Abstract
Diabetic patients with hypertension are approximately twice as likely to develop cardiovascular disease as non-diabetic patients with hypertension. Given that hypertension affects ∼60% of patients with diabetes, effective blood pressure (BP) management is important in this high-risk population. This post-hoc analysis pooled data from six clinical studies to quantify additional BP efficacy achieved when titrating hypertensive diabetic patients from amlodipine 5 mg to 10 mg. Approximately half of the diabetic patients were male (44/98; 44.9%) with a mean (standard deviation [SD]) age of 60.6 (9.6) years and a baseline mean (standard error [SE]) systolic blood pressure/diastolic blood pressure (SBP/DBP) of 150.8 (1.30)/87.5 (0.94) mmHg while on amlodipine 5 mg (159.1 [1.40]/92.6 [0.94] mmHg prior to treatment). In comparison, 350/610 (57.4%) non-diabetic patients were male with a mean (SD) age of 58.7 (11.1) years and baseline mean (SE) SBP/DBP of 150.3 (0.62)/90.9 (0.41) mmHg while on amlodipine 5 mg (160.0 [0.67]/96.2 [0.45] mmHg prior to treatment). Increasing amlodipine from 5 mg to 10 mg lowered sitting SBP by −12.5 mmHg (95% confidence interval (CI): −15.5, −9.5; P<0.0001) and DBP by −6.0 mmHg (−7.4, −4.6; P<0.0001) in diabetic patients; and SBP by −12.4 mmHg (−13.5, −11.3; P<0.0001) and DBP by −7.3 mmHg (−8.0, −6.7; P<0.0001) in non-diabetic patients. In total, 12.0% (95% CI: 6.4, 20.0) of diabetic patients achieved their BP goal versus 46.4% (42.4, 50.4) of non-diabetic patients after titration to amlodipine 10 mg. Overall, 22.0% of diabetic patients experienced 31 adverse events (AEs) and 28.9% of non-diabetic patients experienced 282 AEs. Serious AEs were reported by one (1.0%) diabetic and five (0.8%) non-diabetic patients. In this analysis, increasing amlodipine from 5 mg to 10 mg produced a clinically significant reduction in the BP of diabetic hypertensive patients, similar to non-diabetic patients, highlighting the importance of optimizing amlodipine titration in this high-risk population.
Background
Hypertension and diabetes each represent a major public health challenge worldwide. They frequently co-exist, thus compounding the risk of vascular morbidity and mortality associated with these conditions individually. The worldwide prevalence of hypertension in the year 2000 was estimated to be 26.4% (972 million adults), with a predicted rise of approximately 60% to a total of 1.56 billion adults (29.2%) by 2025.Citation1 Recent estimates put global diabetes prevalence in the year 2011 at 8.3% (366 million adults), increasing to 9.9% (552 million adults; approximately 50% rise) by 2030.Citation2
Higher levels of blood pressure (BP) are a powerful predictor of cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke.Citation3,Citation4 Hypertension affects approximately 60% of patients with diabetes,Citation5 and is twice as likely to occur in diabetic patients compared with their age-matched non-diabetic counterparts.Citation6 Compared with diabetic individuals who are normotensive, those with concomitant hypertension exhibit about a two-fold increase in the risk of cardiovascular events and death.Citation7–Citation9 Moreover, hypertension has been found to be a stronger predictor of cardiovascular events as compared with diabetes.Citation9
Given the high prevalence of hypertension in diabetic patients, combined with the observed increased cardiovascular risk attributable to hypertension, appropriate BP management strategies should be employed to mitigate the risk of future CVD events. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) advocate a sitting BP goal of 140/90 mmHg for hypertensive patients with diabetes and/or chronic kidney disease patients.Citation10 Similarly, the American Diabetes Association (ADA) guidelines and joint guidelines from the European Society of Hypertension (ESH)/European Society of Cardiology (ESC) recommend a BP goal of <140/80 mmHgCitation11 and <140/85 mmHg,Citation12 respectively, for patients with diabetes. The caveat is that a lower systolic blood pressure (SBP) target (ie, <130 mmHg) may be appropriate for some diabetic individuals, such as younger patients or those at higher risk of stroke, if it can be achieved with fewer drugs and without adverse effects.Citation11 A similarly revised BP goal of <140/85 mmHg has been proposed for those with diabetes in the latest joint guidelines for hypertension management from the ESH/ESC.Citation12 However, effective therapeutic control of BP in patients with concomitant diabetes and hypertension can be challenging, and many patients are not achieving desired BP goals despite antihypertensive medication use.Citation13–Citation18
