Abstract
Purpose
The performance of Omron HEM-1026 (HCR-1901T2 / HCR-1902T2) for monitoring blood pressure (BP) in the upper arm was validated in accordance with the International Organization for Standardization (ISO) 81060-2:2018+amendment (Amd)1:2020 protocol.
Methods
The device was assessed in 101 participants who fulfilled the inclusion criteria, including arm circumference range and systolic and diastolic BP provided by the protocol. Data validation and analysis were performed according to the manufacturer’s instructions.
Results
In the ISO 81060-2:2018+Amd 1:2020 validation procedure (criterion 1), the mean ± standard deviation (SD) of the differences between the test device and reference BP was −2.1 ± 7.24/-0.6 ± 5.63 mmHg (systolic/diastolic). These data fulfilled the ISO81060-2:2018+Amd1:2020 requirement of ≤5±≤8 mmHg. The mean differences between the two observers and Omron HEM-1026 were −2.1 ± 5.71 mmHg for systolic BP and −0.6 ± 4.81 mmHg for diastolic BP, fulfilling criterion 2 with an SD of ≤ 6.62 for systolic BP and ≤ 6.91 for diastolic BP. The two ISO criteria were fulfilled.
Conclusion
The Omron HEM-1026 BP monitor fulfilled the requirements of the ISO 81060-2:2018+Amd 1:2020 validation standard and can be recommended for home BP measurements in the general population.
Introduction
A blood pressure (BP) monitor used at home should make it as easy as possible to measure BP without paying attention to it. It may not be easy for the general population to wrap the cuff properly around the arm, the direction and position of the cuff, and the extent of the wrapping influence the BP readings.Citation1 HEM-1026 can easily measure BP by inserting the arm into a cylinder that encapsulates the cuff of the device, and the positioning of the arm and wrapping of the cuff are arranged all by itself. This device is recommended for home use in the general population at home.
The most important feature of BP monitoring is its accuracy. Therefore, scientific data is required for device validation.Citation2 Therefore, international protocolsCitation3–6 for the validation of BP measuring devices have been proposed. Accordingly, clinical guidelines for the diagnosis and treatment of hypertension in many countries recommend the use of validated devices according to international protocols.Citation7,Citation8 In this study, the performance of Omron HEM-1026 was validated according to the latest protocol (ISO 81060–2:2018+Amd 1:2020).
Materials and Methods
Test Device
Omron HEM-1026 (HCR-1901T2 / HCR-1902T2) (Omron Healthcare Co. Ltd. Kyoto, Japan) () is an automatic oscillometric device for blood pressure (BP) measurements in the upper arm, with a pressure range of 0–299 mmHg and a heart rate range of 40–180 beats/min. The systolic BP (SBP), diastolic BP (DBP), and pulse rate were measured using a liquid crystal digital (LCD) monitor. The entire weight of the device was approximately 1700 g (without accessories or options), and its dimensions were approximately 179 mm × 211 mm × 246 mm (width × height × depth). The cuff can be used for arm circumference in the range of 17.0–32.0 cm. Omron HEM-1026 (HCR-1901T2 / HCR-1902T2) detects and displays the position mark, irregular heartbeat, and body movement during the BP measurement, and measures BP during deflation of the cuff. This device is a user-friendly BP monitor that can easily measure BP by inserting the arm into a molded plastic cylinder without paying attention to the position of the arm and wrapping of the cuff. In addition, the measured BP and pulse rate data were easily and correctly transferred to a smartphone and could be processed into tables and graphs; however, these data are not presented herein.
Blood Pressure Measurements
The manufacturer provided standard production device models. The validation team consisted of three observers with experience in BP measurement. They were trained using the British and Irish Hypertension Society’s online program.Citation9 BP was measured following the Korotkoff method, using a mercury sphygmomanometer (ACOMA Medical Industry Co., Ltd., Tokyo, Japan) and the tested device. Simultaneous auscultation was performed by two observers using a double stethoscope (Y-tube) while performing measurements following the Korotkoff method. The two observers were blinded to each other’s readings and a third observer served as a supervisor to verify their BP readings of the two observers.
Participant Selection
All individuals participated in the study voluntarily and all procedures complied with the requirements of ISO 14155:2011. This study was performed in accordance with the ethical standards of Declaration of Helsinki and its amendments and was approved by the Institutional Review Boards of Biwako Yoikuin Hospital and Omron Healthcare Co., Ltd., and written informed consent was obtained from each participant. The study was performed in the measurement room of Omron Healthcare Co., Ltd. by the staff employed by Omron Healthcare Co., Ltd. (Kyoto, Japan).
Based on the criteria, 101 participants were included in the study. In accordance with the guidelines, participants were screened to ensure that their sex, age, arm circumference, and BP readings fulfilled the participation requirements described in the protocols. Participants with arrhythmias, those who moved their arms or bodies during BP measurements, and those with unclear DBP readings during Phase V of Korotkoff sounds were excluded from the study. The device was calibrated with the mercury sphygmomanometer before the entry of the study.
