Abstract
Background
The safety and efficacy of olmesartan 40 mg and hydrochlorothiazide (HCTZ) as a fixed-dose combination has been investigated in clinical trials leading to its approval. The aims of the present study were to confirm these data in an unselected patient population in daily practice and to determine the impact of physical activity on blood pressure control.
Methods
In a multicenter, noninterventional study, 3,333 patients with either insufficient blood pressure control on olmesartan 40 mg alone or on a fixed/free combination of olmesartan 40 mg and HCTZ 12.5/25 mg were primarily assessed for safety and tolerability of the fixed-dose combination of olmesartan 40 mg and HCTZ 12.5/25 mg at 24 ± 2 weeks. Secondary objectives were blood pressure reduction, treatment compliance, and impact of physical activity as measured by the sum of weekly energy costs.
Results
The mean patient age was 63.2 ± 11.46 years, mean baseline blood pressure was 159.6 ± 15.28/93.5 ± 9.52 mmHg, and 70.9% had at least one additional cardiovascular risk factor. Adverse drug reactions were rare (n = 19), and no serious adverse drug reactions occurred. Compliance with drug therapy was at least sufficient in more than 99% of patients at the end of the study. Blood pressure at the last available visit was reduced by 26.1 ± 15.5/13.0 ± 10.1 mmHg versus baseline (P < 0.0001), but had reduced effectiveness in patients ≥75 years with diabetes or impaired renal function. In 69% of patients, blood pressure was normalized (<140/90 mmHg). No noteworthy differences in baseline characteristics or baseline blood pressure were found between patients with an activity level (sum of weekly energy costs) above or below the median of 9,460.6. A higher versus lower physical activity score had no impact on blood pressure reduction.
Conclusion
Our data confirm randomized trial data concerning safe and efficient blood pressure reduction using a fixed-dose combination of olmesartan 40 mg and HCTZ 12.5/25 mg in a large, unselected patient population, independent of physical activity level.
Introduction
The majority of patients with hypertension require combination therapy to achieve therapeutic blood pressure (BP) targets and a reduction in cardiovascular risk.Citation1 For this purpose, there is a strong preference for angiotensin-converting enzyme inhibitor/angiotensin receptor blocker-based strategies combined with either calcium channel blockers and/or thiazide-type diuretics.Citation2–Citation4 The choice of any particular combination usually relies on criteria that include compelling indications or contraindications, coexisting conditions, adverse effects, race, and the clinician experience.Citation5
Two-drug combinations of renin-angiotensin blocking agents with diuretics are usually preferred in patients with heart failure, in black patients, and in those with chronic renal failure.Citation1 The combination of olmesartan and hydrochlorothiazide (HCTZ) in particular has been investigated in a number of clinical trials.Citation6–Citation11 These demonstrated that, at mean baseline BP values of 154–167 mmHg systolic and 88–103 mmHg diastolic, a combination of olmesartan 40 mg and HCTZ led to office BP reductions of 22–30 mmHg systolic and 10–22 mmHg diastolic. Because fixed-dose combinations are not only efficacious but also improve patient compliance with treatment, their use is strongly encouraged.Citation12
The present study was designed to supplement existing clinical trial data with experience from an unselected patient cohort under normal conditions in daily practice. The positive effect of physical exercise on cardiovascular risk reduction is undoubted, but only limited data are available investigating BP reduction according to the level of regular physical exercise undertaken. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and calcium channel blockers are the treatments of choice in physically active patients.Citation13 The BP-lowering effects of exercise were found to be more pronounced in hypertensive people who engage in endurance exercise.Citation14 Therefore, as an exploratory endpoint, we used our large real-life patient cohort to determine whether physical activity would have an impact on BP control in these patients.
Materials and methods
Design
This multicenter, open-label, observational, noninterventional study was performed at 606 centers (including general practitioners, internists, and cardiologists) in Germany and Austria. Ethical approval was obtained prior to commencement of the study from the international ethics committees in Freiburg, Germany, and Vienna, Austria. Written informed consent was obtained from all patients prior to enrollment.
