Abstract
Heart failure remains a major concern across the globe as life expectancies and delivery of health care continue to improve. There has been a dearth of new developments in heart failure therapies in the last decade until last year, with the release of the results from the PARADIGM-HF Trial heralding the arrival of a promising new class of drug, ie, the angiotensin receptor-neprilysin inhibitor. In this review, we discuss the evolution of our incremental understanding of the neurohormonal mechanisms involved in the pathophysiology of heart failure, which has led to our success in modulating its various pathways. We start by examining the renin-angiotensin-aldosterone system, followed by the challenges of modulating the natriuretic peptide system. We then delve deeper into the pharmacology and mechanisms by which angiotensin receptor-neprilysin inhibitors achieve their significant cardiovascular benefits. Finally, we also consider the potential application of this new class of drug in other areas, such as heart failure with preserved ejection fraction, hypertension, patients with renal impairment, and following myocardial infarction.
Introduction
Advances in acute cardiac care and the ever increasing efficacy of treatment for coronary artery disease mean more and more people are surviving acute coronary events. Although a boon for cardiologists, this has also resulted in a steadily increasing number of patients with chronic heart failure (HF). Together with an ageing population, HF is now a public health problem, with 22 million patients worldwide and costing 38 billion USD a year in the USA alone.Citation1 Defined as a clinical syndrome where the heart is unable to maintain cardiac output to meet metabolic demands and accommodate venous return,Citation1 it takes an indolent, progressively worsening course that results in significant morbidity and eventually a premature death. There has been a substantial increase in our understanding of the pathophysiological pathways involved in HF which has helped to define new therapeutic strategies for combating it. In this review, we journey beyond the drugs that affect the renin-angiotensin-aldosterone system (RAAS) to also look at modulators of the natriuretic peptide (NP) system. Firstly, we will look into the pathophysiology of HF with preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF). We then consider our approach toward the development of drugs to treat these conditions, starting with the lessons learnt from the early failures of NP manipulation, to the development of simultaneous NP + RAAS modulation therapy that eventually led to the discovery of the angiotensin receptor-neprilysin inhibitor (ARNI), LCZ 696 (). Thirdly, we study the potential benefits of this novel class of drug in conditions other than HFrEF, such as in HFpEF and hypertension. Finally, we explore its use in other areas, such as left ventricular dysfunction following a myocardial infarction and renal impairment.
Table 1 Pertinent trials in the development of nesiritide, candoxatril, omapatrilat, and LCZ 696
HFpEF: what’s all the fuss about?
We have now started to appreciate that our rather naive view of diagnosing HF solely based on a patient’s left ventricular ejection fraction does not encompass the entire spectrum of HF. There are patients with normal ejection fraction who present with typical symptoms of the disease. The previous label of diastolic dysfunction has been proven to be a misnomer, as detailed echocardiographic studies reveal these patients to also have significant systolic dysfunction in the form of impaired long axis function and systolic twist, torsional dys-synchrony, and reduced myocardial systolic reserve.Citation2 There is also impaired atrial function, especially on exercise.Citation2,Citation3 All of this is in addition to the traditional definition of diastolic HF characterized by increased end diastolic pressure as a result of abnormal active relaxation and increased passive left ventricular stiffness.Citation4,Citation5 Another feature that is frequently seen is stiff and noncompliant chambers, albeit with normal volumes. Westermann et al described chronic interstitial inflammation causing elevated profibroblast growth factor beta which stimulates type 1 and 3 collagen formation, resulting in increased myocardial wall stiffness and translating into higher resting ventricular pressures.Citation6 Fittingly, diastolic dysfunction has now been replaced by the term HF with preserved ejection fraction to describe this cohort of patients.
It is important to note that HFpEF patients have similar survival rates to HFrEF patients,Citation7,Citation8 ranging from 8%Citation9 to 23%,Citation10 depending on the region and cut-offs for the definition of HFpEF. Also, the fact that approximately 50% of the patients with symptomatic HF actually have HFpEFCitation11 underscores the importance of correctly identifying and treating these patients. The European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic HF identify elderly, obese, and female patients to be at increased risk to develop HFpEF; they also note that patients with HFpEF are more likely to have underlying hypertension, atrial fibrillation, and no previous history of coronary artery disease.Citation12 In fact, hypertension and the resultant chronic pressure overload is the single most important predictor for HFpEF across registry, epidemiological, and controlled trials.Citation13 The diagnosis of HFpEF is one of exclusion, and is considered only when cardiac and non-cardiac factors have been ruled out. Non-cardiac considerations include differentials like obstructive sleep apnea, chronic obstructive airway disease, obesity, and liver disease. On the other hand, when investigating cardiac causes for symptoms of HF in the setting of preserved ejection fraction, one has to rule out valvular and pericardial pathologies, as well as high-output conditions (anemia, thyrotoxicosis, arterio-venous fistula) and left ventricular hypertrophy before a final diagnosis of HFpEF is reached.Citation14 Despite the progress we have made in understanding the pathophysiology of HFpEF, we have yet to identify treatment that can confer a significant mortality benefit. Trials studying various agents like angiotensin-converting enzyme (ACE) inhibitors,Citation15 angiotensin receptor blockers (ARBs),Citation16,Citation17 and spironolactoneCitation18,Citation19 have all failed to demonstrate a definite mortality benefit, although some did indicate a signal toward improved morbidity.Citation17,Citation19
Neurohormonal mechanisms of HFrEF: RAAS and NP system
There are a myriad causes of HFrEF, the commonest being hypertension and ischemic heart disease,Citation20,Citation21 as well as a host of less common etiologies like cardiomyopathy, valvular heart disease, myocarditis, and cardiotoxic agents.Citation1,Citation21 There is also a small group of patients with no discernible cause, ie, the so-called idiopathic HF.Citation21 Whatever the cause, the resultant symptom complex of systemic and pulmonary congestion remains the same, and is a key element in diagnosing HF.
