsmarREffect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implicationsAm J Cardiovasc Drugs2001138739714728020
- RomanMJDevereuxRBKizerJRCentral pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart StudyHypertension20075019720317485598
- AsmarRGLondonGMO’RourkeMESafarMEREASON Project Coordinators and InvestigatorsImprovement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenololHypertension20013892292611641310
- WilliamsBLacyPSThomSMCAFE InvestigatorsAnglo-Scandinavian Cardiac Outcomes Trial InvestigatorsCAFE Steering Committee and Writing CommitteeDifferential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) studyCirculation20061131213122516476843
- KampusPSergMKalsJDifferential effects of nebivolol and metoprolol on central aortic pressure and left wall thicknessHypertension20115761122112821536983
- ProtogerouADPapaioannouTGBlacherJPapamichaelCMLekakisJPSafarMECentral blood pressures: do we need them in the management of cardiovascular disease? Is it a feasible therapeutic target?J Hypertens20072526527217211229
- ProtogerouADEffect of antihypertensive drugs on central blood pressure over and above brachial blood pressure: focusing on blood pressure amplificationMedicographia201032254261
- BoutouyriePAchoubaATrunetSLaurentSEXPLOR Trialist GroupAmlodipine-valsartan combination decrease central systolic blood pressure more effectively than amlodipine-atenolol combination: the EXPLOR studyHypertension20105561314132220404219