Patient population and schedule
Patients with essential hypertension and insufficient BP control with olmesartan 40 mg either alone or in fixed combination with 12.5 mg HCTZ and those on a free combination of olmesartan 40 mg and HCTZ were considered for inclusion. Patients with contraindications as outlined in the summary of product characteristics (ie, hypersensitivity to one of the components or to sulfonamide derivatives, impaired renal function, resistant hypokalemia, hypercalcemia, and hyponatremia, symptomatic hyperuricemia, moderate to severe liver impairment, pregnancy in the second or third trimester) were defined as protocol violators. As the number of protocol violators did not exceed 20%, they were not excluded from the efficacy analysis. Follow-up was at 24 ± 2 weeks with optional interim visits after 8 ± 2 and 16 ± 2 weeks.
Objectives
The principal objective of the present study was to provide safety and tolerability data for the fixed combination of olmesartan 40 mg and HCTZ 12.5 mg and olmesartan 40 mg and HCTZ 25 mg in an unselected patient cohort in daily practice. Adverse drug reactions were graded according to their severity (mild, moderate, severe) and duration (start and end dates, or if continuing at final examination), and whether the reaction constituted a serious adverse event. This was defined as an event which was fatal or life-threatening, resulted in persistent or significant disability/incapacity, constituted a congenital anomaly/birth defect, required inpatient hospitalization or prolongation of existing hospitalization, or was medically significant.
The following parameters were used for BP-related endpoint evaluation: BP target achievement (<140/<90 mmHg); BP response (<140/<90 mmHg, systolic BP reduction ≥20 mmHg, or diastolic BP reduction ≥10 mmHg); proportion of patients in the different hypertension grades; and average change in systolic and diastolic BP from baseline to end of follow-up.
BP measurement
Sitting office BP was recorded at each visit using a calibrated standard sphygmomanometer and appropriately sized cuff. All subsequent readings were done after the patient had been resting for at least 5 minutes in a sitting position with the arm supported at the level of the heart. The investigator was advised to report the mean of three single measurements.
Physical activity
Physical activity was evaluated by inquiring about the number of times per week and duration that the patient spent performing typical physical activities. A sum of weekly energy costs (SWECs) was calculated for each patient, using the physical activity ratio (PAR) suggested and defined by James and Schofield.Citation15 PAR is “the actual energy cost of an activity per minute divided by the energy cost of a basal metabolic rate per minute.” The weekly energy cost (WEC) is calculated based on the time that is spent for the activity within one week. In a last step, the SWEC is calculated by summing up the WECs of all asked for activities from the questionnaire.Citation15
Statistical analyses
Data were recorded on a paper case report form and entered into a Microsofit Access 2003 database. A subset (30%) of physicians was randomly selected to complete a faxed questionnaire containing selected patient variables. These were compared with the case report forms. In the event of discrepancies, physicians were approached for clarification.
Analyses were conducted by the Department for Biostatistics and Data Operations at Daiichi Sankyo Europe (Munich, Germany) and a subcontracted CRO (effect GmbH, Deutsch-Evern, Germany) using SAS version 9.2 (SAS Institute, Cary, NC, USA). The safety set included all patients who took at least one dose of study medication. The full analysis set represented the safety set with valid non-missing data for systolic and diastolic BP at baseline and at least one available post baseline visit. The full analysis set was used for efficacy analysis.
Qualitative parameters were summarized using absolute and percentage numbers within the various categories. Quantitative parameters were summarized using standard statistics ie, amount of non-missing and missing data, mean, standard deviation, minimum, lower quartile, median, upper quartile, and maximum. For changes in systolic and diastolic BP over time, P-values are presented based on a paired t-test. For the comparison of different subgroups according to gender or age, the P-values presented are based on a t-test for two independent samples. Because no confirmatory analysis was performed, all P-values presented have to be interpreted in a purely descriptive way.
Results
Between June 2010 and October 2011, a total of 3,333 patients were included, comprising 3,063 in Germany and 270 in Austria. The safety set (at least one dose of study medication) comprised 3,318 patients and the full analysis set (valid BP measurement at baseline and at least one follow-up visit) included 3,303 patients.
Patient characteristics
The mean age of the safety set was 63.2 ± 11.5 years, 48.6% were women, and mean body mass index was 29.1 ± 4.82 kg/m2 (). The majority of participants (90.1%) had already been treated with antihypertensive drugs before study entry. A lack of BP control on prior treatment was the principal reason for switching treatment to the fixed-dose combination (80.0%).
Table 1 Patient baseline characteristicsTable Footnote*
At least one cardiovascular risk factor was documented for 70.9% of the patients. The most frequent ones were diabetes (28.9%), metabolic syndrome (23.8%), and smoking (21.0%). In total, 85.7% of the baseline population received at least one non-antihypertensive comedication, including lipid-lowering drugs (57.2%) and acetylsalicylic acid (37.1%).