The pathophysiology of HFrEF has long been elucidated. The first neurohormonal compensatory mechanism to be studied,Citation22 and arguably the most influential, is the RAAS. The RAAS, with its cascade of hormones, generates a multifaceted response affecting the heart, kidneys, and systemic vasculature, in an attempt to reverse the adverse effects of HF. However, a vigorous and sustained activation of this mechanism often results in permanent and deleterious alterations in the patient’s cardiovascular physiology. For instance, the left ventricular hypertrophy triggered by angiotensin (Ang) IICitation23,Citation24 and aldosterone to achieve higher stroke volumes will also increase myocardial oxygen demand, predisposing to or worsening any underlying coronary artery disease.Citation25 Besides that, the cellular proliferative effect of these hormones on cardiac myocytes also increases cell apoptosis and fibroblast activity, thus replacing healthy myocardium with non-contractile fibrotic tissue,Citation26,Citation27 resulting in adverse ventricular remodeling. Pulmonary and systemic congestion, on the other hand, is exacerbated by increased intravascular volume due to the enhanced sodium and water retention, also mediated by both Ang II and aldosterone.Citation28,Citation29 The increased peripheral vascular resistance generated by Ang II and sympathetic hyperactivity will not only diminish peripheral blood supply, but also contribute to elevated blood pressure (BP).Citation30 All of this increases the strain on the heart and sets up a vicious cycle that steadily diminishes left ventricular function.
Remarkably, the heart has yet another mechanism to counteract this chronically activated compensatory system, in the form of NPs. The NP system plays a crucial role in maintaining cardiorenal homeostasis using an arsenal of structurally similar but genetically and physiologically distinct peptides.Citation31 Of the four known NPs, atrial natriuretic peptide (ANP) is released by the atria in response to myocardial stretching, signifying increased intravascular volume,Citation32 whereas brain natriuretic peptide (BNP) is released by the ventricles in response to increased ventricular volume and pressures.Citation33,Citation34 Both ANP and BNP have similar physiological effects, inducing diuresis and natriuresis and vasodilatation as well as inhibiting the sympathetic nervous system (by reduced catecholamine secretion) and RAAS (by reducing renin section).Citation35 C-type natriuretic peptide (CNP), on the other hand, is released by the endothelium of the heart, lungs, kidneys, and vasculature in response to shearing stressors.Citation36,Citation37 CNP possesses minimal diuretic capabilities but is a potent vasodilator and prevents vascular cell growth.Citation37 Finally, D-type NP is also associated with chronic HF, but not much of its physiology and significance is known.Citation38 These peptides exert their effects via transmembrane receptors from the guanylyl cyclase family. There are three types of natriuretic peptide receptors (NPRs), namely NPR-A, NPR-B, and NPR-C; however, their nomenclature is not consistent with that of their ligands.Citation39 NPR-A and NPR-B have similar effects and bind to all three NPs, but NPR-A has a stronger affinity for ANP and BNP than for CNP. Conversely, NPR-B is more selective toward CNP.Citation40 When the extracellular binding domain of both these receptors is activated by an NP, the receptor catalyzes the intracellular formation of cyclic guanosine monophosphate from guanosine triphosphate.Citation41 The cyclic guanosine monophosphate then acts as a second messenger to mediate the effects of the NP. NPR-C, on the other hand, has a very different effect, in that its function is to remove NPs from the circulation. It internalizes the bound NP for lysosomal degradation within the cell. This is a minor catabolic pathway for NPs. NPs are predominantly catabolized via enzymatic cleavage by a membrane-bound enzyme called neprilysin, also known as neutral endopeptidase or membrane metallo-endopeptidase.Citation33 Neprilysin, which is widely expressed but most abundant in the kidneys,Citation42 cleaves the ring structures of the NPs, thus rendering them biologically inactive.Citation43 There has been much interest in the positive physiological effects of the NP system, with various groups studying its possible application in treating acute decompensated HF, for instance with BNP (the VMAC trial)Citation44 (discussed below), and more recently with uraglitide (TRUE-HF,Citation45 ClinicalTrials.gov identifier NCT01661634), also in retarding the progress of chronic HF,Citation46,Citation47 and some are even considering its use in preventing symptomatic HF (stage C) among asymptomatic but structurally compromised stage B patients.Citation31 There are still others who are developing chimeric NPs that are superior to their endogenous counterparts in terms of potency and duration of action.Citation48,Citation49
In spite of the enthusiasm, the counter-regulatory effects of the NP are no match for the far more potent effects of the RAAS and other facets of the neurohormonal system. In an attempt to mitigate these effects, we have developed therapy that block the RAAS cascade at almost every level. Right from its origin with the renin inhibitor, aliskiren and beta-blockers that reduce renin secretion, to ACE inhibitors that prevent the enzymatic conversion of Ang I to Ang II, to ARBs that block the binding of Ang II to the angiotensin II type I (AT1) receptors, and finally mineralocorticoid receptor antagonists that act at the very end of the RAAS cascade, by blocking the binding of aldosterone to its receptors on the distal convoluted tubules. In spite of this multi-level blockade on a key pathogenetic cascade, we realize that we still have not won the battle with this complex disease. Hence, it is only natural that we have now ventured beyond the RAAS to identify new targets in other cascades that may be addressed to treat patients with HF. One therapeutic target that has shown much promise is the NP system itself. Its physiological benefits are clear, but studies have shown reduced biological activity of NPs among patients with chronic HF, indeed some patients even develop resistance to NP activity over prolonged periods of exposure.Citation50 Researchers have long tried to augment the NP system, initially by adding recombinant NPs and later by inhibiting its breakdown enzyme, eventually leading to the breakthrough discussed in the following sections ().
Figure 1 Overview of the mechanisms and effects of nesiritide, candoxatril, omapatrilat, and LCZ 696.
Abbreviations: ARNI, angiotensin receptor-neprilysin inhibitor; NEP, neprilysin; NP, natriuretic peptide; RAAS, renin-angiotensin-aldosterone system; ACE, angiotensin-converting enzyme; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNP, C-type natriuretic peptide.