Antihypertensive regimens based on angiotensin-converting-enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), calcium channel blockers (CCBs), and diuretics/beta-blockers are all broadly comparable for reducing cardiovascular risk in patients with and without diabetes.Citation19 Amlodipine (Pfizer Inc., New York, NY, USA) is a well-established, long-acting CCB for the treatment of hypertension, including patients with diabetes.Citation20–Citation22 Studies in hypertensive patients have highlighted the additional benefit of titrating amlodipine from 5 mg to 10 mg once daily with regard to lowering BP,Citation23–Citation27 including Asian patients.Citation26 However, because the efficacy of increasing amlodipine dose from 5 mg to 10 mg has thus far only been evaluated in a small number of patients with hypertension and diabetes, we conducted this post-hoc analysis of 710 patients with known diabetic status from six clinical trials, which included 100 diabetic patients. The objective of this analysis was to assess the incremental effect of amlodipine titration to 10 mg daily on BP-lowering efficacy in patients with concomitant diabetes and hypertension who had not responded sufficiently to treatment with the 5 mg dose.
Methods
Study selection criteria
Studies selected for this analysis were all Pfizer-sponsored studies of amlodipine treatment for mild to moderate hypertension for which there was patient-level data, diabetic status was known, and dose was titrated from 5 mg to 10 mg.
Study design and participants
This post-hoc analysis used pooled data from six randomized controlled or open-label clinical studies assessing the efficacy and safety of amlodipine 5 mg daily titrated to 10 mg daily in patients with mild or moderate hypertension (). Although total study durations varied depending on the design, patients in this analysis received amlodipine at a dose of 5 mg daily for 4–8 weeks followed by titration up to 10 mg daily as required for an additional 4–8 weeks. Patients were stratified into two subgroups based on their diabetes status at the time of entry into the study (those with diabetes versus those without diabetes), derived from their medical history.
Table 1 Studies used in the pooled analysisTable Footnotea
Study endpoints and assessments
Efficacy endpoints were change from baseline in sitting SBP and diastolic blood pressure (DBP) to the specified time point, and proportion of patients who achieved the sitting BP goal at follow-up. Depending on the study, sitting BP was measured once or twice at each visit. Each patient’s response for a given visit was the average of the multiple measurements, or the single measurement if only one sitting BP was available. The sitting BP goal was <130/80 mmHg for patients with diabetes and <140/90 mmHg for patients without diabetes. The BP goal was also assessed according to recently revised guidelines of a sitting BP goal of <140/80 mmHg (ADA guidelines)Citation11 and a sitting BP goal for age <60 years, 140/90 mmHg; age ≥60 years, 150/90 mmHg (JNC 8 guidelines)Citation10 for both subgroups.
Safety endpoints were frequency of treatment-emergent adverse events (AEs) (all causality), treatment-emergent serious AEs (all causality), and AEs resulting in withdrawal from treatment (all causality).
Statistical analyses
Patients without a diabetes status at study entry were excluded from the analysis. The efficacy analysis included all patients in the intent-to-treat population who received at least one dose of amlodipine, were titrated from 5 mg to 10 mg daily, and had both a baseline and at least one follow-up BP measurement (last observation carried forward analysis). The safety analysis included all patients who received a least one dose of amlodipine and were titrated from 5 mg to 10 mg daily.
Baseline values were established while patients were on amlodipine 5 mg treatment (ie, prior to amlodipine titration). Baseline characteristics were compared between the two subgroups using analysis of variance for numeric variables with study and subgroup in the model, and using the Cochran–Mantel–Haenszel statistic stratified by study for dichotomous variables. Blood pressure data were analyzed using descriptive statistics, paired Student’s t-tests, and corresponding confidence intervals (CIs). An exact CI based on the binomial distribution was used for the proportion of responders. The proportion of common AEs was compared between subgroups using the Cochran–Mantel–Haenszel statistic stratified by study. Given the sparseness of events across studies, a corresponding unconditional exact CI was calculated on the pooled data using the statistical software StatXact Version 10 by Cytel (Cambridge, MA, USA).Citation28 All tests used an alpha of 0.05 with corresponding 95% CIs.
