Abstract
Background and aim
Chronic kidney disease (CKD) is frequent in type 2 diabetes mellitus (T2DM), and therapeutic management of diabetes is more challenging in patients with renal impairment (RI). The place of metformin is of particular interest since most scientific societies now recommend using half the dosage in moderate RI and abstaining from use in severe RI, while the classic contraindication with RI has not been removed from the label. This study aimed to assess the therapeutic management, in particular the use of metformin, of T2DM patients with CKD in real life.
Methods
This was a French cross-sectional observational study: 3,704 patients with T2DM diagnosed for over 1 year and pharmacologically treated were recruited in two cohorts (two-thirds were considered to have renal disease [CKD patients] and one-third were not [non-CKD patients]) by 968 physicians (81% general practitioners) in 2012.
Results
CKD versus non-CKD patients were significantly older with longer diabetes history, more diabetic complications, and less strict glycemic control (mean glycated hemoglobin [HbA1c] 7.5% versus 7.1%; 25% of CKD patients had HbA1c ≥8% versus 15% of non-CKD patients). Fifteen percent of CKD patients had severe RI, and 66% moderate RI. Therapeutic management of T2DM was clearly distinct in CKD, with less use of metformin (62% versus 86%) but at similar mean daily doses (~2 g/d). Of patients with severe RI, 33% were still treated with metformin, at similar doses. For other oral anti-diabetics, a distinct pattern of use was seen across renal function (RF): use of sulfonylureas (32%, 31%, and 20% in normal RF, moderate RI, and severe RI, respectively) and DPP4-i (dipeptidyl peptidase-4 inhibitors) (41%, 36%, and 25%, respectively) decreased with RF, while that of glinides increased (8%, 14%, and 18%, respectively). CKD patients were more frequently treated with insulin (40% versus 16% of non-CKD patients), and use of insulin increased with deterioration of RF (19%, 39%, and 61% of patients with normal RF, moderate RI, and severe RI, respectively). Treatment was modified at the end of the study-visit in 34% of CKD patients, primarily to stop or reduce metformin. However, metformin was stopped in only 40% of the severe RI patients.
Conclusion
Despite a fairly good detection of CKD in patients with T2DM, RI was insufficiently taken into account for adjusting anti-diabetic treatment.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic progressive disease, dramatically increasing worldwide, with about 371 million patients in 2012, thus presenting a major health care burden.Citation1 T2DM is also the leading cause of chronic kidney disease (CKD)Citation2 even where it is not related to histologic diabetic nephropathy.Citation3 Complications of T2DM, especially end-stage renal disease (ESRD), account for the largest portion of the cost of the disease.Citation4 The prevalence of CKD in T2DM patients is estimated to be 25%–40% worldwideCitation5–Citation7 and was almost 30% in France in the 2007 ENTRED survey (the Échantillon National Témoin REprésentatif des personnes Diabétiques, a large cross-sectional survey of adults with diabetes conducted to monitor the health status of diabetic patients in France), likely underestimated because of inadequate screening.Citation8 Renal impairment (RI) may often go undetected,Citation9 which is a concern for two reasons: firstly, patients without a documented diagnosis of RI are more likely to progress to ESRD compared with those who are diagnosed, and secondly, patients may be prescribed inappropriate drugs or dosages.Citation10 Monitoring of renal function (RF) should be done by calculating the estimated glomerular filtration rate (eGFR), with two main techniques in widespread use: the Cockcroft-Gault (CG) formula, which gives creatinine clearance but has strong limitations in diabetes and should therefore be avoided, and the Modification of Diet in Renal Disease (MDRD) formula, which gives eGFR.Citation11 MDRD tends to underestimate eGFR at higher levels, but performs better at lower eGFRs (<60 mL/min per 1.73 m2). Latest recommendations propose using the recent Chronic Kidney Disease – Epidemiology Collaboration (CKD-EPI) equation, which generally results in a lower prevalence of CKD and a more accurate assessment of prognosis.Citation12
Improving glucose control slows progression of nephropathy in people with diabetes,Citation13 even if the ideal glycemic target remains elusive, in the absence of interventional trials of intensive glucose control in patients with CKD.Citation14 Current guidelinesCitation15 thus recommend aiming for good glycemic control while balancing benefit/risk – in particular, the risk of severe hypoglycemiaCitation16 – and feasibility with the available therapeutic options. RI impacts the therapeutic management of T2DM and limits the use of certain oral anti-diabetic drugs (OADs) due to drug contraindications, need for dose adjustments and/or regular monitoring, specific pharmacokinetic considerations, and the high risk and more severe consequences of hypoglycemia.Citation10,Citation11,Citation15,Citation17 The place of metformin is of particular interest since most scientific societies now recommend using half the dosage for eGFR between 60 and 30 mL/min per 1.73 m2 and abstaining from using under 30 mL/min per 1.73 m2,Citation15,Citation18,Citation19 while the classic absolute contraindication with RI has not been removed from the official label.Citation20
This study aimed to assess, in real life, the therapeutic management of T2DM patients with and without CKD by general practitioners (GPs) and by diabetologists (DBs) in France, and to evaluate how RF is monitored and taken into account for treatment decisions. In addition, we looked at differences between real-life practices and prescriptions versus theoretical knowledge.
