Abstract
Background
Recently, high-sensitive troponin (hsTrop) assays consistent with professional societies’ recommendations became available. We aimed to summarize the evidence on the diagnostic accuracy of hsTrop on presentation.
Methods
We searched electronic databases for studies evaluating the diagnostic accuracy of hsTrop in suspected acute coronary syndrome (ACS) patients. Random effect meta-analyses and meta-regression were performed. Primary and secondary analyses were restricted to studies using conventional Trop and hsTrop in the reference standard, respectively.
Results
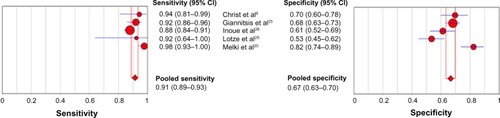
Fifteen studies with a total of 8,628 patients met the inclusion criteria for the primary analysis. hsTrop T (Hoffman-La Roche Ltd) and hsTrop I (Siemens) had sensitivities of 0.89 (95% confidence interval [CI]: 0.86–0.91) and 0.90 (95% CI: 0.87–0.92) and specificities of 0.79 (95% CI: 0.77–0.80) and 0.89 (95% CI: 0.87–0.90), respectively. There was no statistically significant difference in the area under the curve between hsTrop (95% CI: 0.920) and conventional Trop (95% CI: 0.929) at the 99th percentile (P=0.62). hsTrop at the level of detection had a sensitivity of 0.97 (95% CI: 0.96–0.98) and a specificity of 0.41 (95% CI: 0.40–0.42). The studies using a cut-off at coefficient of variance <10% as opposed to the 99th percentile for the conventional assay used for diagnosis reported higher diagnostic accuracy (relative diagnostic odds ratio =2.13, P=0.02). Five studies were included in the secondary analysis; hsTrop T (Hoffman-La Roche Ltd) had a sensitivity of 0.91 (95% CI: 0.89–0.93) and a specificity of 0.67 (95% CI: 0.63–0.70). There was significant heterogeneity among the studies.
Conclusion
hsTrop have excellent diagnostic accuracy for myocardial infarction on presentation, but may not outperform conventional Trop assays. The variation among the studies can be explained, in part, by the cut-off used for conventional Trop assays.
Background
Each year in the US, more than seven million people visit the emergency department with complaints of chest pain and related symptoms.Citation1 Many such patients require further evaluation including cardiac biomarkers. Cardiac troponins T or I (Trop T or I) are the preferred biomarkers for the evaluation of such patients with suspected acute coronary syndrome (ACS).Citation2 Trop T or I not only help in the rapid diagnosis of acute myocardial infarction (AMI), but also in risk stratification and selection of an appropriate treatment strategy. The European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation (ESC/ACC/AHA/WHF) task force consensus document recommends an assay-specific cut-off of 99th percentile of a normal healthy population as the decision level, in addition to a rise and fall pattern, for the diagnosis of AMI.Citation3,Citation4 In order to reliably detect rise and fall, assays with a coefficient of variation (CV) <10% at the 99th percentile are considered to have optimal precision.Citation4 Contemporary Trop assays lack such a precision at the 99th percentile, and therefore suffer from reduced sensitivity, especially during the early hours of AMI. To fulfill these criteria, sensitive or high-sensitive troponin (sTrop or hsTrop) assays with improved sensitivity and precision (CV <10% at the 99th percentile) have been developed. Initial studies have reported improved diagnostic accuracy of sTrop/hsTrop (s/hsTrop) assays on presentation to the emergency room at the recommended 99th percentile cut-off.Citation5,Citation6 Subsequent studies have proposed a novel cut-off at the level of detection (LOD) in order to achieve 100% sensitivity on the initial evaluation and rapidly rule out patients with symptoms suggestive of ACS.Citation7–Citation9 Although a test with high sensitivity is desired in the emergency room setting, the elevations in Trop, as measured by the s/hsTrop assays in conditions other than AMI, may significantly compromise its specificity. In order to synthesize the evidence on currently available s/hsTrop assays, we undertook a systematic review and meta-analysis to assess the diagnostic accuracy of s/hsTrop on initial presentation in patients with symptoms suggestive of ACS.