Safety and tolerability
According to the physicians’ assessment, tolerability was deemed to be “very good” in 73.2% and “good” in 25.4%, with no relevant differences between HCTZ doses at the last available visit. Sixteen patients (0.48%) experienced at least one drug-related adverse drug reaction (). Twelve patients discontinued treatment due to drug-related adverse drug reactions (0.36%), the most frequent of which were skin and subcutaneous tissue disorders (three cases of pruritus, one adverse skin reaction). Dizziness was reported three times, hypotension twice, and renal complications once. No peripheral edema, cough, or flushing occurred. No serious suspected adverse drug reactions were recorded. No medically relevant pathologic laboratory values were noted during the observation period, in particular no increase in serum potassium or creatinine concentrations.
Table 2 Overview of adverse drug reactions or serious adverse drug reactions based on the safety set
BP reduction and target achievement
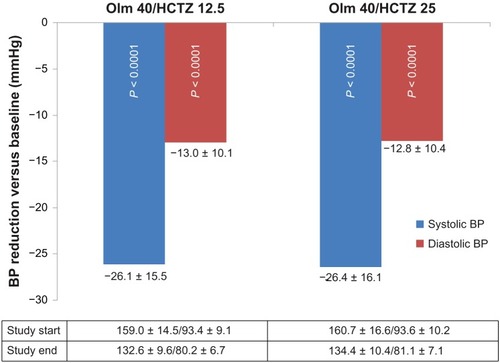
Patients who had previously received olmesartan 40 mg alone or a free combination of olmesartan and HCTZ reduced their BP with olmesartan 40 mg and HCTZ 12.5 mg by 26.1 ± 15.49/13.0 ± 10.09 mmHg from a mean of 159.0 ± 14.50/93.4 ± 9.14 mmHg at baseline (P < 0.0001 for either value, ). Blood pressure reduction with olmesartan 40 mg and HCTZ 25 mg at study end was 26.4 ± 16.14/12.8 ± 10.38 mmHg from a mean of 160.7 ± 16.62/93.6 ± 10.21 mmHg at baseline (P < 0.0001 for both values).
Figure 1 Blood pressure reduction (mmHg) between study start and end.
The therapeutic efficacy of the fixed-dose combination was also reflected in decreased pulse pressure, with a drop of −13.8 mmHg on olmesartan 40 mg + HCTZ 12.5 mg and by −13.0 mmHg on olmesartan 40 mg + HCTZ 25 mg.
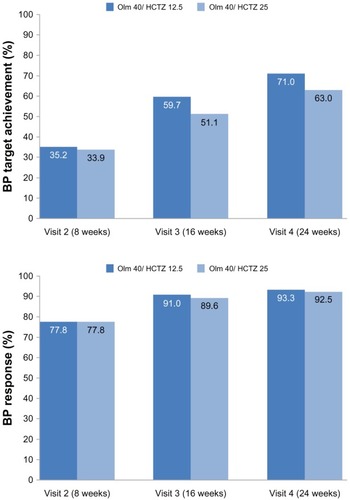
Of the 3,318 patients, 70.7% achieved target BP (<140/90 mmHg) at 24 weeks on the HCTZ 12.5 mg combination and 63.3% on the HCTZ 25 mg combination, respectively. The response rate (either BP target achieved or at least a 20 mmHg systolic reduction or 10 mmHg diastolic reduction) was 93.3%/92.5% at 24 weeks on the 12.5/25 mg HCTZ combination ().
Figure 2 BP target achievement* and BP response** (% of patients).
Abbreviations: BP, blood pressure; LAE, last available examination; Olm, olmesartan; HCTZ, hydrochlorothiazide.
The percentage of patients with optimal, normal, or high normal BP according to the European Society of Hypertension/European Society of Cardiology 2007 BP categories (systolic BP<139 mmHg, diastolic BP<89 mmHg) increased from 4.5% at baseline to 67.8% at study end (66.7% at the last available visit). The severity of hypertensive disease (assessed as stage 1, 2, or 3) decreased accordingly ().