Materials and methods
The OREDIA study (Observation of patients with REnal disease and DIAbetes) was a multicentric, cross-sectional observational study conducted in France between June 1, 2012 and January 28, 2013. A total of 15,582 physicians nationwide (GPs and DBs) were invited to participate; 1,000 accepted and 968 (813 GPs and 155 DBs) were active in recruiting patients. Each participating physician had to include the first two consecutive outpatients with T2DM who were considered to have “renal disease” (CKD patients) and the first patient who was considered by the physician not to (non-CKD patients). No specified definition of “renal disease” was provided to the physicians. Patients included in the study were adults diagnosed with T2DM more than 1 year ago and treated with OAD ± insulin or insulin alone, in addition to lifestyle management. Of note, in 2012, thiazolidinediones were no longer available on the French market and thus were not included here. Patients with any form of secondary diabetes and patients already included in an interventional clinical trial or who had participated in one in the last 3 months could not be included.
Regardless of their inclusion in the CKD or non-CKD cohorts by the physicians, we further classified patients by their actual eGFR status (MDRD formula, on the basis of serum creatinine collected in the data form), in normal RF (eGFR ≥60 mL/min per 1.73 m2), moderate RI (eGFR 30–60 mL/min per 1.73 m2), and severe RI (eGFR <30 mL/min per 1.73 m2).
Clinical and biological data were collected during the unique study visit on a specific data form collecting information on: sociodemographic data, clinical data (disease history, comorbidities, diabetes complications, cardiovascular [CV] risk factors, and concomitant therapies), available biological data including urinary albumin excretion rate (UAER) (no test was required by the protocol in this observational study), and current anti-diabetic treatments. In addition, physicians were asked whether they had adapted anti-diabetic treatment at the end of the study visit.
After recording patients’ data and returning all their patients’ data completion forms, physicians received a general questionnaire about their knowledge and usual practices in monitoring and managing patients with T2DM and renal disease.
Assessments
This study aimed to describe the therapeutic management in patients with T2DM considered by the participating physicians with and without CKD and to assess the impact of CKD on patients’ treatment. The primary assessment was to evaluate the percentage of patients receiving metformin in patients considered by the physician with and without CKD (without protocol-specific definition) and, as a second step, according to their actual eGFR status.
Secondary objectives were to describe RF monitoring and overall management of T2DM (other OADs and insulin) in patients with/without CKD when included by GPs and by DBs, as well as drugs for CV risk management.
Statistical analyses
Quantitative or continuous variables were described by mean and standard deviation and, in some cases, median and range. Qualitative variables were described by absolute frequency and percentage per modality. Quantitative variables were compared between groups by Student’s t-tests in case of normal distribution and Wilcoxon–Mann–Whitney test otherwise. Qualitative variables were compared between groups using the Pearson chi-squared test when all theoretical sample sizes were >5 and the Fisher exact test when <5. All tests were adjusted with a significance level of 5%. All the analyses were performed on the overall population of patients analyzed (with CKD and non-CKD cohorts) and in those included by GPs and by DBs. Missing data were not replaced. The analyzed population was defined as all patients who fulfilled all inclusion criteria with no major protocol deviations.
Sample size was set to guarantee sufficient accuracy (±5%) of the proportion of patients receiving a treatment by metformin in the subgroup of patients with eGFR <30 mL/min per 1.73 m2, which is the main endpoint of the study. All statistical analyses were performed using SAS 8.2 software (SAS Institute, Cary, NC, USA).
Ethics
This observational study was conducted in accordance with the rules of the French Order of Physicians and Good Practices for Epidemiological Studies. Candidates for inclusion were provided with full information about the study in writing. All data processing was carried out in compliance with French Information Technology and Privacy Law.
Results
Demographic and disease characteristics of the diabetic population in the two cohorts
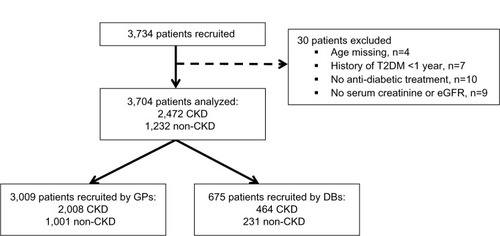
Of the 3,734 patients recruited, 3,704 patients were kept for the analysis, in two cohorts: two-thirds who were considered by the physician to have renal disease (CKD patients) and one-third who were considered by the physician not to (non-CKD patients) ().
Figure 1 Patient disposition.
CKD versus non-CKD patients were significantly older (mean age of 71 versus 63 years; with 40% of the CKD versus 15% of the non-CKD patients being ≥75 years), had longer diabetes history (mean duration of 13 versus 9 years; with 38% of the CKD versus 18% of the non-CKD patients having ≥15 years of disease duration) but similar sex-ratio (62% male) and body mass index (BMI; 29.3 kg/m2). CV risk factors were highly prevalent overall (98% and 90% in the CKD and non-CKD cohorts, respectively) driven by hypertension (91% and 71%) and dyslipidemia (79% and 65%). Diabetic complications were more prevalent in CKD patients (84% versus 29%) driven by nephropathy (71% versus 5%), CV disease (40% versus 17%), and retinopathy (20% versus 8%). Mean eGFR was 49 and 90 mL/min per 1.73 m2 in the CKD and non-CKD cohorts, respectively. Glycemic control was less strict in the CKD population, which had a mean glycated hemoglobin (HbA1c) of 7.5% versus 7.1% in the non-CKD; 25% of the CKD patients had an HbA1c ≥8% versus 15% of the non-CKD patients. Demographic characteristics are presented in in more detail, by specialty (GP and DB). For both cohorts, patients included by DB versus GP were significantly older, had longer diabetes history, more micro-and macrovascular complications, and the prevalence of all CV risk factors was higher ().