Results
Study population
A total of 710 patients with known diabetic status were included in this analysis (693 with complete BP data). Of the 100 patients (14.1%) with diabetes at study entry, four patients (4.0%) discontinued from the study; all four discontinuations were due to AEs. Of the 610 patients (85.9%) without diabetes at study entry, 44 patients (7.2%) discontinued from the study. Reasons for discontinuation among non-diabetic patients included: AEs (n=33; 5.4%), no longer willing to participate (n=4; 0.7%), protocol violation (n=2; 0.3%), insufficient clinical response (n=1; 0.2%), and other reason (n=4; 0.7%).
Patients with diabetes were mostly female (55.1%) with a mean (standard deviation [SD]) age of 60.6 (9.6) years and a baseline mean (standard error [SE]) SBP/DBP prior to amlodipine treatment of 159.1 (1.40)/92.6 (0.94) mmHg (); mean (SE) SBP/DBP was 150.8 (1.30)/87.5 (0.94) mmHg while on amlodipine 5 mg treatment. Patients without diabetes were mostly male (57.4%) with a mean (SD) age of 58.7 (11.1) years and a baseline mean (SE) SBP/DBP prior to amlodipine treatment of 160.0 (0.67)/96.2 (0.45) mmHg (); mean (SE) SBP/DBP was 150.3 (0.62)/90.9 (0.41) mmHg while on amlodipine 5 mg treatment. Patients with diabetes weighed more and had a higher body mass index than their non-diabetic counterparts ().
Table 2 Baseline characteristics of the study population stratified by diabetes status
Blood pressure levels and goal attainment
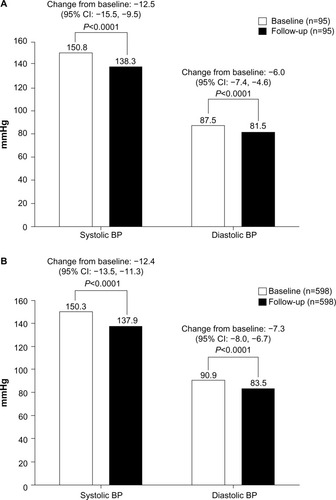
In patients with diabetes, titration of amlodipine 5 mg to 10 mg lowered sitting SBP by –12.5 mmHg (95% CI: −15.5, −9.5; P<0.0001) and DBP by −6.0 mmHg (95% CI: −7.4, −4.6; P<0.0001) from baseline (established while patients were on amlodipine 5 mg treatment) to follow-up (). Mean (SE) SBP/DBP at follow-up in diabetic patients was 138.3 (1.35)/81.5 (0.86) mmHg. Similarly, in patients without diabetes, titration to amlodipine 10 mg lowered sitting SBP by −12.4 mmHg (95% CI: −13.5, −11.3; P<0.0001) and DBP by −7.3 mmHg (95% CI: −8.0, −6.7; P<0.0001) from baseline (established on amlodipine 5 mg treatment) to follow-up (). Mean (SE) SBP/DBP at follow-up in non-diabetic patients was 137.9 (0.56)/83.5 (0.36) mmHg.
Figure 1 Sitting blood pressure at baseline and follow-up in (A) patients with diabetes and (B) patients without diabetes titrated from amlodipine 5 mg to 10 mg.
Abbreviations: BP, blood pressure; CI, confidence interval.
Not surprisingly, fewer patients with diabetes achieved their desired BP goal compared with non-diabetic patients. In total, 12/100 patients with diabetes (12.0%; 95% CI: 6.4, 20.0) attained a BP <130/80 mmHg; 283/610 patients without diabetes (46.4%; 95% CI: 42.4, 50.4) attained a BP <140/90 mmHg. Slightly more diabetic patients (25/100 [25%; 95% CI: 16.9, 34.7]) achieved the newly revised ADA target BP levels of <140/80 mmHg. Even more diabetic patients (64/100 [64%; 95% CI: 53.8, 73.4]) achieved the new JNC 8 target BP goals of 140/90 (aged <60 years) or 150/90 (aged ≥60 years) mmHg.