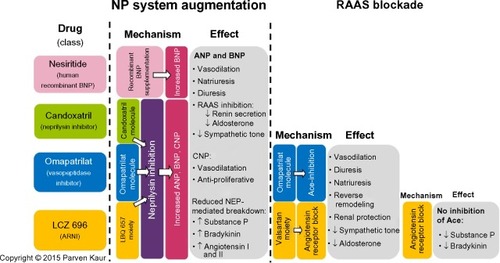
Nesiritide: supplementing the NP system
Encouraged by the wide-ranging cardiovascular effects of the NP system, researchers first attempted to harness the system by supplementing it with recombinant human BNP in the form of nesiritideCitation51 (). The early VMAC trial demonstrated marked improvements in pulmonary capillary wedge pressures and subjective measurements of dyspnea when compared with other vasodilators during the acute phase of decompensated HF.Citation44,Citation52 They also demonstrated its supposed superiority against dobutamine with regard to ventricular ectopy and average heart rate.Citation53,Citation54 In 2001, it was approved by the US Food and Drug Administration for early relief of dyspnea in acute HF.Citation55 However, concerns followed about the renal toxicity and mortality associated with the use of nesiritide in acute HF. A meta-analysis of three trials revealed a signal for increased 30-day mortality (hazard ratio after adjusting for study, 1.80; 95% confidence interval 0.98–3.31; P=0.057) among patients treated with nesiritide compared with controls.Citation56 In another meta-analysis of five trials pooling 1,269 patients to study the renal effects of nesiritide in acutely decompensated HF patients, Sackner-Bernstein et al demonstrated that treatment with nesiritide increased the risk of worsening renal function (defined as an increase of serum creatinine more than 44 μmol/L) by 1.52 (95% confidence interval 1.16–2.00; P=0.003) and 1.54 (95% confidence interval 1.19–1.98; P=0.001) when compared with a non-inotrope-based control or any (inotrope or non-inotrope) control, respectively.Citation57 An independent panel was convened to evaluate this issue, which prompted the ASCEND-HFCitation58 trial (n=7,141), which showed that nesiritide had no effect on mortality or 30-day rehospitalization rates but a small, non-significant effect on dyspnea when used in combination with other therapies. It was not associated with a worsening of renal function, but was associated with an increase in rates of hypotension. On the basis of those results, nesiritide could not be recommended for routine use in the broad population of patients with acute HF.Citation59 A subgroup analysis was also performed on approximately two thirds of the patients in whom urinary data were available, but failed to show a diuretic effect attributable to nesiritide.Citation60 One could speculate that recombinant BNP is inferior to its endogenous counterpart, or perhaps its effect may become blunted in the acute decompensated phase of HF which itself sees a surge in endogenous BNP levels.
Candoxatril: preventing breakdown of NPs
Following the failure of simply adding NPs, researchers turned to inhibiting their enzymatic breakdown instead. Targeting the enzyme neprilysin with the drug candoxatril, researchers were able to prevent the breakdown of ANP, BNP, and CNP,Citation61,Citation62 thereby prolonging their effects on the cardiovascular system (). In addition, neprilysin has a vast array of other substrates including enkephalins, oxytocin, gastrin, Ang I and II, endothelin-1, substance P, and bradykinin,Citation63,Citation64 highlighting the sheer complexity of manipulating such a system. Initial results with this class of drug were encouraging, with significant reductions in pulmonary capillary wedge pressuresCitation65 and improved exercise capacity.Citation66 Researchers also found a significant diuretic effect without the concomitant RAAS activation that is usually seen with the use of furosemide.Citation67,Citation68 However, they were unable to demonstrate a significant BP-lowering effect.Citation69 Indeed, one study reported BP elevations among normotensive patients who were given candoxatril.Citation70 This was likely the direct result of increased Ang IICitation71 and endothelin-1Citation72 activity as a consequence of neprilysin inhibition. Other groups researching the effect of candoxatril on left ventricular dysfunction were also disappointed when they discovered a dose-dependent increase in peripheral vascular resistance and a reduction of cardiac index in the treatment arm.Citation73 Following a string of negative or equivocal publications, researchers investigating candoxatril conceded that pure neprilysin inhibitors lacked efficacy when used as monotherapy in hypertension and HF.Citation61
Omapatrilat: simultaneously modulating the NP system and RAAS
The next phase of evolution was that of dual inhibition; simultaneously inhibiting neprilysin and ACE to potentiate the beneficial physiological effects of the NP system and undermine the harmful effects of RAAS (). This produced a new class of drug, referred to as vasopeptidase inhibitors, of which omapatrilat has been the most developed.Citation74 It was hoped that concurrent ACE inhibition would be able to address the downstream effects of neprilysin inhibition, and early evidence submitted by the authors of the IMPRESS trial were tantalizingly promising. Studying chronic HF patients, they demonstrated improvements in the composite of death and hospitalization, as well as improved New York Heart Association (NYHA) functional class when compared with lisinopril.Citation75 However, the results of larger and more definitive trials, such as OVERTURE and OCTAVE, failed to support this conclusion. OVERTURE, which enrolled nearly 6,000 HF patients to compare omapatrilat and enalapril, did not show any significant difference in death and HF hospitalization rates between groups.Citation76 However, post hoc analysis of a modified definition of its primary end point did reveal a significantly lower risk in the active comparator arm.Citation76 Unfortunately, analysis of its safety revealed a higher incidence of severe angioedema than with any other ACE inhibitor. OCTAVE, which was designed primarily to determine if lower doses of omapatrilat would be safer, enrolled 25,000 hypertensive patients to compare omapatrilat and enalapril. Although it did demonstrate a mean 3/2 mmHg reduction in BP and a larger number of patients achieving goal BPCitation74 at 6 months in the omapatrilat arm, it failed to show a lower incidence of angioedema.Citation77 Investigators discovered that patients on omapatrilat were 3.2 times more likely to develop angioedema than those on enalapril, and the events were also more likely to be severe. This risk of angioedema was more pronounced among blacks and smokers. This included two patients with life-threatening airway compromise, one of which required mechanical ventilation.Citation78 We now believe this is due to a surge in bradykinin and substance P levels as a result of the dual inhibition of their breakdown enzymes, ACE and neprilysin.Citation79,Citation80 The sponsors of the study were quick to point out that the potential benefits of BP reduction by omapatrilat outweighed the risks of angioedema, but failed to convince regulators and were denied approval for the drug.Citation78
ARNI: a new approach to dual inhibition
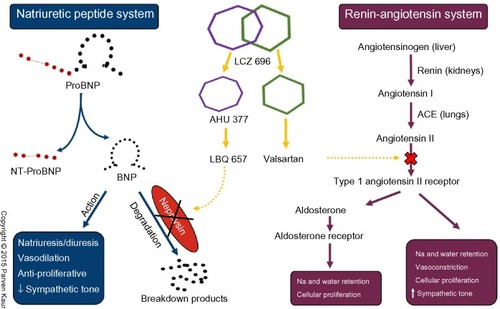
Recognizing the potential of dual neprilysin and RAAS blockade, researchers persevered in trying to address the concerns of angioedema associated with omapatrilat. The elegant solution to this safety concern came in the form of shifting the RAAS target from ACE inhibition to angiotensin receptor blockade. By doing so, the potentially harmful accumulation of substance P and bradykinin is prevented, as they can now be broken down by the uninhibited ACE (). The first in class molecule that was able to achieve this dual angiotensin receptor and neprilysin inhibition was LCZ 696, which is composed of two anionic moieties in a 1:1 molar ratio,Citation80,Citation81 namely AHU 377, which confers potent neprilysin inhibition, and valsartan, a well-studied ARB with a proven track record in treating cardiovascular diseaseCitation82 ().