Methods
We systematically searched PubMed/MEDLINE and the EMBASE database for clinical studies evaluating the diagnostic accuracy of s/hsTrop assays in unselected patient populations suspected of ACS. The following keywords were used: “high-sensitive/sensitivity troponin”, “Roche troponin”, “Abbott troponin”, and “Siemens troponin”. The reference list of included studies and relevant review articles were hand-searched to identify additional studies. Citations with manuscripts published in peer-reviewed journals were included. Conference abstracts were excluded due to the inability to assess the relevant study characteristics. No language restrictions were imposed. The last search was performed on November 9, 2012.
For the purpose of this review, s/hsTrop assays were defined as assays with: 1) LOD <99th percentile of the healthy population and 2) CV ≤10% at the 99th percentile for the assay.
The following inclusion criteria were used: prospective, retrospective, or observational studies evaluating the diagnostic accuracy of s/hsTrop assays, as defined above, on initial presentation in unselected patients presenting with symptoms suggestive of ACS. In addition, the final diagnosis was adjudicated by a contemporary reference standard that comprised a review of the clinical data, serial Trop testing, and available supplementary investigations to diagnose or exclude AMI (or ACS) in accordance with the existing guidelines.
Exclusion criteria: studies only evaluating specific patients such as those with negative biomarkers on presentation, abnormal electrocardiogram, or undergoing invasive therapy were excluded. Also, studies using creatine kinase/creatine kinase myocardial band for the diagnosis of AMI were excluded.
Data extraction and synthesis
Two authors (AS and AB) independently assessed all studies for inclusion in the systematic review and meta-analysis. Data on study characteristics – mean/median age, % males, inclusion/exclusion criteria, and adjudication of diagnosis – were extracted from the included studies (Table S1). The enrollment period and study sites for the data collection were specifically noted to prevent duplication. In the presence of more than one study from the same site and enrollment period, the study with the higher number of patients and latest publication date was included. The studies reporting accuracy of more than one s/hsTrop assay were treated as separate datasets. Among other study characteristics, inclusion of patients with ST elevation myocardial infarction (STEMI), dialysis, and prevalence of coronary artery disease (CAD) were noted. If the prevalence of CAD was not reported, then the most prevalent surrogate, ie, angina, myocardial infarction, or coronary revascularization was used. The cut-off value used for both s/hsTrop and conventional Trop assays was noted. There was a provision of third author evaluation in the case of disagreement regarding study inclusion or extracted characteristics, but no significant discordance was encountered.
The primary quantitative analysis was restricted to studies evaluating diagnostic accuracy for AMI using conventional Trop testing in the reference standard. A systematic review of studies evaluating diagnostic accuracy for ACS instead of AMI was reported separately. A secondary quantitative and qualitative analysis was performed on the studies evaluating diagnostic accuracy of s/hsTrop for AMI using serial s/hsTrop testing instead of conventional Trop in the reference standard.
Statistical analysis
Absolute numbers of true-positive, true-negative, false-positive, and false-negative were extracted or calculated for the individual studies. From the extracted data pooled, sensitivity, specificity, and negative and positive likelihood ratio were calculated using the DerSimonian and Laird method (random effect model). As studies with the same diagnostic cut-off, ie, 99th percentile or LOD were used to calculate pooled estimates, threshold analysis was not undertaken.
Heterogeneity between studies was assessed using Cochran’s Q test and ICitation2 (Inconsistency index). The source of heterogeneity among the studies was explored by performing a meta-regression of study characteristics on the diagnostic odds ratio (DOR) by using the restricted maximum likelihood method weighted by the inverse of study variance and inclusion of threshold effect in the model.
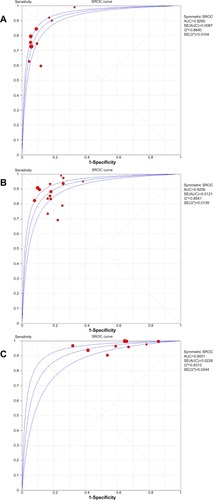
The summary receiver operating curve (SROC) was estimated with the area under the curve (AUC) as the measure of diagnostic accuracy. An AUC of 0.5 indicated poor discrimination, whereas value of 1 suggested perfect discrimination between those with and without disease. The curve was plotted on the basis of change in DOR using the DerSimonian and Laird method. The areas under the curve were compared using two-tailed t-tests. A P-value <0.05 was considered statistically significant. Statistical analysis was performed using Review Manager (RevMan; v5.2; The Nordic Cochrane Centre, Copenhagen, Denmark) and Meta-DiSc (v1.4; Clinical Biostatistics Unit, Ramon y Cajal Hospital, Madrid, Spain).Citation10
The Review Manager was used for quality assessment of included studies based on the QUADAS (Quality Assessment tool for Diagnostic Accuracy Studies) questionnaire.