Procedure
The participants were seated in a quiet room at a comfortable temperature and instructed to maintain silence during the procedure. BP measurements were performed after a 5-minute rest period. Participants sat on a chair with their legs uncrossed and feet flat on the floor. The chair had a supportive back as well as elbow and forearm rests. The arm circumference of each participant was measured and the cuff size was adjusted for auscultation. All BP measurements were performed on the left arm of the participants at the height of left ventricle of the heart. The devices were validated using the same arm and sequential method specified in the ISO. In detail, the validation team for the studies of the two devices consisted of three observers who were experienced in measuring BP measurement. The control data were defined as the measurements obtained with a standard mercury sphygmomanometer by the observers; ie, two of the three observers measured BP using a teaching stethoscope with two Y-tube headsets for simultaneous measurements with one standard mercury sphygmomanometer. The BP measurement components were carefully checked before the study; the third observer was the supervisor, who checked that the BP values obtained by the two observers agreed. The two observers performing the BP measurements were blinded to each other’s readings. The patient underwent nine sequential BP measurements with 1 minute interval as follows:
| (1) | Entry BP A with a mercury sphygmomanometer by observers 1 and 2 (used to separate the patients into appropriate BP range groups). | ||||
| (2) | Entry BP with test device. | ||||
| (3) | BP1 by observers 1 and 2 with a mercury sphygmomanometer. | ||||
| (4) | BP2 by the supervisor with the test device. | ||||
| (5) | BP3 by observers 1 and 2 with a mercury sphygmomanometer. | ||||
| (6) | BP4 by the supervisor with the test device. | ||||
| (7) | BP5 by observers 1 and 2 with a mercury sphygmomanometer. | ||||
| (8) | BP6 by the supervisor with the test device. | ||||
| (9) | BP7 by observers 1 and 2 with a mercury sphygmomanometer. | ||||
Analysis
Data analysis was performed according to the ISO requirements. Data are expressed as mean ± standard deviation (SD), and the minimum and maximum values with ranges were calculated. The mean of each measurement pair before and after the BP measurement with the test device was used as a reference value. The difference between the mean observer and test values was calculated according to the protocols and presented in Bland-Altman plots against the mean between the two values.
Results
Based on the ISO protocol, 101 participants were screened to determine their eligibility to participate in the experiment. After excluding 9 subjects according to the criteria specified in the subject selection (), 92 individuals (43 men, 46.7%; 49 women, 53.3%) fulfilled the inclusion criteria. The participants’ mean age was 63.2 ± 10.6 years (range, 39–81 years). The mean arm circumference was 25.1 ± 4.5 cm (range, 17.0–32.0 cm). Approximately 27.2% (criteria: ≥ 20%), 20.7% (≥ 20%), 20.7% (≥ 20%), 31.4% (≥ 20%), 10.9% (≥ 10%) and 16.3% (≥ 10%) of the participants had arm circumference measurements of 17.0–20.7 cm (first quarter), 20.8–24.5 cm (second quarter), 24.6–28.2 cm (third quarter), 28.3–32.0 cm (fourth quarter), 17.0–18.8 cm (lowest octile), and 30.2–32.0 cm (highest octile), respectively ().
Table 1 Screening and Recruitment Details
Table 2 Baseline Characteristics of the Subjects
Using the standard mercury sphygmomanometer, the mean value of the 276 measurements were 126.0±24.4 (range, 77.8–202.5) for SBP and 77.0±13.9 (range, 51.5–118.3) mmHg for DBP.
The percentages of patients with high SBP (≥160 mmHg), medium SBP (≥140 mmHg), and low SBP (≤100 mmHg) were 7.6% (criteria ≥5%), 30.4% (≥20%), and 15.6% (≥5%), respectively. The percentages of high (≥100 mmHg), medium (≥85 mmHg), and low (≤60 mmHg) DBPs were 5.1% (criteria ≥5%), 30.1% (≥20%), and 14.1% (≥5%), respectively ().
Table 3 Blood Pressure Distribution
The differences between the measurements of the two observers were −0.3±1.1 mmHg and −0.1±1.2 mmHg for SBP and DBP, respectively. The mean differences between observer measurements and Omron HEM-1026 readings in 276 pairs were −2.1±7.24 mmHg (range, −31.0 to 19.5 mmHg) for SBP and −0.6±5.63 mmHg (range, −16.0 to 13.5 mmHg) for DBP according to criterion 1 (). These data fulfilled the ISO81060-2: 2018+Amd1:2020 requirements of ≤5±≤8 mmHg. The mean differences in each 3 sets of measurements in 92 participants between the observer measurements and Omron HEM-1026 readings were −2.1±5.71 mmHg (range, −23.3 to 9.8 mmHg) for SBP and −0.6±4.81 mmHg (range, −13.7 to 10.1 mmHg) for DBP according to criterion 2. Thereby, the SD values were calculated as less than 6.62 mmHg for SBP and 6.91 mmHg for DBP based on criterion 2 (). These results are in accordance with the ISO81060-2:2018+Amd1:2020 requirements for criterion 2. Therefore, Omron HEM-1026 fulfills the validation criteria outlined in the ISO81060-2:2018+Amd1:2020 requirements.