Figure 2 Schematic representation of mechanism of action of LCZ 696 on the natriuretic peptide and renin angiotensin systems.
Abbreviations: ACE, angiotensin-converting enzyme; BNP, brain natriuretic peptide; Na, sodium; NT, N-terminal.
Pharmacology of LCZ 696
As detailed above, LCZ 696 contains equal molar amounts of AHU 377 and valsartan. AHU 377 is a prodrug that is rapidly metabolized into the biologically active neprilysin inhibitor, LBQ 657, by enzymatic cleavage of its ethyl ester,Citation83 reaching its peak concentration in 1.8–2.7 hours. Valsartan, on the other hand, is biologically active in its original form and has a peak concentration at 1.6–4.9 hours, which is comparable with that of LBQ 657.Citation81 With a plasma half-life of approximately 18 hours, LCZ 696 is suitable for once-daily administration,Citation81 although it may also be administered twice daily for indications like HF.Citation84 It is important to note that proBNP, which is triggered by failing ventricles, remains biologically inactive as long as it is attached to its N-terminal polypeptide chain. Only after the N-terminal portion is cleaved from proBNP does it become BNP and start to exert its cardiovascular effects. It should also be remembered that circulating BNP levels do not influence the secretion of proBNP because it is only secreted in response to ventricular volume and pressure overload. This understanding is key to appreciating why proBNP and NT-proBNP levels remain relevant markers of disease severity in patients treated with LCZ 696, simply because they are not substrates of neprilysinCitation80,Citation85 ().
ARNI in heart failure
Heart failure with reduced ejection fraction
The recently published PARADIGM-HF trial was the first in nearly two decades to generate excitement in the HF community.Citation86 This Phase III active comparator trial conducted across 47 countries enrolled approximately 8,400 patients with NYHA functional class II–IV HF, an ejection fraction <35% and elevated NT-proBNP levels, who were already on evidence-based treatment for HF.Citation87 The investigators aimed to compare LCZ 696 against enalapril, an ACE inhibitor with proven mortality benefits,Citation88,Citation89 with a primary end point of composite cardiovascular death or hospitalization for HF. They also looked at all-cause mortality, 8-month quality of life score (Kansas City Cardiomyopathy Questionnaire), time to new atrial fibrillation, and renal dysfunction. In an attempt to address the safety concerns with previous attempts at dual RAAS-neprilysin inhibition, the authors elected to have an active run-in phase in which all patients were given a single blind run-in with enalapril for 2 weeks followed by an additional 4–6 weeks of LCZ 696 titrated to a target dose of 200 mg (equivalent to 160 mg of valsartan) twice daily. Only after demonstrating tolerability to both drugs would a patient be randomized to either arm of the trial.Citation87
The baseline characteristics of the cohort included a mean age of 63 years, left ventricular ejection fraction 29%±6%, background therapy of ACE/ARB (99%), beta-blockers (93%), mineralocorticoid receptor antagonists (56%), and predominantly NYHA Class II functional status (70%).Citation90 The outcomes of the trial were so overwhelmingly positive that it was stopped early by its data monitoring committee. With a median follow-up of 27 months, the investigators demonstrated a 20% relative risk reduction in the composite of cardiovascular death or hospitalization for HF and a 16% relative risk improvement in all-cause mortalityCitation91 with a number needed to treat of 35.Citation86 They also showed a significant 3 mmHg BP reduction as well as higher quality of life scores in the treatment arm at 8 months. In all, 11% of the recruited patients withdrew due to adverse events to either drug during the run-in period. Following randomization, a further 10.7% from the LCZ 696 arm and 12.3% from the enalapril arm withdrew due to adverse events (P=0.03), leading the investigators to claim that LCZ 696 is at least as safe as enalapril.Citation91
Following the initial enthusiasm about these remarkable results, a number of commentators started to raise some concerns regarding the design and conclusions drawn from the trial. Efficacy concerns revolved around the use of moderate dose enalapril (20 mg/day) instead of its maximal dose (40 mg/day) against 400 mg/day LCZ 696 which delivered an equivalent valsartan dose of 320 mg/day (the maximal daily dose for valsartan). Hence, the supposed benefits may have simply been a result of more effective RAAS blockade conferred by the higher valsartan dose in the intervention arm.Citation92 On the other hand, the safety concerns raised were the use of an active run-in period which would have excluded patients with adverse events from being randomized, thus underestimating the true adverse event risk and possibly even overestimating the efficacy of the study drug. In an edifying paper published in 1998 about the implications of an active run-in design, Pablos-Mendez et al discussed these concerns and added that the applicability of such trials may be diluted because of a trial cohort that cannot be readily defined and reproduced in a clinical setting.Citation93 Apart from that, the community is also concerned that black patients (who are most susceptible to angioedema) and the elderly were under-represented in the trial. In spite of these issues, the general consensus remains positive about the prospects of LCZ 696; it has been granted US Food and Drug Administration fast track status and a rolling submission was completed at the end of 2014. Further post marketing study into the drug will undoubtedly address all the concerns raised.