Results
Fifteen studies with a total of 8,628 patients met the inclusion criteria for the primary analysis as shown in – eight studies with 3,115 patients used hsTrop T, six studies with 4,415 patients used s/hsTrop I, and one study with 1,098 patients used both hsTrop T and sTrop I. Among these studies, ten studies also reported on the diagnostic accuracy of conventional Trop assays on initial presentation at a cut-off of 99th percentile. The mean/median age was 54–67.6 years, and 49.2%–71.3% patients were male. Other relevant study characteristics are shown in Table S1. All hsTrop T studies used the assay manufactured by Roche (Roche High-Sensitive Troponin T; Hoffman-La Roche Ltd, Basel, Switzerland). Out of the seven studies on s/hsTrop I, four used Trop I Ultra (Siemens Healthcare Diagnostics, Erlangen, Germany), one used Architect STAT High-Sensitive Trop (Abbott Laboratories, Abbott Park, IL, USA), one used VITROS Trop I assay (Ortho-Clinical Diagnostics; Johnson & Johnson, New Brunswick, NJ, USA), and one used Singulex Erenna Trop I (Alameda, CA, USA). Two additional studies that reported diagnostic accuracy of s/hsTrop for ACS only were identified (Table S1). Seven studies reported diagnostic accuracy of hsTrop using serial s/hsTrop assays in the reference standard and were included in the secondary analysis (Table S2).
Figure 1 Study selection flow chart.
hsTrop T
Eight out of the nine studies reported diagnostic accuracy of hsTrop T on presentation at a 99th percentile cut-off of a healthy population. Kurz et alCitation19 used a different diagnostic cut-off for hsTrop T, and therefore, was not included in the calculation of pooled point estimates. The pooled sensitivity and specificity were 0.885 (95% CI: 0.863–0.905, χ2=32.87, P≤0.001, ICitation2=78.7%) and 0.783 (95% CI: 0.768–0.797, χ2=47.5, P<0.001, ICitation2=85.3%), respectively, as shown in . The pooled positive and negative likelihood ratios were 3.999 (95% CI: 3.360–4.760, χ2=38.6, P<0.001, ICitation2=81.9%) and 0.137 (95% CI: 0.092–0.205, χ2=27.82, P<0.001, ICitation2=74.8%), respectively (). There was significant heterogeneity among the studies.
Figure 2 Forest plots showing pooled sensitivities and specificities.
Abbreviation: CI, confidence interval.
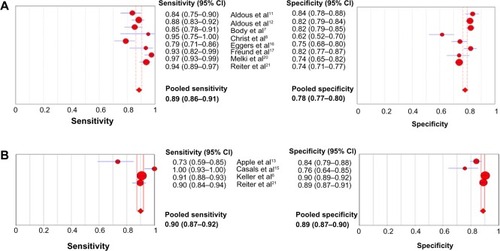
Table 1 (A) Pooled estimates of diagnostic accuracy for myocardial infarction of studies included in primary analysis; (B) results of meta-regression analysis of study level covariates on diagnostic odds ratio
s/hsTrop I
The assay types were more variable in the s/hsTrop I studies. Therefore, in addition to cumulative point estimates, a subgroup analysis of studies using a sensitive assay – Siemens Trop I Ultra – was performed. The pooled sensitivity and specificity of all s/hsTrop I studies at a 99th percentile cutoff were 0.867 (95% CI: 0.845–0.887, χ2=43.46, P<0.001, ICitation2=86.2%) and 0.879 (95% CI: 0.869–0.888, χ2=80.34, P<0.001, ICitation2=92.5%), respectively. The pooled sensitivity and specificity of the studies using Siemens trop I Ultra were 0.899 (95% CI: 0.874–0.886, χ2=21.62, P<0.001, ICitation2=86.1%) and 0.886 (95% CI: 0.874–0.898, χ2=19.26, P<0.001, ICitation2=84.4%), respectively, as shown in and . The pooled positive and negative likelihood ratios for this subgroup were 6.30 (95% CI: 4.400–9.022, χ2=27, P<0.001, ICitation2= 88.9%) and 0.135 (95% CI: 0.067–0.269, χ2=20.37, P<0.001, ICitation2=85.3%), respectively, as shown in . There was significant heterogeneity among the studies for all pooled estimates.