Safety
Overall, 22/100 patients with diabetes (22.0%) experienced 31 AEs and 176/610 patients without diabetes (28.9%) experienced 282 AEs (). The AEs most frequently reported by patients with diabetes were: peripheral edema (n=4; 4.0%), sinusitis (n=2; 2.0%), and upper respiratory tract infection (n=2; 2.0%). The AEs most frequently reported by patients without diabetes were: peripheral edema (n=46; 7.5%), nasopharyngitis (n=24; 3.9%), back pain (n=8; 1.3%), and upper respiratory tract infection (n=6; 1.0%).
Table 3 Incidence of all-cause adverse events in patients with and without diabetes titrated from amlodipine 5 mg to 10 mg
Serious AEs were reported by one patient (0.1%) with diabetes (event: ankle fracture) and five patients (0.8%) without diabetes (events: cardiomyopathy, pneumonia, facial bone fracture, intervertebral disk protrusion, gingival cancer, cerebrovascular accident, cerebrovascular disorder, ). Three patients with diabetes (3.0%) and 20 patients without diabetes (3.3%) withdrew from treatment due to an AE (). Adverse events that led to treatment withdrawal in patients with diabetes included: peripheral edema (n=1; 1.0%), headache (n=1; 1.0%), and hypertension (n=1; 1.0%). AEs that led to treatment withdrawal in patients without diabetes included: peripheral edema (n=8; 1.3%), joint swelling (n=2; 0.3%), and dyspnea (n=2; 0.3%).
Discussion
This retrospective, pooled analysis has shown that increasing amlodipine from 5 mg to 10 mg daily provided a clinically and statistically significant incremental reduction in sitting SBP and DBP in patients with concomitant diabetes and hypertension who had not responded sufficiently to treatment with the 5 mg dose. Furthermore, the incremental benefit on BP lowering achieved with amlodipine titration was similar in both diabetic and non-diabetic patient populations. This is similar to what has previously been observed in other patient populations, such as Asian patients with hypertension.Citation26 Amlodipine 10 mg was also well tolerated in patients with diabetes, demonstrating a safety profile similar to that observed in the non-diabetic patient group and previous studies involving high-dose amlodipine.Citation23,Citation24,Citation26,Citation29,Citation30
The age-adjusted prevalence of diagnosed diabetes in the general US adult population has recently been estimated at 8.5%.Citation31 However, in this analysis, diabetes prevalence in this patient population with mild or moderate hypertension was nearly double that of the current national estimate, at 14.1%. Patients with hypertension have been shown to have a 2.5-fold excess risk of developing diabetes over 6 years of follow-up compared with non-hypertensive individuals (relative risk: 2.43; 95% CI: 2.16, 2.73).Citation32 Similarly, hypertension is twice as likely to occur in patients with diabetes versus those without diabetes, with prevalence in diabetic patients of approximately 60%.Citation5,Citation6 Although hypertension and diabetes individually predict cardiovascular morbidity and mortality, the concomitance of these two conditions only serves to exacerbate the cardiovascular risk associated with either condition in isolation.Citation7–Citation9 Hence, appropriate management of cardiovascular risk factors, with particular emphasis on the early detection and effective treatment of hypertension in patients with diabetes, is paramount to reduce CVD burden in these high-risk individuals.
Even modest reductions in BP have the potential to substantially reduce cardiovascular morbidity and mortality. A meta-analysis of data from 61 prospective observational studies on deaths from vascular disease among individuals with no known vascular disease at baseline found that a 2 mmHg reduction in SBP could lower mortality from stroke by 10% and from ischemic heart disease or other vascular causes by 7%.Citation4 Similarly, the results of a prospective, observational study in patients with diabetes showed that a 10 mmHg decrease in SBP was associated with reductions in the risk of diabetes-related complications (including myocardial infarction, sudden death, angina, and stroke; relative risk reduction: 12%; 95% CI: 10%, 14%; P<0.0001), diabetes-related deaths (including death from myocardial infarction, sudden death, and stroke; relative risk reduction: 15%; 95% CI: 12%, 18%; P<0.0001), and myocardial infarction (relative risk reduction: 11%; 95% CI: 7%, 14%; P<0.0001).Citation33 Thus, the observed incremental reduction in BP by uptitrating amlodipine to 10 mg (−12.5 mmHg in SBP, −6.0 mmHg in DBP) in diabetic patients would be expected to drastically reduce the occurrence of diabetes-related complications.