Heart failure with preserved ejection fraction
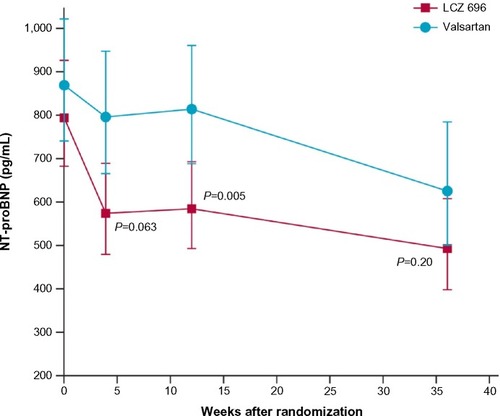
The PARAMOUNT trial was the first to evaluate the use of LCZ 696 in patients with HF. It was a Phase II trial studying 300 patients with signs and symptoms of HF, NYHA class II–III with an ejection fraction of ≥45%, and NT-proBNP >400 pg/mL, comparing LCZ 696 200 mg twice daily against a bioequivalent dose of valsartan 160 mg twice daily.Citation94 The primary outcome of a significant reduction in NT-proBNP at 12 weeks was achieved by 4 weeks and sustained up to the 36-week extension phase of the trial. However, the patients in the valsartan arm demonstrated a slow but steady reduction in NT-proBNP levels, resulting in loss of significance between the two groups at 36 weeks (). Besides that, the extension phase also revealed improved NYHA scores and left atrial size (an important predictor of outcomes in HFpEFCitation95) in the LCZ 696 arm. The investigators also reported that patients on LCZ 696 had a significantly greater BP reduction than those on valsartan in both the 12-week and 36-week intervals. Just as importantly, they noted no significant difference in side effect profiles between the groups.Citation94
Figure 3 Results from the PARAMOUNT Trial NT-proBNP at 4, 12, and 36 weeks in the LCZ 696 and valsartan groups.
Abbreviation: NT-proBNP, N-terminal pro-brain natriuretic peptide.
The proof of concept PARAMOUNT trial has now lead to the recently initiated PARAGON-HF trial (ClinicalTrials.gov identifier NCT01920711) which aims to compare LCZ 696 against valsartan in approximately 4,000 HFpEF patients to look for hard outcomes like composite cardiovascular death and hospitalization. The authors are also aiming to look for functional improvement as well as other secondary end points like new-onset atrial fibrillation, stroke, myocardial infarction, and all-cause mortality. Patients in NYHA class II–IV, with ejection fraction >45%, structural heart disease (left atrial enlargement/left ventricular hypertrophy), and requiring diuretic therapy will be eligible for the trial. The results for this study are expected in 2019.
ARNI: broadening the horizons
Hypertension
Hypertension was the first indication studied for LCZ 696. In 2010, Ruilope et alCitation96 evaluated 1,300 patients with mild-to-moderate hypertension for 8 weeks to measure the mean difference of three pairwise comparisons of sitting diastolic BP between LCZ 696 and bioequivalent doses of valsartan (ie, LCZ 696 100 mg versus valsartan 80 mg, LCZ 696 200 mg versus valsartan 160 mg, and LCZ 696 400 mg versus valsartan 320 mg) as well as a pairwise comparison between AHU 377 200 mg and placebo. They included secondary outcomes such as systolic BP reduction, pulse pressure, ambulatory BP, and safety parameters, as well as markers for other cardiovascular diseases. Their results demonstrated a significantly greater reduction of BP in the 200 mg and 400 mg LCZ 696 pairs (−5/−3 mmHg and −6/−3 mmHg, respectively) and a greater but non-significant reduction in the 100 mg LCZ 696 versus 80 mg valsartan pair. This effect was also seen in 24-hour ambulatory BP measurements. Interestingly, the researchers pointed out that the BP reduction conferred by LCZ 696 400 mg compared with bioequivalent doses of its components (200 mg AHU 377 and 320 mg valsartan) was fully additive in diastolic BP reduction and more than fully additive in systolic BP reduction, highlighting the complementary effects of dual RAAS and neprilysin inhibition. They also noted positive signals in markers of improvement for HF and pulmonary hypertension.Citation96 Researchers in Japan have also recently confirmed the safety and efficacy of LCZ 696 as an antihypertensive in the Asian population.Citation97
Renal impairment
Patients with chronic kidney disease have an excess risk of developing cardiovascular complications as a result of substantial changes in their internal milieu.Citation98 Numerous studies have demonstrated progressively increasing cardiovascular risk with a worsening glomerular filtration rate. Additionally, patients with albuminuria, even if mild, see a near doubling of cardiovascular mortality risk.Citation99 Treating this cohort of patients with ARNIs offers the exciting prospect of not only improving cardiovascular risk, but also delaying the progression to renal replacement therapy. The evidence for the potential renoprotective effects of ARNIs is currently inferred from animal models using other vasopeptidase inhibitors like omapatrilat and ilepatril, which have shown promise in reversing proteinuria and halting glomerulosclerosis and tubulointerstitial fibrosis.Citation100,Citation101 Reassuringly, secondary outcomes of the two major HF trials conducted so far also do not signal any negative impact of ARNIs on renal function. The PARAMOUNT trial showed no significant difference in new-onset renal dysfunction, hyperkalemia, or a >50% reduction in glomerular filtration rate between the LCZ 696 and valsartan arms.Citation94 In fact, there was a higher degree of change in estimated glomerular filtration rate in the valsartan group (LCZ 696, −1.6 mL/min/1.73 m2 versus valsartan, −5.2 mL/min/1.73 m2; P=0.007). The analysis from PARADIGM-HF was also as expected, with no significant difference in protocol-defined decline in renal function or progression to end-stage renal disease.Citation91 In an effort to explore this potential benefit further, the pilot UK HARP-III StudyCitation102 (ISRCTN 11958993), with the primary objective of documenting the change in renal function over 6 months, aims to recruit 360 patients with chronic kidney disease (estimated glomerular filtration rate 20–60 mL/min/1.73 m2 or urinary albumin to creatinine ratio ≥30 mg/mol) to compare LCZ 696 with irbesartan.