Table 3 Efficacy according to European Society of Hypertension/European Society of Cardiology 2007 blood pressure (BP) categories (n = 3301)
Efficacy according to patient subgroup
A post hoc analysis of subgroups with specified risk constellations showed a numerically similar effective BP reduction in all groups investigated, with the exception of a smaller effect in the event of coexisting risk factors (). Patients aged ≥75 years and those with impaired renal function had smaller changes in BP between baseline and the last available visit. Due to the low numbers of patients in the subgroups and the retrospective nature of the analysis, this result has to be interpreted with caution.
Table 4 Efficacy according to patient subgroup at last available visit (n = 3,301)
Physical activity
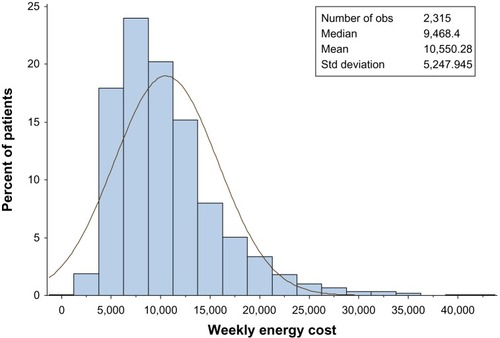
Baseline data on physical activity were available for 70.1% of patients (n = 2,327, ), 27.6% of whom reported some form of weekly regular sporting activity, most often cycling (20.1%, for a mean of 3.4 hours per week), walking (17.6%, for a mean of 2.0 hours per week), and swimming (16.8%, for a mean of 2.2 hours per week). Two groups were defined for comparison of more and less active patients, ie, a first group with sum of weekly energy costs up to and including the median and a second with sum of weekly energy costs exceeding the median. The mean sum of weekly energy costs was 10,550.3 ± 5,247.95, with a median of 9,468.4 (). More women than men belonged to the group of less physically active people. The body weight of patients below the mean sum of weekly energy costs was 1.9 kg lower than the weight of less active patients.
Table 5 Efficacy and safety of the fixed-dose combination of olmesartan 40 mg and hydrochlorothiazide 12.5/25 mg in more and less physically active patients (n = 2327)
The efficacy of olmesartan 40 mg and HCTZ 12.5/25 mg was independent of the level of physical activity. Both groups started with very similar baseline BP levels and achieved a comparable BP reduction. The Spearman’s correlation coefficient between patient physical activity (summarized by sum of weekly energy costs) and physician assessment of efficacy was −0.060, and thus negligible. Neither body weight at the start of the study nor beta-blocker comedication showed any noteworthy differences between more and less active patients.
Discussion
Efficacy
There already exists a large number of clinical trials that have analyzed the efficacy of treatment with olmesartan and HCTZ in different dose combination and competitive angiotensin receptor blocking agents. Systematic reviews found that a combination of olmesartan and HCTZ is a well tolerated, effective short-term and long-term option for patients who fail to respond to monotherapy. It can also be used as initial therapy in those who need large reductions in systolic or diastolic BP to achieve goal BP.Citation16,Citation17 A recent review integrating the relevant approval studies for the fixed-dose combination of olmesartan and HCTZ with and without amlodipine found that the BP-lowering effect of the combination of olmesartan and HCTZ was superior to other combination therapies, and discussed a rationale for choosing HCTZ over another diuretic, ie, chlorthalidone, based on pharmacokinetic differences, clinical concerns, and trends in use.Citation18 One of the largest studies tested the combination of olmesartan and HCTZ in 502 patients against placebo and the respective monotherapies, and found greater reductions in BP than with the individual components for all six dose combinations. The highest dose combination (olmesartan 40 mg and HCTZ 25 mg) reduced seated systolic/diastolic BP by 26.8/21.9 mmHg, 87.2% of patients achieved their systolic BP target, and 79.5% achieved a diastolic BP<90 mmHg.Citation6 The double-blind, placebo-controlled BENIFORCE (Benicar Efficacy: New Investigative Findings show Olmesartan medoxomil safely and effectively Reduces blood pressure Compared with placEbo) titration study in 276 patients with stage 1 or 2 hypertension reported mean reductions in seated BP of 22.3/12.1 mmHg versus 0.1/−0.8 mmHg (P < 0.0001) after 12 weeks.Citation10 A recent subgroup analysis indicated that significant improvements in BP reduction were achieved with olmesartan-based therapy versus placebo, regardless of race, age, or gender.Citation19 We also found a powerful BP-lowering effect in our analysis regardless of gender and risk factors, but quantitatively a slightly lower BP reduction depending on the presence of risk factors, mainly age ≥75 years, diabetes, and impaired renal function. However, the study was not powered for this subgroup analysis, so the data have to be interpreted with caution.