Table 1 Demographic and disease characteristics in the two cohorts (CKD and non-CKD), according to physicians
Assessment of RF
Recent creatinine levels (<1 year) were available in all patients (mean of 144 and 80 μmol/L, in the CKD and non-CKD patients, respectively) and results for eGFR/creatinine clearance in 82%. The CG formula was more frequently used by GPs than DBs (38% versus 18%) and the MDRD formula more frequently used by DBs than GPs (75% versus 52%). The use of the CKD-EPI formula was extremely marginal (less than 1%). Overall, 70% of patients had been screened for proteinuria within the past year.
Most CKD/non-CKD patients were correctly classified when taking into account both their actual eGFR status and their UAER, by all physicians. When classified by their actual eGFR status, 96% of patients with moderate RI and 99% of those with severe RI were included in the CKD cohort. Only about 19% of CKD patients had normal RF, 66% of them had moderate RI (versus 8% of non-CKD), and 15% had severe RI (versus 0.4% of non-CKD patients). Among the CKD patients who had normal RF, the vast majority (82%) had an abnormal UAER and thus were correctly classified. Overall, 79% of CKD patients (versus 14% of non-CKD patients) had abnormal UAER, 50% had microalbuminuria (versus 11%) and 29% had proteinuria (versus 3%).
Therapeutic management of T2DM in the CKD and non-CKD cohorts
Therapeutic management of T2DM was clearly distinct in the two cohorts, with significantly less use of metformin in CKD patients (61.7% versus 86.4%; P<0.001) but without any reduction in mean daily doses (~2 g/d). The duration of metformin use was longer in the CKD cohort, in line with the mean disease history (9.4±6.5 versus 7.2±5.3 years). There was also less use of dipeptidyl peptidase-4 inhibitors (DPP4-i) in the CKD cohort (35.6% versus 45.3%; P<0.001), while patients were much more frequently treated with insulin (40.3% versus 16.2% of non-CKD; P<0.001) and twice more often with glinides (14.2% versus 7.1%; P<0.001). In contrast, sulfonylureas (SUs) use was basically the same regardless of the cohort: 30.1% and 31.3% in CKD and non-CKD, respectively.
Overall, anti-diabetic therapy was based on oral agents alone in 83.8% of the non-CKD patients (38% as a single agent and 45% as dual therapy) and in 59.7% of CKD patients (36% as a single agent and 49% as dual therapy).
Therapeutic management of T2DM by eGFR status
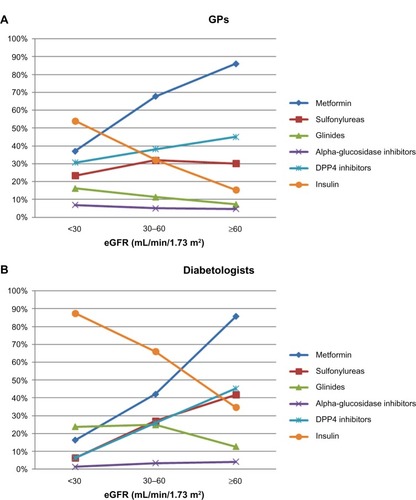
Similar observations were found when considering the pattern of use of anti-diabetics across RF. Overall, with increasing degree of renal insufficiency, the use of metformin, SUs, and DPP4-i decreased, while that of glinides and insulin increased. Metformin was used by 86% of patients with normal RF, and 63% and 33% of those with moderate and severe RI, respectively. Of note, in the population with severe RI, the mean daily dose of metformin however remained the same at 2 g. Furthermore, proportions of patients treated with metformin still receiving doses >2 g/d and doses ≥3 g/d were fairly similar across RF: 25% and 22% in severe RI, 31% and 20% in moderate RI, and 31% and 19% in normal RF, respectively. SU use decreased with RF (32%, 31%, and 20% in normal RF, moderate RI, and severe RI, respectively) as did the use of DPP4-i (41%, 36%, and 25%, respectively), while that of glinides (8%, 14%, and 18%, respectively) and of insulin (19%, 39%, and 61%, respectively) clearly increased. Alpha-glucosidase inhibitors were the only anti-diabetics which were used consistently in about 5% of patients regardless of RF. Use of anti-diabetics according to the degree of renal alteration is presented by specialty (GPs and DBs) in .
Management of the CV risk
In line with the high prevalence of associated CV risk factors, most patients received concomitant therapies, even more so in the CKD cohort (92% and 72% of non-CKD patients, respectively): the mean number of co-medications was 4.8±2.7 in the CKD cohort and 3.0±2.2 in the non-CKD cohort (P<0.01), primarily represented by antihypertensive drugs (91.0% and 71.0%) followed by lipid lowering agents (77.5% and 63.3%) and antiplatelet agents (51.2% and 29.9%). Overall, 51.2% of the CKD patients and 29.9% of the non-CKD patients received a combination of the three (antihypertensive + lipid-lowering + antiplatelet agents). The number of antihypertensive drugs was also higher in CKD patients, with 35% of the patients receiving at least a triple antihypertensive therapy (versus 17% of non-CKD patients); among the 1,069 patients overall (in both cohorts) treated with three antihypertensive drugs, the combination of a renin angiotensin aldosterone system (RAAS) blocker + diuretic + calcium-channel blocker represented 44.8% of the cases. The management of CV risk factors by DBs and by GPs is detailed in .