s/hsTrop at the LOD
Nine studies/substudies reported diagnostic accuracy of s/hsTrop at the cut-off of LOD on presentation. Reiter et alCitation21 did not report diagnostic accuracy at LOD. However, a prior publication from the same cohort did,Citation6 and was included in the current analysis. The pooled sensitivity and specificity were 0.974 (95% CI: 0.963–0.983, χ2=44.4, P<0.001, ICitation2=82%) and 0.410 (95% CI: 0.396–0.424, χ2=533.2, P<0.001, ICitation2= 98.5%), respectively. The pooled positive and negative likelihood ratios were 1.646 (95% CI: 1.337–2.027, χ2=609.87, P<0.001, ICitation2= 98.7%) and 0.079 (95% CI: 0.042–0.148, χ2=17.45, P=0.026, ICitation2= 54.1%), respectively ().
Effect of study covariates
To explore the heterogeneity among the studies, meta-regression analysis of four study level covariates: inclusion of patients with STEMI and dialysis, prevalence of CAD, and cut-off of conventional assays used to diagnose AMIs on the DOR was performed. There was no association between diagnostic accuracy and the other three study characteristics, but use of a cut-off CV <10% for the conventional assay instead of the 99th percentile significantly improved the diagnostic accuracy of s/hsTrop on presentation as shown in .
Comparison of conventional and hsTrop
Among the included studies, ten studies reported the diagnostic accuracy of conventional trop assays on presentation. The SROC of conventional Trop assays, s/hsTrop assays at a cut-off of 99th percentile, and the LOD is shown in . There was no statistically significant difference between the AUC of conventional Trop and s/hsTrop at two different cut-offs.
Figure 3 Summary receiver operating plots: (A) conventional troponin, (B) sensitive/high-sensitive troponin at 99th percentile cut-off, and (C) high-sensitive troponin at the level of detection.
Abbreviations: AUC, area under the curve; SE, standard error; SROC, summary receiver operating curve.
ACS
Two studies not included in the primary analysis reported diagnostic accuracy for ACS only (Table S1). Also, Keller et alCitation18 and Reiter et alCitation21 reported diagnostic accuracy for ACS in addition to AMI. As shown in Figure S1, the sensitivity ranged 0.56–0.77 and the specificity ranged 0.76–0.94.
s/hsTrop for adjudication of diagnosis
Seven additional studies that used serial s/hsTrop testing in the reference standard, instead of conventional Trop, to diagnosis the AMI were identified (Table S2). In addition to conventional assays, Christ et alCitation8 and Melki et alCitation20 reported diagnostic accuracy on presentation using serial hsTrop T testing to diagnose AMI. Clearly, the outcomes of these studies suffer from incorporation bias. Out of these nine studies, seven used hsTrop T (Hoffman-La Roche Ltd) and two used trop I Ultra (Siemens Healthcare Diagnostics). The studies were very heterogeneous in terms of inclusion and exclusion criteria, as well as diagnostic cut-off. KellyCitation27 and Khan et alCitation28 excluded patients with any alternate diagnosis and Scharnhorst et alCitation31 used clinical diagnosis to adjudicate the AMI. The pooled sensitivity and specificity for hsTrop T (Hoffman-La Roche Ltd) on admission, after exclusion of data from Khan et al, were 0.91 (95% CI: 0.89–0.93) and 0.67 (0.63–0.70), respectively, as shown in .
Quality assessment
The methodological quality summary based on the QUADAS questionnaire is shown in Figure S2. The majority of the studies were thought to have included a representative spectrum of patients presenting to the emergency department or admitted to the hospital, except for the studies by Aldous et al,Citation12 Bhardwaj et al,Citation23 Casals et al,Citation15 and Schreiber et al,Citation22 because of the use of a convenience sample, incompletely defined inclusion criteria, or unrealistic exclusion criteria. The index test was not blinded in the study by Christ et al.Citation8 No studies reported intermediate or uninterpretable test results.