Post myocardial infarction
Following a myocardial infarction, the risk for further fatal and non-fatal ischemic events continues to increase.Citation103,Citation104 Although factors such as age, sex, and comorbidities play an important role, the degree of left ventricular dysfunction remains the primary determinant of survivability.Citation105 Trials such as EPHE-SUSCitation106 and SAVECitation105 have demonstrated the undeniable importance of attenuating left ventricular dysfunction in this group of patients, and LCZ 696 seems poised to support that theory. Von Lueder et alCitation107,Citation108 have been conducting preclinical studies of the possible benefits of ARNI on cardiac remodeling in rats with induced myocardial infarction. In a recently published article, Von Lueder et al demonstrated lower cardiac weight and reduced fibrosis in the peri-infarct and remote myocardium in the LCZ 696 group compared with a placebo group. The LCZ 696 group also had a lower left ventricular end-diastolic diameter, a higher left ventricular ejection fraction, and higher circular and diastolic wall strain, confirming improved left ventricular function 4 weeks after treatment. Using cell cultures, they showed the LBQ 657 component of LCZ 696 inhibiting hypertrophy but not fibrosis, and the valsartan component inhibiting both hypertrophy and fibrosis. Importantly, they also showed that the inhibitory properties of valsartan monotherapy were augmented by the dual inhibition of LCZ 696, which at the highest doses was able to completely cancel the Ang II-mediated effects.Citation108 These are very promising findings but, to the best of our knowledge, there have not been any groups studying this effect clinically.
Conclusion
ARNIs and its first in class molecule, LCZ 696, have shown much potential in becoming the next big thing in the field of cardiovascular therapeutics. Our knowledge and experience with the drug remains very much in flux, but will undoubtedly mature as more research is carried out. However, it is imperative that we remain mindful of our previous eagerness to embrace new therapeutic agents before a thorough and rigorous evaluation of its safety profile. It is important for us to maintain a healthy dose of skepticism and foster open discussions when evaluating the merits of this novel class of drug. If proven, however, ARNIs may well supersede ACE inhibitors and ARBs, heralding a new age of multisystem modulation that will revolutionize the way we understand and treat cardiovascular disease.
Acknowledgments
The authors wish to thank Ms Parven Kaur for designing the original illustrations used in this review.
Disclosure
CCL has received consultancy fees and research support for the conduct of PARADIGM-HF and PARAGON-HF from Novartis. JSSS has received funding from the European Foundation for the Study of Diabetes for a trial on sodium-glucose cotransporter 2 II inhibitors in heart failure.
References
- KempCDConteJVThe pathophysiology of heart failureCardiovasc Pathol201321536537122227365
- TanYTWenzelburgerFLeeEThe pathophysiology of heart failure with normal ejection fraction exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motionJ Am Coll Cardiol2009541364619555838
- BorlaugBAOlsonTPLamCSGlobal cardiovascular reserve dysfunction in heart failure with preserved ejection fractionJ Am Coll Cardiol2010561184585420813282
- ZileMRBaicuCFBonnemaDDDiastolic heart failure: definitions and terminologyProg Cardiovasc Dis200547530731316003645
- ZileMRBaicuCFGaaschWHDiastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricleN Engl J Med2004350191953195915128895
- WestermannDLindnerDKasnerMCardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fractionCirc Heart Fail201141445221075869
- VasanRSLarsonMGBenjaminEJEvansJCReissCKLevyDCongestive heart failure in subjects with normal versus reduced left ventricular ejection fraction prevalence and mortality in a population-based cohortJ Am Coll Cardiol19993371948195510362198
- BhatiaRSTuJVLeeDSOutcome of heart failure with preserved ejection fraction in a population-based studyN Engl J Med2006355326026916855266
- CohnJNJohnsonGHeart failure with normal ejection fraction The V-HeFT Study. Veterans Administration Cooperative Study GroupCirculation1990812 SupplIII48III532404638
- Digitalis Investigation GroupThe effect of digoxin on mortality and morbidity in patients with heart failureN Engl J Med199733685255339036306
- YancyCWJessupMBozkurtB2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice GuidelinesJ Am Coll Cardiol20136216e147e23923747642
- McMurrayJJAdamopoulosSAnkerSDESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012Eur J Heart Fail201214880386922828712
- Rose-JonesLJRommelJJChangPPHeart failure with preserved ejection fraction: an ongoing enigmaCardiol Clin2014321151-x24286586
- LindenfeldJAlbertNMBoehmerJPHFSA 2010 Comprehensive Heart Failure Practice GuidelineJ Card Fail2010166475539
- ClelandJGTenderaMAdamusJFreemantleNPolonskiLTaylorJThe perindopril in elderly people with chronic heart failure (PEP-CHF) studyEur Heart J200627192338234516963472
- MassieBMCarsonPEMcMurrayJJVIrbesartan in patients with heart failure and preserved ejection fractionN Engl J Med2008359232456246719001508
- YusufSPfefferMASwedbergKEffects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved TrialLancet2003362938677778113678871
- EdelmannFWachterRSchmidtAGEffect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the ALDO-DHF randomized controlled trialJAMA2013309878179123443441
- PittBPfefferMAAssmannSFSpironolactone for heart failure with preserved ejection fractionN Engl J Med2014370151383139224716680
- GoASMozaffarianDRogerVLHeart Disease and Stroke Statistics – 2014 update: a report from the American Heart AssociationCirculation20141293e28e29224352519
- McMurrayJJVPfefferMAHeart failureLancet200536594741877188915924986
- SayerGBhatGThe renin-angiotensin-aldosterone system and heart failureCardiol Clin201432121-vii24286576
- CrowleySDGurleySBHerreraMJAngiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidneyProc Natl Acad Sci U S A200610347179851799017090678