Analysis by physicians: management of patients included by GPs versus those included by DBs
When patients were included by a GP, 48.1% of the patients in the CKD cohort and 26.0% of those in the non-CKD cohort were also followed by a DB. Less than half of the CKD patients had ever seen a nephrologist (47.6% of those included by DBs and 36.4% of those included by GPs), while cardiologists were largely involved (almost 80% of patients in both cases had seen a cardiologist) ().
T2DM therapeutic management of patients included by DBs significantly differed to that of patients included by GPs: “DB patients” with CKD received less metformin (42.2% versus 66.1% of “GP patients”), slightly less SU (26.1% versus 31.0% of “GP patients”) and less DPP4-i (24.6% versus 38.1%), but considerably more insulin (67.7% versus 34.0% of “GP patients”) and more glinides (23.5% versus 12.1% of “GP patients”). In patients included by DBs, SUs were prescribed almost half as much for patients with CKD than for those without (26.1% versus 40.7%), while no such difference was seen among patients included by GPs (with SUs used in 31.0% and 29.2% of CKD and non-CKD patients, respectively). The mean daily dose of metformin did not differ significantly among patients included by GPs and DBs (mean daily dose =2 g/d; one-third had a dose >2 g/d, and 20% a dose of 3 g/d).
Adaptation of anti-diabetic therapy with the degree of RI was more pronounced in patients included by DBs than in those included by GPs but followed the same trends: in the population with severe RI, a higher proportion of patients were receiving insulin (87.5% versus 54.0% of patients included by GPs) and a much smaller proportion were receiving metformin (16.3% versus 37.1% of patients included by GPs) and SUs (6.3% versus 23.4%). DBs tended to use more glinides in this severe RI population (23.8%) than GPs (16.2%). Use of anti-diabetics according to RF is presented by specialty (GPs and DBs) in .
Adaptation of anti-diabetic therapy at the end of the visit
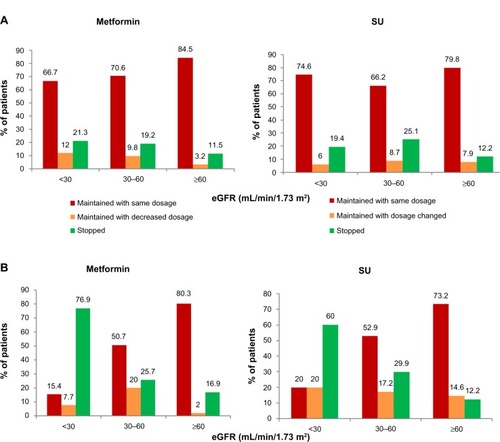
Overall, treatment was modified at the end of the visit in about 34% of CKD patients, primarily to stop or reduce metformin. In the subgroup of patients with severe RI (eGFR <30 mL/min per 1.73 m2), metformin was reduced/stopped in only 40% of the patients, and significantly less often for patients included by GPs (about 33%) than for those included by DBs (85%). In this same group of patients with severe RI, SUs were stopped in only 19% of the patients included by GPs but in 60% of those included by DBs ().
Figure 3 Adaptation of anti-diabetic treatment at the end of the visit. (A) Adaptation of metformin and SU for patients included by GPs. (B) Adaptation of metformin and SU for patients included by DBs.
Theoretical knowledge (based on the physician questionnaire on renal monitoring and their own prescribing practices)
Based on the general questionnaire, physicians declared monitoring RF at least once a year in their diabetic patients, mostly by eGFR, using the MDRD formula by DBs (92.1% versus 67.4% of GPs; P<0.001) and the CG formula by GPs (60.2% versus 32.5% of DBs; P<0.001). Less than 5% declared using plasma creatinine levels only. Of the physicians, 68.8% (65.3% of GPs and 86.1% of DBs) declared assessing UAER once a year.
Both GPs and DBs declared decreasing metformin doses at an equivalent mean threshold of 54±11 mL/min per 1.73 m2 and stopping its use at 35±12 and 33±7 mL/min per 1.73 m2, respectively. For the overall cohort of CKD patients, GPs declared using SUs (24%) slightly more often than DBs (21%), whereas DBs declared using insulin (44%) more than GPs (29%; P<0.001). In patients with severe RI, GPs declared using primarily insulin (61%) followed by DPP4-i (30%) and, to a lesser extent, glinides (11%) or SUs (10%), while DBs, who also declared using mostly insulin (64%), were much more inclined to prescribe glinides (37%) and much less SUs (2%).
Discussion
Patients considered with or without CKD by the physicians, without any protocol pre-specified criteria, were mostly entered in the proper cohort and thus accurately classified in our study. However, despite this relatively good diagnosis of CKD, our data show that RF was not sufficiently taken into account for anti-diabetic treatment adjustments in T2DM, especially for metformin. Nonetheless, theoretical knowledge about the advisable adjustments existed.