Discussion
The introduction of s/hsTrop assays with enhanced analytic performance, consistent with the recommendations of the ESC/ACCF/AHA/WHF consensus statement, is considered a major advancement in cardiovascular medicine. These assays are being used in many parts of the world, and are awaiting approval in the US and recently became approved for use in Canada. There is no standardized definition for the term hsTrop assays in the current literature, and it has been used for assays with variable properties. Most experts advocate the use of the term “hsTrop assays” for the assays that detect cardiac Trop in the majority of the reference population.Citation32 Optimal precision, ie, CV <10% at the 99th percentile as defined by the ESC/ACCF/WHF consensus document is another desired characteristic feature of hsTrop assays. These features make s/hsTrop assays more sensitive compared to conventional Trop assays, especially near the upper reference limit. In the absence of a widely accepted definition of high-sensitive assays, we included studies evaluating assays with LOD at <99th percentile and CV ≤10% at the 99th percentile to be consistent with the ESC/AHA/ACCF/WHF consensus statement.
The 99th percentile is the cut-off recommended by the Joint ESC/ACCF/AHA/WHF task force as the decision limit, in addition to a rise and fall pattern, for the diagnosis of AMI. Similar to clinical practice, this decision limit is not consistently used for conventional Trop assays by contemporary studies evaluating the accuracy of s/hsTrop for the diagnosis of AMI.Citation5,Citation6 As pointed out previously,Citation32 we found that studies using a cut-off of CV <10% (which is achieved at a level greater than the 99th percentile for all of the included conventional assays) instead of the 99th percentile, reported a higher diagnostic accuracy for s/hsTrop assays at presentation, as reflected by a relative diagnostic odds ratio of 2.15 (). Although DOR does not allow separate weighing of true and false positive rates, it appears logical that the use of a cut-off higher than the 99th percentile will decrease the true positive rate for AMI; this may therefore enhance the diagnostic accuracy of s/hsTrop on presentation. Further, comparing the AUC of SROC for s/hsTrop and conventional Trop on presentation at the 99th percentile cutoff failed to show any difference in supporting the current recommendation of using the 99th percentile, instead of at a CV level of <10%, as the decision limit.
Some authors have suggested that using a novel cut-off at the LOD for hsTrop on presentation can be an effective rapid rule out strategy, which is often desired in the emergency room setting.Citation7,Citation8 As shown in , at this novel cut-off, s/hsTrop assays provide an excellent sensitivity of 0.97. The negative predictive value will vary by the disease prevalence, but in general, may not reduce the historically reported rate of missed diagnosis of about 2%.Citation33 Also, it is important to note that excellent sensitivity for the diagnosis of AMI may not be used to rule out ACS. Although no data was available for the novel cut-off, at the 99th percentile, s/hsTrop assays had a sensitivity ranging from 0.56–0.77; therefore, it certainly cannot be used as rapid rule out strategy underscoring the importance of complete clinical evaluation of the individual patient.
Evaluation of more sensitive assays against less sensitive reference standards is one of the limitations of the studies included in the primary analysis, as it may potentially inflate sensitivity with apparent loss of specificity. As Trop may be detected with s/hsTrop assays in some patients diagnosed as having unstable angina using conventional Trop assays, it may be useful to evaluate specificity for ACS rather than AMI. The specificity of s/hsTrop assays for ACS ranged 0.76–0.90 (Figure S1), which may not be an improvement over specificity for AMI (). Furthermore, a systematic review of studies using s/hsTrop assays in the reference standard for the diagnosis of AMI (Table S2) shows that studies using very stringent exclusion criteria, ie, excluding all alternate diagnoses, cardiomyopathies, heart failure, etc, may achieve a specificity exceeding 0.9. However, the studies with a more representative spectrum of the patient population using contemporary diagnostic methods have lower specificity (). Prior studies have shown that ACS patients testing positive for Trop by conventional assays derive additional benefit from glycoprotein IIb/IIIA inhibitors and anticoagulant therapies.Citation34,Citation35 Similarly, these patients appear to benefit from invasive therapy as opposed to conservative management.Citation36 However, it remains unknown if ACS patients testing positive with hsTrop assays but negative with conventional Trop assays will benefit from these therapies.
s/hsTrop assays may detect elevation greater than at the 99th percentile in patients with non-ACS conditions including stable CAD and heart failure.Citation37–Citation39 Therefore, one of other major concerns with hsTrop assays is the false positive rates, which exceed 30% even when hsTrop assays were used in the reference standard (). It is likely these patients who tested positive for elevated s/hsTrop in routine clinical practice will undergo additional testing that may not have been otherwise warranted. The cost incurred by this undesirable consequence of s/hsTrop assay use needs to be evaluated in future studies.
Limitations
This was a study level meta-analysis with significant heterogeneity among the studies not completely explained by meta-regression analysis. The power to detect statistically significant relationships between study level covariates such as the prevalence of CAD and inclusion of dialysis and diagnostic accuracy of s/hsTrop was limited due to the relatively small number of studies. The effect of time elapsed between symptom onset to presentation and diagnostic accuracy could not be explored in the present analysis.