Numerous clinical trials of antihypertensive agents have demonstrated the benefit of BP lowering on cardiovascular outcomes in a range of patient populations, including patients with diabetes.Citation34–Citation36 The UK Prospective Diabetes Study compared tight control of BP to <150/85 mmHg with less rigorous control of BP to <180/105 mmHg in hypertensive patients with diabetes.Citation34 Patients allocated to tight BP control experienced a 24% reduction in the relative risk of diabetes-related endpoints (including sudden death, myocardial infarction, angina, and stroke; 95% CI: 8%, 38%; P=0.0046), a 32% reduction in diabetes-related deaths (including death from myocardial infarction, sudden death, and stroke; 95% CI: 6%, 51%; P=0.019), and a 44% reduction in the risk of stroke (95% CI: 11%, 65%; P=0.013) compared with patients allocated to less rigorous BP control. The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial demonstrated that significant reductions in SBP and DBP (−5.6 mmHg and −2.2 mmHg, respectively; both P<0.001) to mean follow-up levels of 134.7/74.8 mmHg reduced the relative risk of major macrovascular or microvascular events (including CVD death, non-fatal stroke, and non-fatal myocardial infarction) by 9% (hazard ratio [HR]: 0.91; 95% CI: 0.83, 1.00; P=0.04), and CVD death by 18% (HR: 0.82; 95% CI: 0.68, 0.98; P=0.03).Citation35 A subgroup analysis of 1,501 patients with diabetes enrolled in the Hypertension Optimal Treatment (HOT) trial, where hypertensive patients were randomly assigned to a target DBP, showed that the risk of major cardiovascular events (non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death) was halved in those randomized to a DBP ≤80 mmHg compared with ≤90 mmHg (relative risk for 90 mmHg versus 80 mmHg: 2.06; 95% CI: 1.24, 3.44; P for trend =0.005).Citation36
However, recent results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD)Citation37 and International Verapamil SR-Trandolapril Study (INVEST)Citation38 trials have questioned the clinical benefit obtained with aggressive control of SBP levels to <120 mmHg, or even <130 mmHg. For example, the ACCORD trial, where patients with diabetes were randomized to a SBP <120 mmHg versus <140 mmHg, failed to demonstrate a significant reduction in the relative risk of major cardiovascular events, although a significant reduction in the relative risk of stroke was observed (HR: 0.59; 95% CI: 0.39, 0.89; P=0.01).Citation37 Moreover, the incidence of serious AEs attributed to antihypertensive treatment was significantly higher in the intensive-therapy group (3.3%) compared with the standard-therapy group (1.3%; P<0.001), indicating the potential for harm with aggressive BP lowering to within the normotensive range in this patient population. With this in mind, recent updates to treatment guidelines for the management of BP in patients with diabetes have seen therapeutic targets revised to <140/80 mmHg (ADA),Citation11 or <140/85 mmHg (ESH/ESC),Citation12 with the option of a lower SBP goal (ie, <130 mmHg) for some individuals, such as younger patients or those at very high risk of stroke, if it can be achieved with few drugs and without adverse effects.Citation11
For patients with diabetes, current clinical practice guidelines advocate the use of renin–angiotensin system (RAS) inhibitors such as an ACEI or an ARB, with the addition of a CCB such as amlodipine, and/or hydrochlorothiazide, as needed to achieve BP goals.Citation11 Studies in hypertensive patients have highlighted the incremental benefit of titrating amlodipine up to 10 mg on BP lowering.Citation23–Citation26 Moreover, higher doses of amlodipine have been shown to be equally effective or superior, either as monotherapy or in combination with another agent, in reducing cardiovascular outcomes compared with other treatment regimens,Citation39–Citation42 even in patients with diabetes.Citation16 The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trialCitation16 demonstrated that combination therapy with benazepril and amlodipine (mean achieved BP: 131.5/72.6 mmHg) reduced the relative risk of a cardiovascular event (cardiovascular death, myocardial infarction, stroke, hospitalization for angina, resuscitated arrest, and coronary revascularization) by 21% compared with benazepril and hydrochlorothiazide (mean achieved BP: 132.7/73.7 mmHg) in patients with concomitant diabetes and hypertension (HR: 0.79; 95% CI: 0.68, 0.92; P<0.003).