To assess the representativeness of our OREDIA population, it was of interest to compare our patients without CKD to the general diabetic population of the French ENTRED 2007 surveyCitation8,Citation21 even though the diabetic population of ENTRED included a significant proportion of patients with CKD (about 30%). Patient characteristics were fairly similar in many aspects: ENTRED participants had a mean age of 66 years (versus 63 years in non-CKD patients of OREDIA), and the mean duration of diabetes (9 years), mean BMI (29 kg/m2), and mean glycemic control (HbA1c of 7.1%) were identical in ENTRED and OREDIA. Of the ENTRED participants, 20.8% had coronary heart disease; of the patients in OREDIA, 17% had macrovascular complications, and 75% versus 70%, respectively, received an antihypertensive treatment. The rate of retinopathy was similar in both cases (8%). In populations with CKD, data are sparser: in the small cohort described in Bouee et al,Citation22 mean age of patients with moderate/severe RI was 74 versus 62 for patients without RI. In another primary care survey conducted from a GP panel in 2011 in France, 297 patients with eGFR <60 mL/min per 1.73 m2 also had similar characteristics: 75 years old on average, with a long disease duration of 12–14 years, a mean BMI of 29 kg/m2, and well-controlled diabetes, with a mean HbA1c of 7.1%.Citation23
Degree of glycemic control in our population
Glycemic control was less strict in the CKD patients which, in most cases, is expected since it is an older population with more advanced disease and more complications and thus a more flexible target is likely appropriate. This could also explain why glycemic control tended to be less strict in patients included by DBs (7.5%) versus GPs (7.1%) in view of the differences seen in populations. However, one-quarter of the CKD patients had an HbA1c >8%, which is certainly not sufficient, possibly due to a mix of clinical inertia and to increasing treatment complexity. In contrast, some patients may have too intensive glucose control since it is probably not beneficial to go lower than 6.5% in this particular population. Indeed, the relationship between HbA1c and outcomes in diabetic people with CKD was recently examined in a large Canadian registry dataset of people with diabetes and CKD not requiring dialysis.Citation24 It showed that a higher HbA1c level was strongly and independently associated with markedly worse clinical outcomes (increased risk of death, hospitalization, myocardial infarction, stroke, and ESRD) regardless of baseline eGFR. However, the association with mortality was U-shaped, with increases in the risk of mortality apparent at HbA1c levels lower than 6.5% and higher than 8.0%.Citation24 Recent guidelinesCitation2 balance the need for tight glycemic control and the increased risk of severe hypoglycemia, with approaches targeting near normal glycemia, and recommend a general HbA1c of ~7.0% in the CKD population. Maintaining good glycemic control may require a combination of anti-diabetic drugs, which need to be carefully adjusted to the degree of RI.Citation2
Use of OADs
Metformin
There are some gray zones and major inconsistencies between the current – very restrictive – prescribing information/labels worldwide, and evidence from the literature suggesting that patients with mild–moderate RI gain more benefit than harm from using metformin.Citation25–Citation27 Indeed, data showing harmless use of metformin in moderate RI has been accumulating. The Reduction of Atherothrombosis for Continued Health (REACH) Registry 2004 showed that metformin use was associated with a decreased mortality in secondary prevention, including in patients with moderate RI.Citation28 In 19,691 diabetic patients with established atherothrombosis treated with or without metformin, the adjusted hazard ratio for 2-year mortality rates was 0.76 (0.65–0.89; P<0.01) overall, and was 0.64 (95% confidence interval, 0.48–0.86; P=0.03) in the subgroup of patients with an eGFR of 30–60 mL/min per 1.73 m2.Citation28 Other data from 51,675 patients of the Swedish National Diabetes Register, followed for 4 years, showed that patients with RI (eGFR 30–45 mL/min per 1.73 m2) treated with metformin compared with other OADs, had no increased risk of CV disease, all-cause mortality, or acidosis/serious infection.Citation29 The Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter StudyCitation30 (in 19 outpatient diabetes clinics in years 2007–2008, evaluating 15,773 T2DM patients) showed that metformin was associated with lower CV event rates, even in elderly adults with RI.
This contradiction between current prescribing guidelines and recent data from the literature may explain why it is not so clear-cut for the prescribers in practice. In fact, published evidence clearly supports the safe use of appropriate doses of metformin in patients with chronic stable RI, and highlights the greater risks of the alternatives, most notably severe hypoglycemia in patients taking SUs and/or insulin, and fluid retention in patients taking a thiazolidinedione. However, once patients reach the stage of severe RI, all international guidelines indicate that metformin should be withdrawn.Citation10,Citation15,Citation19,Citation31
In our study, use of metformin was still prominent (63%) in patients with moderate RI, even if clearly decreased from that in patients with normal RF (86%). Interestingly, 33% of all patients with severe RI (eGFR <30 mL/min per 1.73 m2) were still receiving metformin. It is worth noting that, as a rule, the daily dose was not adjusted as would be needed in CKD patients with declining RF. However, at the end of the study visit, a large majority of DBs stopped the treatment in patients with severe RI, while it was not the case with GPs (metformin was stopped/reduced in only about one-third of the patients included by GPs, but in 85% of those included by DBs). This is in sharp contrast to what all physicians, both GPs and DBs, answered in the questionnaire about their practice, in which they declared (regardless of their specialty) reducing the doses at a mean threshold of 54 mL/min per 1.73 m2 and stopping its use at 35 or 33 mL/min per 1.73 m2. In the RIACE Italian study in real-life conditions,Citation30 use of agents that are not recommended in individuals with RI was also frequent: in particular, use of metformin in moderate and severe RI was 41.4% and 14.5%, respectively, not unlike our findings.