- GrayMOLongCSKalinyakJELiH-TKarlinerJSAngiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-β1 and endothelin-1 from fibroblastsCardiovasc Res19984023523639893729
- LorellBHCarabelloBALeft ventricular hypertrophy: pathogenesis, detection, and prognosisCirculation2000102447047910908222
- WeberKTExtracellular matrix remodeling in heart failure: a role for de novo angiotensin II generationCirculation19979611406540829403633
- PengJGurantzDTranVCowlingRTGreenbergBHTumor necrosis factor-α-induced AT1 receptor upregulation enhances angiotensin II-mediated cardiac fibroblast responses that favor fibrosisCirc Res200291121119112612480812
- BartBATreatment of congestion in congestive heart failure: ultrafiltration is the only rational initial treatment of volume overload in decompensated heart failureCirc Heart Fail20092549950419808381
- SchrierRWRole of diminished renal function in cardiovascular mortality: marker or pathogenetic factor?J Am Coll Cardiol20064711816386657
- AtlasSAThe renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibitionJ Manag Care Pharm2007138S917605505
- McKiePBurnettJJrRationale and therapeutic opportunities for natriuretic peptide system augmentation in heart failureCurr Heart Fail Rep201512171425331110
- ThibaultGAmiriFGarciaRRegulation of natriuretic peptide secretion by the heartAnnu Rev Physiol199961119321710099687
- SteinBCLevinRINatriuretic peptides: physiology, therapeutic potential, and risk stratification in ischemic heart diseaseAm Heart J199813559149239588425
- GackowskiAIsnardRGolmardJ-LComparison of echocardiography and plasma B-type natriuretic peptide for monitoring the response to treatment in acute heart failureEur Heart J200425201788179615474693
- NathisuwanSTalbertRLA review of vasopeptidase inhibitors: a new modality in the treatment of hypertension and chronic heart failurePharmacotherapy2002221274211794428
- MoyesAJKhambataRSVillarIEndothelial C-type natriuretic peptide maintains vascular homeostasisJ Clin Invest201412494039405125105365
- CheungBYKumanaCRNatriuretic peptides – relevance in cardiovascular diseaseJAMA199828023198319849863839
- LisyOJougasakiMHeubleinDMRenal actions of synthetic dendroaspis natriuretic peptideKidney Int199956250250810432389
- SugaSNakaoKHosodaKReceptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptideEndocrinology199213012292391309330
- HanksSKQuinnAMHunterTThe protein kinase family: conserved features and deduced phylogeny of the catalytic domainsScience1988241486142523291115
- ChinkersMGarbersDLThe protein kinase domain of the ANP receptor is required for signalingScience19892454924139213942571188
- GrafKKoehnePGräfeMZhangMAuch-SchwelkWFleckERegulation and differential expression of neutral endopeptidase 24.11 in human endothelial cellsHypertension19952622302357635530
- VannesteYMichelADimalineRNajdovskiTDeschodt-LanckmanMHydrolysis of alpha-human atrial natriuretic peptide in vitro by human kidney membranes and purified endopeptidase-24.11. Evidence for a novel cleavage siteBiochem J198825425315372972276
- YoungJBAbrahamWTStevensonLWHortonDPResults of the VMAC trial: vasodilation in the management of acute congestive heart failureCirculation20001022227942794
- Cardiorentis. TRUE-AHF Phase III clinical trial Available from: http://www.cardiorentis.com/de/rd/true-ahf.phpAccessed January 26, 2015
- AhmadTFelkerGMSubcutaneous B-type natriuretic peptide for treatment of heart failure: a dying therapy reborn?J Am Coll Cardiol201260222313231523122792
- ChenHHGlocknerJFSchirgerJACataliottiARedfieldMMBurnettJCNovel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptideJ Am Coll Cardiol201260222305231223122795
- McKiePMCataliottiABoerrigterGA novel atrial natriuretic peptide based therapeutic in experimental angiotensin II mediated acute hypertensionHypertension20105661152115920975033
- LisyOHuntleyBKMcCormickDJKurlanskyPABurnettJCDesign, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NPJ Am Coll Cardiol2008521606818582636
- ChenHHHeart failure: a state of brain natriuretic peptide deficiency or resistance or both!*J Am Coll Cardiol200749101089109117349889
- Natrecor® [Product Information]Fremont, CA, USAScios Inc2013 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/20920s033lbl.pdf
- ColucciWSElkayamUHortonDPIntravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study GroupN Engl J Med2000343424625310911006
- SilverMAHortonDPGhaliJKElkayamUEffect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failureJ Am Coll Cardiol200239579880311869844
- BurgerAJHortonDPLeJemtelTEffect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT studyAm Heart J200214461102110812486437
- KesselheimASFischerMAAvornJThe rise and fall of Natrecor for congestive heart failure: implications for drug policyHealth Aff (Millwood)20062541095110216835191
- Sackner-BernsteinJDKowalskiMFoxMAaronsonKShort-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trialsJAMA2005293151900190515840865
- Sackner-BernsteinJDSkopickiHAAaronsonKDRisk of worsening renal function with nesiritide in patients with acutely decompensated heart failureCirculation2005111121487149115781736
- HernandezAFO’ConnorCMStarlingRCRationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF)Am Heart J2009157227127719185633
- O’ConnorCMStarlingRCHernandezAFEffect of nesiritide in patients with acute decompensated heart failureN Engl J Med20113651324321732835
- GottliebSSStebbinsAVoorsAAEffects of nesiritide and predictors of urine output in acute decompensated heart failure: results from ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure)J Am Coll Cardiol201362131177118323747790
- RichardsAMVasopeptidase inhibitors for heart failure: where do we go from here?Eur J Heart Fail20035441141312921800
- LangCCMotwaniJCoutieWJStruthersADInfluence of candoxatril on plasma brain natriuretic peptide in heart failureLancet199133887612551676808
- NorthridgeDBJardineAGAlabasterCTEffects of UK 69 578: a novel atriopeptidase inhibitorLancet1989286635915932570286
- ErdosEGSkidgelRANeutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormonesFASEB J1989321451512521610
- KahnJCPateyMDubois-RandeJLEffect of sinorphan on plasma atrial natriuretic factor in congestive heart failureLancet199033586811181191967410