In 2012, Germino et al published data on 176 elderly patients with stage 1, 2, or isolated systolic hypertension and of mean age of 72 years, who were uptitrated every 3 weeks from olmesartan 20 mg, olmesartan 40 mg, a combination of olmesartan 40 mg and HCTZ 12.5 mg, and a combination of olmesartan 40 mg and HCTZ 25 mg in the event of insufficient target achievement with the previous dosage.Citation20 BP goals <140/90 mmHg were reached by 88.3%, 56.0%, and 72.4% of the stage 1, 2, and isolated systolic hypertension cohorts, respectively. A high-dose European study in patients with stage 2 or 3 hypertension found a reduction in seated systolic/diastolic BP of 16.2/11.2 mmHg for the combination of olmesartan 40 mg and HCTZ 25 mg compared with olmesartan 40 mg monotherapy.Citation11
Given the fact that our data represent patients receiving at least olmesartan monotherapy or a low-dose HCTZ combination at baseline, and that all the above-mentioned randomized trial data were mostly tested against placebo, our real-life data show a quite powerful numeric BP reduction in unselected patients as well as a noteworthy reduction of pulse pressure, which is a powerful marker of cardiovascular risk reduction.Citation21,Citation22 We assume that inadequate antihypertensive therapy before entry to the study may in part explain this positive result.
Fogari et al reported 58.5% BP target achievement after 8 weeks of a combination of olmesartan 40 mg and HCTZ 12.5 compared with olmesartan 40 mg monotherapy.Citation23 Another study investigating the combination of olmesartan 40 mg and HCTZ using HCTZ doses up to 50 mg/day found a 70.4% BP target achievement with HCTZ 25 mg after 8 weeks, increasing to 77.5% with HCTZ 50 mg after a further 4 weeks.Citation24 In the present study, comparable target achievement rates were reached later than 8 weeks after the start of the study, which is in contrast with the above-mentioned studies.
Patients with isolated systolic hypertension at baseline warrant special attention. Quite a lot of them shifted to high normal at the last available visit. However, the overall frequency did not change in a noteworthy way, because patients with mild, moderate, or severe hypertension at baseline shifted to isolated systolic hypertension at the last available visit.
Safety
Overall, the safety and tolerability of treatment in our study was excellent. The total number of suspected adverse drug reactions was 19 in 16 of 3318 patients (0.48%) who received the study drug at least once. None of them were regarded as serious. The most frequent suspected adverse drug reactions (dizziness and hypotension) have to be interpreted in light of the underlying cardiac condition. Given the large number of patients, this rate is very low and coincides well with the literature. In the above-mentioned BENIFORCE trial, the rate of drug-related adverse reactions was 2.2%–7.6% across titration steps in the treatment arm receiving olmesartan and HCTZ compared with 2.1%–9.5% in the placebo arm. Another trial in type 2 diabetic patients, who present a higher comorbidity profile than a non-diabetic population, reported dizziness at a rate of 0.7%.Citation25 Drug-related treatment-emergent adverse events ranged from 0.5% to 7.6% across the titration steps for olmesartan and HCTZ. The most commonly reported treatment-emergent adverse event was arthralgia and extremity pain, at 2.1%. Germino et al reported the rate of drug-related hypotension to be less than 3% in elderly patients with stage 1, 2, or isolated systolic hypertension.Citation20
A large pooled analysis of 20 post-authorization surveys of olmesartan involving 156,682 hypertensive patients further supports the placebo-like tolerability of olmesartan and HCTZ. Olmesartan was used as monotherapy or in combination with other antihypertensive drugs, for example, HCTZ.Citation26 The frequency of adverse drug reactions was 0.4% and not altered by dose, age ≥65 years, or presence of comorbidities.