SUs
Because of decreased renal neoglucogenesis, hypoglycemia is already more common in patients with RI,Citation32 independent of the anti-diabetic treatment. In addition, hypoglycemia poses greater threat to patient safety in CKD, as suggested by a large retrospective analysis of patients cared for at the Veterans Health Administration in which hypoglycemia was associated with excessive mortality.Citation17 SU use sharply increases the risk of hypoglycemia, a risk which further rises with older age and decreasing RF.Citation33 Besides the hypoglycemic risk, SU users, compared with metformin, were shown to be at increased risk for decline in eGFR, diagnosis of ESRD, or death, in a retrospective cohort of 93,577 diabetic patients with baseline normal RF from the national Veterans Administration database.Citation34 Despite these limitations with SUs, the ENTRED surveyCitation35 in 2007 found that 41% of diabetic patients with an eGFR <45 mL/min per 1.73 m2 and 33% of those with eGFR <30 mL/min per 1.73 m2 were still taking an SU. In the OREDIA study, in spite of the availability since 2007 of the newer class of DPP4-i with considerably lower hypoglycemic risk, the use of SUs remained high, with over 20% of the patients with severe RI (eGFR <30 mL/min per 1.73 m2) included by GPs still treated with SUs. Moreover, in this group of patients with severe RI, SUs were stopped in only 19% of the patients included by GPs (but in 60% of those included by DBs). Similar findings were seen in the RIACE study,Citation36 where the use of SUs remained frequent in moderate and severe RI (34.2% and 18.1%, respectively). Furthermore, a worrisome finding in RIACE was that inappropriate prescription of these agents, especially SUs, increased with age.Citation36
Glinides
Glinides were more likely to be used by DBs than by GPs, and their use steeply increased with declining RF.Citation37 This is not surprising since glinides have primarily a role in the treatment of T2DM when an oral agent is needed in case of RI.Citation38 It is, however, unclear that glinides offer any advantages over SUs in terms of hypoglycemia, and several meta-analyses from the literature have concluded against any hypoglycemic benefit of this class compared with SUs.Citation39,Citation40 Nonetheless, glinides seem to have acquired a special place with DBs for their RI patients, as shown here, based on a unique publication on this fieldCitation41 (an open-label study of 3-months duration which included 66 patients with creatinine clearance <60 mL/min per 1.73 m2).
DPP4-i
DPP4-i improve glycemic control in a glucose-dependent manner and are associated with a low incidence of hypoglycemia, a distinct benefit in this RI population at high risk, and most are now approved for use in patients with moderate or severe RI.Citation33 Data assessing the use of these novel agents in RI patients have been recently published, with vildagliptinCitation42,Citation43 (including in severe RICitation44 and in elderly patientsCitation45), as well as with sitagliptin,Citation46,Citation47 saxagliptin,Citation48 and linagliptin.Citation49
In our study, while the class was largely prescribed, especially among GPs, the potential benefit in RI did not seem to be well recognized. Actually, there was a very clear trend to decreased-use of DPP4-i with declining RF, both in patients included by GPs and DBs. This could be due, in part, to the very recent changes in label and unavailability for some drugs of the appropriate dosage when the study was conducted.
In addition, a potential protective effect of DPP4-i on diabetic kidney disease, beyond their glucose lowering properties, has recently been suggested, with a reduction of microalbuminuria observed on top of RAAS inhibition.Citation50 This effect was also independent of changes in HbA1c or systolic blood pressure.Citation50 As reviewed in Haluzik et al,Citation51 possible mechanisms include reduction of oxidative stress and inflammation and improvement of endothelial dysfunction in the kidney.Citation51 This question will need further confirmation in larger clinical trials (one ongoing “Efficacy, Safety and Modification of Albuminuria in Type 2 Diabetes Subjects With Renal Disease With Linagliptin”; NCT01792518).
Burden of treatment and use of antihypertensive agents
Concomitant therapies for managing CV risk were highly prevalent, with about five co-medications on average for patients in the CKD cohort. Antihypertensive agents were prescribed quite intensively overall and even more so in CKD patients. Indeed, it is well recognized that reaching blood pressure goals in hypertensive patients with nephropathy usually requires combination therapy.Citation52 Even when patients were heavily treated, only about half the patients taking at least three antihypertensive drugs were receiving the NICE (National Institute for Health and Clinical Excellence) recommended combination of an RAAS blocker + diuretic + calcium-channel blocker.Citation53 However, since information regarding blood pressure control was not collected, success of this intensive antihypertensive therapy cannot be assessed. All classes of antihypertensive drugs can be used in patients with T2DM, even if RAAS blockers may be preferred in the presence of nephropathy with microalbuminuria or overt proteinuria.Citation52 If we consider that nearly 80% of our CKD patients had abnormal levels of proteinuria, the rate of RAAS-blocker prescriptions is as expected (about 75% for CKD patients, as assessed in ENTRED).Citation8 Moreover, it should be pointed out that very few patients received a dual RAAS blockade, which is currently not recommended in diabetes and in nephropathy, even though it could potentially be effective in reducing proteinuria.Citation52,Citation54
Limitations
The current observational study has several limitations. The design was cross-sectional, and thus did not allow the assessment of the impact of treatment adaptation and further management of patients. DB investigators tended to be overrepresented in our study, which is therefore not necessarily representative of the distribution of physicians involved in T2DM management in France. Data were collected based on what physicians declared, and may be subject to bias. Like in all these studies, it is possible that the very focus on CKD could have influenced the quality of renal monitoring for patients included (with a relatively high frequency of UAER measures, for example), which may not be representative of all physicians. In addition, a patient “included” by a DB was not necessarily “followed” by a DB but could be managed by their GP and only occasionally seen at the study visit by a DB, so that findings do not necessarily reflect the true differences in practice by specialty. Moreover, no explanation was provided on why physicians did not adapt the doses and medications as they know would be appropriate: we are thus left to speculate on possible reasons for this apparent contradiction. To some extent, patients that have been known for a very long time and are routinely seen, may be “taken for granted” and not really reassessed. It is possible that, in these cases, physicians simply renew the regular prescriptions without reconsidering disease evolution.