- NorthridgeDBCurriePFNewbyDEPlacebo-controlled comparison of candoxatril, an orally active neutral endopeptidase inhibitor, and captopril in patients with chronic heart failureEur J Heart Fail199911677210937982
- NorthridgeDBNewbyDERooneyENorrieJDargieHJComparison of the short-term effects of candoxatril, an orally active neutral endopeptidase inhibitor, and frusemide in the treatment of patients with chronic heart failureAm Heart J19991386 Pt 11149115710577447
- WestheimASBostromPChristensenCCParikkaHRykkeEOToivonenLHemodynamic and neuroendocrine effects for candoxatril and frusemide in mild stable chronic heart failureJ Am Coll Cardiol19993461794180110577572
- BevanEGConnellJMDoyleJCandoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertensionJ Hypertens19921076076131321186
- AndoSRahmanMAButlerGCSennBLFlorasJSComparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelinHypertension1995266 Pt 2116011667498988
- RichardsAMWittertGACrozierIGChronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin IIJ Hypertens19931144074168390508
- FerroCJSprattJCHaynesWGWebbDJInhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivoCirculation19989723232323309639376
- KentschMOtterWDrummerCNotgesAGerzerRMuller-EschGNeutral endopeptidase 24.11 inhibition may not exhibit beneficial haemodynamic effects in patients with congestive heart failureEur J Clin Pharmacol1996513–42692729010697
- MangiaficoSCostello-BoerrigterLCAndersenIACataliottiABurnettJCNeutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeuticsEur Heart J2013341288689322942338
- RouleauJLPfefferMAStewartDJComparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trialLancet2000356923061562010968433
- PackerMCaliffRMKonstamMAComparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE)Circulation2002106892092612186794
- KostisJBPackerMBlackHRSchmiederRHenryDLevyEOmapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trialAm J Hypertens200417210311114751650
- PickeringTGThe rise and fall of omapatrilatJ Clin Hypertens200245371373
- ByrdJBTouzinKSileSDipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedemaHypertension200851114114718025295
- LangCCStruthersADTargeting the renin-angiotensin-aldosterone system in heart failureNat Rev Cardiol201310312513423319100
- GuJNoeAChandraPPharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi)J Clin Pharmacol201050440141419934029
- MistryNBWestheimASKjeldsenSEThe angiotensin receptor antagonist valsartan: a review of the literature with a focus on clinical trialsExpert Opin Pharmacother20067557558116553573
- KsanderGMGhaiRDde JesusRDicarboxylic acid dipeptide neutral endopeptidase inhibitorsJ Med Chem19953810168917007752193
- LangenickelTHDoleWPAngiotensin receptor-neprilysin inhibition with LCZ696: a novel approach for the treatment of heart failureDrug Discov Today Ther Strateg201294e131e139
- VardenyOTachenyTSolomonSDFirst-in-class angiotensin receptor neprilysin inhibitor in heart failureClin Pharmacol Ther201394444544823872864
- JessupMNeprilysin inhibition – a novel therapy for heart failureN Engl J Med2014371111062106425176014
- McMurrayJJVPackerMDesaiASDual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF)Eur J Heart Fail20131591062107323563576
- SwedbergKKjekshusJEffects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)J Am Coll Cardiol198862260A66A
- [No authors listed]Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD InvestigatorsN Engl J Med199132552933022057034
- McMurrayJJVPackerMDesaiASBaseline characteristics and treatment of patients in Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF)Eur J Heart Fail201416781782524828035
- McMurrayJJVPackerMDesaiASAngiotensin-neprilysin inhibition versus enalapril in heart failureN Engl J Med201437111993100425176015
- StilesSAfter sinking in, PARADIGM-HF critiqued at HFSA sessionsMedscape Available from: http://www.medscape.com/viewarticle/8322909252014
- Pablos-MendezABarrRGSheaSRun-in periods in randomized trials: implications for the application of results in clinical practiceJAMA199827932222259438743
- SolomonSDZileMPieskeBThe angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trialLancet201238098511387139522932717
- ZileMRGottdienerJSHetzelSJPrevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fractionCirculation2011124232491250122064591
- RuilopeLMDukatABohmMLacourciereYGongJLefkowitzMPBlood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator studyLancet201037597221255126620236700
- KarioKSunNChiangF-TEfficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled studyHypertension201463469870524446062
- GansevoortRTCorrea-RotterRHemmelgarnBRChronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and preventionLancet2013382988933935223727170
- MatsushitaKvan der VeldeMAstorBCAssociation of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysisLancet201037597312073208120483451
- CaoZBurrellLMTikkanenIBonnetFCooperMEGilbertREVasopeptidase inhibition attenuates the progression of renal injury in subtotal nephrectomized ratsKidney Int200160271572111473654
- BenigniAZojaCZatelliCVasopeptidase inhibitor restores the balance of vasoactive hormones in progressive nephropathyKidney Int20046651959196515496167
- ISRCTN RegistryUK Heart and Renal Protection (UK HARP-III) Available from: http://www.isrctn.com/ISRCTN11958993Accessed January 26, 2015
- MukharjiJRudeREPooleWKRisk factors for sudden death after acute myocardial infarction: two-year follow-upJ Am Coll Cardiol19845413136
- [No authors listed]Risk stratification and survival after myocardial infarctionN Engl J Med198330963313366866068
- PfefferMABraunwaldEMoyéLAEffect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarctionN Engl J Med1992327106696771386652
- PittBRemmeWZannadFEplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarctionN Engl J Med2003348141309132112668699
- Von LuederTGWangBKompaAWebbRJordaanPKrumHAngiotensin-receptor neprilysin-inhibition (ARNi) attenuates ischemic cardiac failure in vivo and angiotensin-II-induced cellular cardiac hypertrophy and fibrosisGlobal Heart201491 Suppl 1e237
- von LuederTGWangBHKompaARThe angiotensin-receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophyCirc Heart Fail201581717825362207