Conclusion
s/hsTrop assays have excellent diagnostic accuracy for AMI on initial presentation at the currently recommended cut-off of 99th percentile. At a cut-off of LOD, s/hsTrop provides excellent sensitivity for AMI but may not be ideal for risk-free rapid exclusion for ACS. There is no conclusive evidence that s/hsTrop assays outperform conventional Trop assays when a cut-off of 99th percentile is used for the latter. Studies evaluating clinical endpoints and cost-effectiveness are needed before accepting these assays in routine clinical practice.
Supplementary materials
Figure S1 Summary plot of individual studies reporting sensitivity and specificity for high-sensitive troponin assays at presentation for acute coronary syndrome.
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; TN, true negative; TP, true positive.
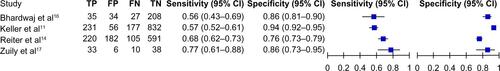
Figure S2 QUADAS analysis of methodological quality of included studies.
Abbreviation: QUADAS, Quality Assessment tool for Diagnostic Accuracy Studies.
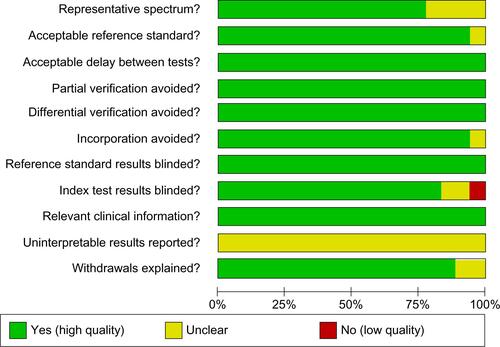
Table S1 Characteristics of studies reporting diagnostic accuracy of high-sensitive troponin assays using conventional troponin in the reference standard
Table S2 Characteristics of studies reporting diagnostic accuracy of high-sensitive troponin assays using high-sensitive troponin in the reference standard
References
- AldousSJFlorkowskiCMCrozierIGComparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency departmentAnn Clin Biochem201148Pt 324124821441390
- AldousSJRichardsMCullenLTroughtonRThanMDiagnostic and prognostic utility of early measurement with high-sensitivity troponin T assay in patients presenting with chest painCMAJ20121845E260E26822291171
- AppleFSSmithSWPearceLALerRMurakamiMUse of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndromeClin Chem200854472372818238833
- AppleFSPearceLASmithSWKaczmarekJMMurakamiMMRole of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse eventsClin Chem200955593093719299542
- BodyRCarleySMcDowellGRapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assayJ Am Coll Cardiol201158131332133921920261
- CasalsGFilellaXBediniJLEvaluation of a new ultrasensitive assay for cardiac troponin IClin Biochem200740181406141317942088
- ChristMPoppSPohlmannHImplementation of high sensitivity cardiac troponin T measurement in the emergency departmentAm J Med2010123121134114220932502
- EggersKMVengePLindahlBHigh-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest painClin Chim Acta201241313–141135114022456003
- FreundYChenevier-GobeauxCBonnetPHigh-sensitivity versus conventional troponin in the emergency department for the diagnosis of acute myocardial infarctionCrit Care2011153R14721663627
- KellerTZellerTPeetzDSensitive troponin I assay in early diagnosis of acute myocardial infarctionN Engl J Med2009361986887719710485
- KellerTZellerTOjedaFSerial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarctionJAMA2011306242684269322203537
- KurzKGiannitsisEBeckerMHessGZdunekDKatusHAComparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndromeClin Res Cardiol2011100320921520852870
- MelkiDLindSAgewallSJernbergTDiagnostic value of high sensitive troponin T in chest pain patients with no persistent ST-elevationsScand Cardiovasc J201145419820421428843
- ReiterMTwerenboldRReichlinTEarly diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assaysEur Heart J201233898899722044927
- SchreiberDHAgboCWuAHShort-term (90 min) diagnostic performance for acute non-ST segment elevation myocardial infarction and 30-day prognostic evaluation of a novel third-generation high sensitivity troponin I assayClin Biochem20124516–171295130122705845
- BhardwajATruongQAPeacockWFA multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency departmentAm Heart J20111622276282. e121835288
- ZuilySChenevier-GobeauxCClaessensYEWahbiKWeberSMeuneCHigh diagnostic performance of a high-sensitivity cardiac troponin T assay in patients with suspected acute coronary syndromeInt J Cardiol2011146111511620965598
- ThygesenKAlpertJSWhiteHDJoint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial InfarctionUniversal definition of myocardial infarctionJ Am Coll Cardiol200750222173219518036459