Despite these limitations, our results were consistent with existing data.Citation55 In fact, another recent study looked at this question in FranceCitation22 from a database based on a panel of 1,200 GPs, and assessed the prescribing of anti-diabetic agents in T2DM patients with CKD. Results on the management of diabetes are in line with ours: daily doses of metformin were rather stable across degree of RI (~1.8 g/d); 21% and 33.7% of patients with severe RI were receiving metformin and SUs, respectively. On the whole, our results, based on a broader population, bring important insights into the way this increasingly important population is taken care of in real life.
Conclusion
In routine clinical practice, despite a fairly good screening of renal complications in patients with T2DM and appropriate knowledge of guidelines, RF was insufficiently taken into account for adjusting anti-diabetic treatments, especially by GPs. While the case of metformin is special, in view of the mounting evidence suggesting its benefit, including in the CKD population with RI, drugs exposing patients at high hypoglycemic risk were also still heavily prescribed. This is of particular concern, since the population of diabetic patients with impaired RF is steadily increasing across the world, and is largely an elderly population (both because RF declines with age and because of diabetic nephropathy), thus more susceptible to the risk of severe hypoglycemia. The benefit of lowering blood glucose would be partly offset by such side-effects as severe hypoglycemia in this vulnerable population.
Improved management of diabetes is clearly required in the RI population, with careful adaptation of anti-diabetic treatments and better use of antihypertensive agents.
Acknowledgments
The authors thank all the patients and investigators at participating sites. This work was funded by Novartis Pharmaceuticals Corporation.
Disclosure
A Penfornis has received fees for consultancy, advisory boards, speaking, travel, or accommodation from Novartis, MSD, Astra-Zeneca, and BMS. JF Blicklé has received fees for advisory boards, conferences or clinical investigations from BMS, Novartis, Sanofi-Aventis, Novo-Nordisk, Takeda, Servier, and invitations to congresses or symposia from Eli Lilly, Novo-Nordisk, Sanofi-Aventis, Novartis, BMS, Astra-Zeneca, Servier, Janssen, MSD, Boehringer Ingelheim, and Merck. B Fiquet, S Quéré, and S Dejager are employees of Novartis Pharma SAS.
References
- International Diabetes FederationDiabetes Atlas Update 20125th edBrussels, BelgiumInternational Diabetes Federation2012
- National Kidney FoundationKDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 UpdateAm J Kidney Dis20126085088623067652
- SharmaSGBombackASRadhakrishnanJThe modern spectrum of renal biopsy findings in patients with diabetesClin J Am Soc Nephrol201381718172423886566
- JoyceATIacovielloJMNagSEnd-stage renal disease- associated managed care costs among patients with and without diabetesDiabetes Care2004272829283515562193
- KoroCELeeBHBowlinSJAntidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United StatesClin Ther2009312608261720110005
- PennoGSoliniABonoraEClinical significance of nonalbuminuric renal impairment in type 2 diabetesJ Hypertens2011291802180921738053
- RemuzziGSchieppatiARuggenentiPClinical practice. Nephropathy in patients with type 2 diabetesN Engl J Med20023461145115111948275
- AssogbaGFCouchoudCRoudierCPrevalence, screening and treatment of chronic kidney disease in people with type 2 diabetes in France: The ENTRED surveys (2001 and 2007)Diabetes Metab20123855856623036461
- MeyersJLCandrilliSDKovacsBType 2 diabetes mellitus and renal impairment in a large outpatient electronic medical records database: rates of diagnosis and antihyperglycemic medication dose adjustmentPostgrad Med201112313314321566423
- KDOQIKDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney DiseaseAm J Kidney Dis200749S12S15417276798
- RigalleauVBeauvieuxMCGonzalezCEstimation of renal function in patients with diabetesDiabetes Metab20113735936621680218
- EarleyAMiskulinDLambEJLeveyASUhligKEstimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic reviewAnn Intern Med201215678527022312131
- HolmanRRPaulSKBethelMAMatthewsDRNeilHA10-year follow-up of intensive glucose control in type 2 diabetesN Engl J Med20083591577158918784090
- ZoungasSChalmersJDiabetes: glycemic control and outcomes in people with diabetes and CKDNat Rev Nephrol2012813313422310949
- BonnetFGauthierEGinHExpert consensus on management of diabetic patients with impairment of renal functionDiabetes Metab201137Suppl 2S1S2522108432
- SlininYIshaniARectorTManagement of hyperglycemia, dyslipidemia, and albuminuria in patients with diabetes and CKD: a systematic review for a KDOQI clinical practice guidelineAm J Kidney Dis20126074776922999165
- MoenMFZhanMHsuVDFrequency of hypoglycemia and its significance in chronic kidney diseaseClin J Am Soc Nephrol200941121112719423569
- LipskaKJBaileyCJInzucchiSEUse of metformin in the setting of mild-to-moderate renal insufficiencyDiabetes Care2011341431143721617112
- National Institute for Health and Clinical ExcellenceType 2 Diabetes: the Management of Type 2 DiabetesNICE clinical guideline 87LondonNational Institute for Health and Clinical Excellence2009 Available from: http://www.nice.org.uk/nicemedia/pdf/CG87NICEGuideline.pdfAccessed March 21, 2014