Physical activity
The positive effect of physical activity on health and cardiovascular risk reduction is common knowledge and was shown in the large LIFE (Losartan Intervention For Endpoint) trial.Citation27 In controlled intervention studies, the weighted net change in conventional BP caused by dynamic aerobic training averaged −5.3 mmHg for systolic BP and −4.8 mmHg for diastolic BP.Citation28 A comprehensive meta-analysis on the effect of endurance training on BP and cardiovascular risk factors in hypertensive patients from 72 trials found net reductions in resting BP of −6.9/−4.9 mmHg (P < 0.001) caused by a reduction in vascular resistance, in which the sympathetic nervous system and the renin-angiotensin system appear to be involved.Citation14 One recent cluster randomized clinical trial evaluated the effect of a program promoting physical activity (PEPAF) on cardiovascular risk reduction. A significant decrease from baseline in systolic and diastolic BP and pulse pressure was observed after 12 months in both groups (−2.93 mmHg, −1.81 mmHg, and −1.15 mmHg, respectively, in the control group, and −3.35 mmHg, −1.4 mmHg, and −1.94 mmHg, respectively, in the PEPAF group). Cardiovascular risk decreased by 0.68 (95% confidence interval 0.13–1.25) in the control group and 0.79 (95% confidence interval 0.22–1.35) in the PEPAF group.Citation29
One study in hypertensive patients showed a decrease in BP at rest and during exercise after 6 months and further reductions after 18 months and 3 years with 60 minutes of aerobic exercise twice a week.Citation30 A recent review of 119 articles found positive effects of primary care-based physical activity and dietary interventions on BP in South Asian populations, but due to a paucity of controlled evaluations, the outcomes were difficult to interpret.Citation31 The BP-lowering efficacy of olmesartan and HCTZ in our study did not differ between more or less active patients. When interpreting this result, it is important to be aware that we measured physical activity only at baseline and did not undertake any formal physical activity program during conduct of the study. This can be interpreted as sufficient therapeutic effect of the medication, regardless of individual variability in the level of physical activity.
Limitations
The present study is a real-life trial with all the known limitations of noninterventional studies when compared with randomized, double-blind, clinical trials, including lack of a control group, randomization, potential selection bias, and less transparent compliance with medication. The advantages are also obvious, ie, unselected patients with a wide range of concomitant diseases and potential contraindications to the study drug, and thereby more accurately representative of real-life practice. The patient group studied reflects the daily medical variety of unselected hypertensive patients needing uptitration of antihypertensive medication. Once more, the BP reductions achieved in the present study underscore the unsolved worldwide problem with achievement of target BP despite a huge amount of scientific research and medical efforts in this area.
Conclusion
The present study documents that a fixed-dose combination of olmesartan 40 mg and HCTZ (12.5 mg or 25 mg) is effective in the control of BP, and has an excellent safety profile in patients with previously uncontrolled arterial hypertension. Treatment with this fixed-dose combination was effective and well tolerated in all patient subgroups, including those with comorbidities, and was independent of the individual physical activity level.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. CZ and PB drafted the manuscript. The other authors revised the manuscript for important intellectual content and all authors gave their final approval for the manuscript to be published.
Acknowledgments
We are indebted to Christine Franzen for organization and management of this trial. The authors also acknowledge the cooperation and commitment of all investigators who contributed to the conduct of the trial, along with their staff.
Disclosure
PB has received consultancy fees, served on advisory boards, and given lectures for a number of pharmaceutical companies, including Daiichi Sankyo Germany. EMF and WPW are employees of Daiichi Sankyo Germany. CZ has no conflicts of interest to declare.
References
- ManciaGFagardRNarkiewiczK2013ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)Eur Heart JJune142013 [Epub ahead of print.]