- WrightRSAndersonJLAdamsCD2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsJ Am Coll Cardiol201157191920195921450428
- ReichlinTHochholzerWBassettiSEarly diagnosis of myocardial infarction with sensitive cardiac troponin assaysN Engl J Med2009361985886719710484
- GiannitsisEKehayovaTVafaieMKatusHACombined testing of high-sensitivity troponin T and copeptin on presentation at prespecified cutoffs improves rapid rule-out of non-ST-segment elevation myocardial infarctionClin Chem201157101452145521807867
- InoueKSuwaSOhtaHHeart fatty acid-binding protein offers similar diagnostic performance to high-sensitivity troponin T in emergency room patients presenting with chest painCirc J201175122813282021937835
- KellyAMPerformance of sensitive troponin assay in the early diagnosis of acute myocardial infarction in the emergency departmentEmerg Med Australas201123218118521489165
- KhanDASharifMSKhanFADiagnostic performance of high-sensitivity troponin T, myeloperoxidase, and pregnancy-associated plasma protein A assays for triage of patients with acute myocardial infarctionKorean J Lab Med201131317217821779191
- LotzeULemmHHeyerAMüllerKCombined determination of highly sensitive Troponin T and copeptin for early exclusion of acute myocardial infarction: first experience in an emergency department of a general hospitalVasc Health Risk Manag2011750951521915168
- OlivieriFGaleazziRGiavarinaDAged-related increase of high sensitive Troponin T and its implication in acute myocardial infarction diagnosis of elderly patientsMech Ageing Dev2012133530030522446505
- ScharnhorstVKrasznaiKvan’t VeerMVMichelsRRapid detection of myocardial infarction with a sensitive troponin testAm J Clin Pathol2011135342442821350097
Disclosure
The authors report no conflicts of interest in this work.
References
- Centers for Disease Control and PreventionNational Hospital Ambulatory Medical Care Survey: 2009 Emergency Department Summary TablesAtlanta, GACenters for Disease Control and Prevention Available from: http://www.cdc.gov/nchs/fastats/ervisits.htmAccessed May 19, 2014
- WrightRSAndersonJLAdamsCD2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsJ Am Coll Cardiol201157191920195921450428
- ThygesenKAlpertJSWhiteHDJoint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial InfarctionUniversal definition of myocardial infarctionJ Am Coll Cardiol200750222173219518036459
- ThygesenKAlpertJSJaffeASJoint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial InfarctionThird universal definition of myocardial infarctionCirculation2012126162020203522923432
- Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarctionEur Heart J200021181502151310973764
- KellerTZellerTPeetzDSensitive troponin I assay in early diagnosis of acute myocardial infarctionN Engl J Med2009361986887719710485
- BodyRCarleySMcDowellGRapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assayJ Am Coll Cardiol201158131332133921920261
- ChristMPoppSPohlmannHImplementation of high sensitivity cardiac troponin T measurement in the emergency departmentAm J Med2010123121134114220932502
- ReichlinTHochholzerWBassettiSEarly diagnosis of myocardial infarction with sensitive cardiac troponin assaysN Engl J Med2009361985886719710484
- ZamoraJAbrairaVMurielAKhanKSCoomarasamyAMeta-DiSc: a software for meta-analysis of test accuracy dataBMC Med Res Method2006631
- AldousSJFlorkowskiCMCrozierIGComparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency departmentAnn Clin Biochem201148Pt 324124821441390
- AldousSJRichardsMCullenLTroughtonRThanMDiagnostic and prognostic utility of early measurement with high-sensitivity troponin T assay in patients presenting with chest painCMAJ20121845E260E26822291171
- AppleFSSmithSWPearceLALerRMurakamiMUse of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndromeClin Chem200854472372818238833
- AppleFSPearceLASmithSWKaczmarekJMMurakamiMMRole of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse eventsClin Chem200955593093719299542
- CasalsGFilellaXBediniJLEvaluation of a new ultrasensitive assay for cardiac troponin IClin Biochem200740181406141317942088
- EggersKMVengePLindahlBHigh-sensitive cardiac troponin T outperforms novel diagnostic biomarkers in patients with acute chest painClin Chim Acta201241313–141135114022456003