- VIDAL informationSummary of products characteristics: metformin Available from: http://www.vidal.fr/recherche/index/q:METFORMINE/Accessed April 15, 2014
- DruetCBourdel-MarchassonIWeillAType 2 diabetes in France: epidemiology, trends of medical care, social and economic burdenPresse Med201342830838 French23566620
- BoueeSGaudinAFAmelineauEBonnetFHypoglycemic treatment in type 2 diabetes patients suffering from moderate to severe renal failure in France. Aim of the studyTherapie2013681926 French23484656
- GrandfilsNDetournayBAttaliCGlucose lowering therapeutic strategies for type 2 diabetic patients with chronic kidney disease in primary care setting in france: a cross-sectional studyInt J Endocrinol2013201364063223653644
- ShurrawSHemmelgarnBLinMAssociation between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort studyArch Intern Med20111711920192722123800
- HolsteinAStumvollMContraindications can damage your health – is metformin a case in point?Diabetologia2005482454245916283245
- NyeHJHerringtonWGMetformin: the safest hypoglycaemic agent in chronic kidney disease?Nephron Clin Pract2011118c380c38321325870
- KajbafFArnoutsPdeBMLalauJDMetformin therapy and kidney disease: a review of guidelines and proposals for metformin withdrawal around the worldPharmacoepidemiol Drug Saf2013221027103523960029
- RousselRTravertFPasquetBMetformin use and mortality among patients with diabetes and atherothrombosisArch Intern Med20101701892189921098347
- EkstromNSchiolerLSvenssonAMEffectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes RegisterBMJ Open201224pii: e001076
- SoliniAPennoGBonoraEDiverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicentre studyDiabetes Care201235114314922124714
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Diabetes Care2012351364137922517736
- GerichJEMeyerCWoerleHJStumvollMRenal gluconeogenesis: its importance in human glucose homeostasisDiabetes Care20012438239111213896
- DejagerSSchweizerAIncretin therapies in the management of patients with type 2 diabetes mellitus and renal impairmentHosp Pract (1995)20124072122615074
- HungAMRoumieCLGreevyRAComparative effectiveness of incident oral antidiabetic drugs on kidney functionKidney Int20128169870622258320
- PornetCBourdel-MarchassonILecomtePTrends in the quality of care for elderly people with type 2 diabetes: the need for improvements in safety and quality (the 2001 and 2007 ENTRED Surveys)Diabetes Metab20113715216121435929
- SoliniAPennoGBonoraEAge, renal dysfunction, cardiovascular disease, and antihyperglycemic treatment in type 2 diabetes mellitus: findings from the Renal Insufficiency and Cardiovascular Events Italian Multicenter StudyJ Am Geriatr Soc2013611253126123889588
- JohansenOEBirkelandKIDefining the role of repaglinide in the management of type 2 diabetes mellitus: a reviewAm J Cardiovasc Drugs2007731933517953471
- SchumacherSAbbasiIWeiseDSingle- and multiple-dose pharmacokinetics of repaglinide in patients with type 2 diabetes and renal impairmentEur J Clin Pharmacol20015714715211417447
- BolenSFeldmanLVassyJSystematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitusAnn Intern Med200714738639917638715
- PhungOJScholleJMTalwarMColemanCIEffect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetesJAMA20103031410141820388897
- HasslacherCSafety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal functionDiabetes Care20032688689112610054
- KothnyWShaoQGroopPHLukashevichVOne-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairmentDiabetes Obes Metab201214111032103922690943
- LukashevichVSchweizerAShaoQGroopPHKothnyWSafety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trialDiabetes Obes Metab20111394795421733061
- LukashevichVSchweizerAFoleyJEDickinsonSGroopPHKothnyWEfficacy of vildagliptin in combination with insulin in patients with type 2 diabetes and severe renal impairmentVasc Health Risk Manag20139212823378769
- SchweizerADejagerSExperience with vildagliptin in patients ≥75 years with type 2 diabetes and moderate or severe renal impairmentDiabetes Ther2013425726723821355
- Arjona FerreiraJCMarreMBarzilaiNEfficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiencyDiabetes Care2013361067107323248197
- ChanJCScottRArjona FerreiraJCSafety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiencyDiabetes Obes Metab20081054555518518892
- NowickiMRychlikIHallerHLong-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety studyInt J Clin Pract2011651230123921977965
- McGillJBSloanLNewmanJLong-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled studyDiabetes Care20133623724423033241
- GroopPHCooperMEPerkovicVEmserAWoerleHJvonEMLinagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunctionDiabetes Care2013363460346824026560
- HaluzikMFrolikJRychlikIRenal effects of DPP-4 inhibitors: a focus on microalbuminuriaInt J Endocrinol2013201389510224089613
- ManciaGFagardRGuidelines for the management of hypertension and target organ damageJ Hypertens2013312464246524220597
- KrauseTLovibondKCaulfieldMMcCormackTWilliamsBManagement of hypertension: summary of NICE guidanceBMJ2011343d489121868454
- de ZeeuwDThe end of dual therapy with renin-angiotensin-aldosterone system blockade?N Engl J Med20133691960196224206456
- DetournayBSimonDGuillausseauPJChronic kidney disease in type 2 diabetes patients in France: prevalence, influence of glycaemic control and implications for the pharmacological management of diabetesDiabetes Metab201238210211222252014