Table 4 Validation Results
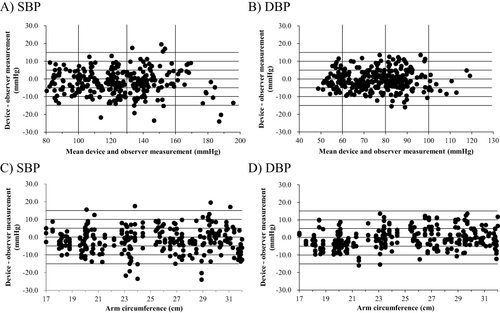
and shows the mean differences between the HEM-1026 SBP and DBP readings and mercury sphygmomanometer SBP and DBP readings. and shows the relationships between arm circumferences and the differences between the Omron HEM-1026 readings and the observer measurements.
Figure 2 The upper panels depict the Bland-Altman plots of the differences between the Omron HEM-1026 (HCR-1901T2/HCR-1902T2) readings and the observer measurements for (A) systolic blood pressure (SBP) and (B) diastolic blood pressure (DBP). The lower panels are scattergrams showing the relationship between arm circumference and differences between Omron HEM-1026 (HCR-1901T2/HCR-1902T2) readings and observer measurements for (C) systolic blood pressure (SBP) and (D) diastolic blood pressure (DBP).
Discussion
A built-in cuff was used in our study, and SBP and DBP were correctly measured within the arm circumference range of 17–32 cm. Because BP was measured using the same arm, the alternative method and time lag of the measurement inevitably caused errors. Based on the understanding of this error in the validation method, the ISO approved a wide range of allowances for the differences between the standard and device measurements of BP as decision criteria. Calculation of the mean data and standard deviation from repeated BP measurements provided sufficient evidence for the accuracy of BP determination. Therefore, the present findings with the HEM-1026 indicate that the device can measure BP accurately according to the ISO 81060–2:2018+Amd1:2020 standard.Citation4,Citation5
Limitations
As the measured BP may be influenced by the activities before measurement, patient’s characteristics, measurement environment, measurement procedure, and device settings, studies including these populations and conditions need to be further researched. In addition, devices deteriorate when used for years. Hence, post-market surveillance of performances of the same device should also be conducted. Finally, this study was performed with employees of the Omron Health Care Co. Ltd. Although it was done fairly and independently from the company with supervision from Dr. H. Takahashi, it is desirable that it be repeated with uninterested parties.
Conclusion
The results of this study showed that Omron HEM-1026 (HCR-1901T2 / HCR-1902T2) meets the requirements of the ISO 81060–2:2018+Amd 1:2020 protocol and can measure BP accurately in the general population with arm circumference of 17–32 cm.
Disclosure
Shingo Yamashita and Nobuki Yakura are employees of the Omron Health Care Co. Ltd., the manufacture of the tested device. The authors report no other conflicts of interest in this work.
Acknowledgment
The authors would like to thank the participants that volunteered their time to participate. We are also grateful to Sayaka Umeki, Kayoko Katsuyama, Manami Taniguchi and Keiko Fujita, research assistants, for the study to measure BP with sphygmomanometer. Many thanks to Omron Health Care Co. Ltd. for the provision of the standard BP monitors used in this study.
References
- Li J, Frick G, Herberigs K, et al. Industry perspectives on the global use of validated blood pressure measuring devices. J Hum Hypertens. 2023;37(2):130–133. doi:10.1038/s41371-022-00717-6
- Bilo G, Sala O, Perego C, et al. Impact of cuff positioning on blood pressure measurement accuracy: may a specially designed cuff make a difference? Hypertens Res. 2017;40(6):573–580. doi:10.1038/hr.2016.184
- Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36(3):472–478. doi:10.1097/HJH.0000000000001634
- Stergiou GS, Palatini P, Asmar R, et al. Recommendations and practical guidance for performing and reporting validation studies according to the universal standard for the validation of blood pressure measuring devices by the association for the advancement of medical instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019;37(3):459–466. doi:10.1097/HJH.0000000000002039
- The International Organization for Standardization (ISO). INTERNATIONAL STANDARD. Non‐Invasive sphygmomanometers – Part 2: clinical investigation of automated measurement type. ISO 81060–3rd ed. 2018.11. Reference number ISO81060-2:2018(E), Available from: https://iso.org/standard/73339.html. Accessed February 21, 2020.
- The International Organization for Standardization (ISO). INTERNATIONAL STANDARD. Non‐Invasive sphygmomanometers – Part 2: clinical investigation of automated measurement type. ISO 81060–Amendment 1 2020.01. Reference number ISO81060-2:2018/Amd.1.2020(E). Available from: https://iso.org/standard/75432.html. Accessed February 21, 2020.
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874–2071. doi:10.1097/HJH.0000000000003480
- Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–1481. doi:10.1038/s41440-019-0284-9
- BP Measurement Auscultatory Tutorials. British and Irish Hypertension Society. Available from: https://bihsoc.org/resources/bp-measurement/bp-measurement-auscultatory-tutorials/. Accessed May 18, 2022.