- FreundYChenevier-GobeauxCBonnetPHigh-sensitivity versus conventional troponin in the emergency department for the diagnosis of acute myocardial infarctionCrit Care2011153R14721663627
- KellerTZellerTOjedaFSerial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarctionJAMA2011306242684269322203537
- KurzKGiannitsisEBeckerMHessGZdunekDKatusHAComparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndromeClin Res Cardiol2011100320921520852870
- MelkiDLindSAgewallSJernbergTDiagnostic value of high sensitive troponin T in chest pain patients with no persistent ST-elevationsScand Cardiovasc J201145419820421428843
- ReiterMTwerenboldRReichlinTEarly diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assaysEur Heart J201233898899722044927
- SchreiberDHAgboCWuAHShort-term (90 min) diagnostic performance for acute non-ST segment elevation myocardial infarction and 30-day prognostic evaluation of a novel third-generation high sensitivity troponin I assayClin Biochem20124516–171295130122705845
- BhardwajATruongQAPeacockWFA multicenter comparison of established and emerging cardiac biomarkers for the diagnostic evaluation of chest pain in the emergency departmentAm Heart J20111622276282. e121835288
- ZuilySChenevier-GobeauxCClaessensYEWahbiKWeberSMeuneCHigh diagnostic performance of a high-sensitivity cardiac troponin T assay in patients with suspected acute coronary syndromeInt J Cardiol2011146111511620965598
- GiannitsisEKehayovaTVafaieMKatusHACombined testing of high-sensitivity troponin T and copeptin on presentation at prespecified cutoffs improves rapid rule-out of non-ST-segment elevation myocardial infarctionClin Chem201157101452145521807867
- InoueKSuwaSOhtaHHeart fatty acid-binding protein offers similar diagnostic performance to high-sensitivity troponin T in emergency room patients presenting with chest painCirc J201175122813282021937835
- KellyAMPerformance of sensitive troponin assay in the early diagnosis of acute myocardial infarction in the emergency departmentEmerg Med Australas201123218118521489165
- KhanDASharifMSKhanFADiagnostic performance of high-sensitivity troponin T, myeloperoxidase, and pregnancy-associated plasma protein A assays for triage of patients with acute myocardial infarctionKorean J Lab Med201131317217821779191
- LotzeULemmHHeyerAMüllerKCombined determination of highly sensitive Troponin T and copeptin for early exclusion of acute myocardial infarction: first experience in an emergency department of a general hospitalVasc Health Risk Manag2011750951521915168
- OlivieriFGaleazziRGiavarinaDAged-related increase of high sensitive Troponin T and its implication in acute myocardial infarction diagnosis of elderly patientsMech Ageing Dev2012133530030522446505
- ScharnhorstVKrasznaiKvan’t VeerMVMichelsRRapid detection of myocardial infarction with a sensitive troponin testAm J Clin Pathol2011135342442821350097
- ThygesenKMairJGiannitsisEStudy Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac CareHow to use high-sensitivity cardiac troponins in acute cardiac careEur Heart J201233182252225722723599
- PopeJHAufderheideTPRuthazerRMissed diagnoses of acute cardiac ischemia in the emergency departmentN Engl J Med2000342161163117010770981
- HammCWHeeschenCGoldmannBBenefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study InvestigatorsN Engl J Med1999340211623162910341274
- LindahlBVengePWallentinLTroponin T identifies patients with unstable coronary artery disease who benefit from long-term anti-thrombotic protection. Fragmin in Unstable Coronary Artery Disease (FRISC) Study GroupJ Am Coll Cardiol199729143488996293
- KleimanNSLakkisNCannonCPTACTICS-TIMI 18 InvestigatorsProspective analysis of creatine kinase muscle-brain fraction and comparison with troponin T to predict cardiac risk and benefit of an invasive strategy in patients with non-ST-elevation acute coronary syndromesJ Am Coll Cardiol20024061044105012354426
- KorosoglouGLehrkeSMuellerDDeterminants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaqueHeart2011971082383120884786
- NdrepepaGBraunSMehilliJPrognostic value of sensitive troponin T in patients with stable and unstable angina and undetectable conventional troponinAm Heart J20111611687521167336
- LatiniRMassonSAnandISVal-HeFT InvestigatorsPrognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failureCirculation2007116111242124917698733