- ManciaGDe BackerGDominiczakA2007 ESH-ESC practice guidelines for the management of arterial hypertension: ESH-ESC Task Force on the Management of Arterial HypertensionJ Hypertens20072591751176217762635
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force documentJ Hypertens200927112121215819838131
- SchmiederREHilgersKFSchlaichMPSchmidtBMRenin-angiotensin system and cardiovascular riskLancet200736995681208121917416265
- BramlagePFixed-dose combinations of renin-angiotensin blocking agents with calcium channel blockers or hydrochlorothiazide in the treatment of hypertensionExpert Opin Pharmacother200910111755176719538001
- ChrysantSGWeberMAWangACHinmanDJEvaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazideAm J Hypertens200417325225915001200
- KereiakesDJNeutelJStoakesKAThe effects of an olmesartan medoxomil-based treatment algorithm on 24-hour blood pressure levels in elderly patients aged 65 and olderJ Clin Hypertens (Greenwich)200911841142119695028
- KereiakesDJNeutelJMSeated cuff blood pressure-lowering efficacy of an olmesartan medoxomil-based treatment regimen in patients with type 2 diabetes mellitusDrugs R D201111325125721777013
- KereiakesDJNeutelJMPunziHAXuJLipkaLJDubielREfficacy and safety of olmesartan medoxomil and hydrochlorothiazide compared with benazepril and amlodipine besylateAm J Cardiovasc Drugs20077536137217953475
- OparilSChrysantSGKereiakesDResults of an olmesartan medoxomil-based treatment regimen in hypertensive patientsJ Clin Hypertens (Greenwich)2008101291192119120717
- RumpLCGirerdXSellinLStegbauerJEffects of high dose olmesartan medoxomil plus hydrochlorothiazide on blood pressure control in patients with grade 2 and grade 3 hypertensionJ Hum Hypertens201125956557421107435
- NarkiewiczKAngiotensin II receptor blocker combinations: from guidelines to clinical practiceBlood Press2012212738121830845
- PescatelloLSFranklinBAFagardRAmerican College of Sports Medicine position stand. Exercise and hypertensionMed Sci Sports Exerc200436353355315076798
- CornelissenVAFagardRHEffects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factorsHypertension200546466767516157788
- JamesWPTSchofieldECHuman Energy Requirements A Manual for Planners and NutritionistsNew York, NYOxford University Press1990
- RuilopeLMClinical efficacy and safety of olmesartan/hydrochlorothiazide combination therapy in patients with essential hypertensionVasc Health Risk Manag2008461237124819337537
- RumpLCSellinLCombination therapy for hypertension: focus on high-dose olmesartan medoxomil (40 mg) plus hydrochlorothiazideExpert Opin Pharmacother201011132231224220707758
- PunziHAIntegrated control of hypertension by olmesartan medoxomil and hydrochlorothiazide and rationale for combinationIntegr Blood Press Control20114738322253546
- OparilSPimentaEEfficacy of an olmesartan medoxomil-based treatment algorithm in patients stratified by age, race, or sexJ Clin Hypertens (Greenwich)201012131320047622
- GerminoFWNeutelJMDubielRMaaJFChavanuKJEfficacy of olmesartan medoxomil and hydrochlorothiazide fixed-dose combination therapy in patients aged 65 years and older with stage 1 and 2 hypertension or isolated systolic hypertensionAm J Cardiovasc Drugs201212532533322920048
- BlacherJStaessenJAGirerdXPulse pressure not mean pressure determines cardiovascular risk in older hypertensive patientsArch Intern Med200016081085108910789600
- LaurentSCockcroftJVan BortelLExpert consensus document on arterial stiffness: methodological issues and clinical applicationsEur Heart J200627212588260517000623
- FogariRTaddeiSHolm-BentzenMBaszakJMelaniLSchumacherKEfficacy and safety of olmesartan medoxomil 40 mg/hydrochlorothiazide 12.5 mg combination therapy versus olmesartan medoxomil 40 mg monotherapy in patients with moderate to severe hypertension: a randomized, double-blind, parallel-group, multicentre, multinational, phase III studyClin Drug Investig2010309581597
- IzzoJLJrNeutelJMSilfaniTDubielRWalkerFTitration of HCTZ to 50 mg daily in individuals with stage 2 systolic hypertension pretreated with an angiotensin receptor blockerJ Clin Hypertens (Greenwich)200791454817215658
- NeutelJMKereiakesDJWaverczakWFStoakesKAXuJShojaeeAEffects of an olmesartan medoxomil based treatment algorithm on 24-hour blood pressure control in patients with hypertension and type 2 diabetesCurr Med Res Opin201026372172820085534
- ScholzeJSchaeferAKreutzRSafety and efficacy of olmesartan: an observational pooled-analysis of 156,682 hypertensive patientsExpert Opin Drug Saf201110218519621254872
- FossumEGleimGWKjeldsenSEThe effect of baseline physical activity on cardiovascular outcomes and new-onset diabetes in patients treated for hypertension and left ventricular hypertrophy: the LIFE studyJ Intern Med2007262443944817875180
- FagardRHThe role of exercise in blood pressure control: supportive evidenceJ Hypertens19951311122312278984117
- Garcia-OrtizLGrandesGSanchez-PerezAEffect on cardiovascular risk of an intervention by family physicians to promote physical exercise among sedentary individualsRev Esp Cardiol201063111244125221070720
- KetelhutRGFranzIWScholzeJRegular exercise as an effective approach in antihypertensive therapyMed Sci Sports Exerc20043614814707760
- ChapmanJQureshiNKaiJEffectiveness of physical activity and dietary interventions in South Asian populations: a systematic reviewBr J Gen Pract201363607104114