This analysis of pooled data from trials investigating the incremental effect of amlodipine titration to 10 mg daily on BP-lowering efficacy in patients with concomitant diabetes and hypertension has revealed that increasing amlodipine from 5 mg to 10 mg daily significantly lowered sitting SBP by −12.5 mmHg and DBP by −6.0 mmHg. Reductions in SBP and DBP of this magnitude are likely to translate to meaningful clinical reductions in cardiovascular outcomes in this high-risk patient group, however, the lack of information on cardiovascular events is an obvious limitation of this study. Nevertheless, the ability to increase amlodipine dose may provide a safe and effective strategy through which to achieve an incremental improvement in BP levels before adding to the medication burden of this patient group, where the average number of antihypertensive medications used to achieve BP goals may be as high as 4.3.Citation43
Conclusion
This retrospective, pooled analysis demonstrates that a significant, incremental improvement in sitting SBP and DBP levels can be safely achieved in patients with and without concomitant diabetes and hypertension by increasing amlodipine dose from 5 mg to 10 mg once daily. Thus, the use of amlodipine as a therapeutic option can be extended in the effective management of hypertension in patients with diabetes.
Author contributions
Barrett W Jeffers, Rahul Bhambri, and Jeffery Robbins were involved in the design of the study. Jeffery Robbins performed the statistical analysis of the data. Barrett W Jeffers, Rahul Bhambri, and Jeffery Robbins interpreted the data, were involved in the drafting of the manuscript, and critically reviewed the manuscript for important intellectual content and accuracy, and made revisions. All authors have approved the final version to be published.
Acknowledgments
Editorial support was provided by Michelle Jenvey, PhD, Shirley Smith, PhD, Sarah Knott and Alexandra Bound, PhD of Engage Scientific and was funded by Pfizer Inc.
Disclosure
This study was funded by Pfizer Inc. Barrett W Jeffers, Rahul Bhambri, and Jeffery Robbins are employees of Pfizer Inc.
References
- KearneyPMWheltonMReynoldsKMuntnerPWheltonPKHeJGlobal burden of hypertension: analysis of worldwide dataLancet2005365945521722315652604
- WhitingDRGuariguataLWeilCShawJIDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030Diabetes Res Clin Pract201194331132122079683
- KannelWBBlood pressure as a cardiovascular risk factor: prevention and treatmentJAMA199627520157115768622248
- LewingtonSClarkeRQizilbashNPetoRCollinsRProspective Studies CollaborationAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236093491903191312493255
- Centers for Disease Control and PreventionAge-Adjusted Percentage of Adults Aged 18 Years or Older with Diagnosed Diabetes Who have Hypertension, United States, 1995–2009 Available from: http://www.cdc.gov/diabetes/statistics/comp/fig8.htmAccessed June 30, 2014
- ChokshiNPGrossmanEMesserliFHBlood pressure and diabetes: vicious twinsHeart201399857758523434624
- [No authors listed]Hypertension in Diabetes Study (HDS). II. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patientsJ Hypertens19931133193258387090
- TurnerRCMillnsHNeilHARisk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23)BMJ199831671348238289549452
- ChenGMcAlisterFAWalkerRLHemmelgarnBRCampbellNRCardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressureHypertension201157589189721403089
- JamesPAOparilSCarterBLEvidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- American Diabetes AssociationStandards of medical care in diabetes – 2013Diabetes Care201336Suppl 1S11S6623264422
- ManciaGFagardRNarkiewiczK2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20133171281135723817082
- BrownMJCastaigneAde LeeuwPWInfluence of diabetes and type of hypertension on response to antihypertensive treatmentHypertension20003551038104210818061
- AmarJChamontinBGenesNCantetCSalvadorMCambouJPWhy is hypertension so frequently uncontrolled in secondary prevention?J Hypertens20032161199120512777958
- ManciaGAmbrosioniERoseiEABlood pressure control and risk of stroke in untreated and treated hypertensive patients screened from clinical practice: results of the ForLife studyJ Hypertens20052381575158116003185
- WeberMABakrisGLJamersonKCardiovascular events during differing hypertension therapies in patients with diabetesJ Am Coll Cardiol2010561778520620720
- KuznikAMardekianJTrends in utilization of lipid- and blood pressure-lowering agents and goal attainment among the US diabetic population, 1999–2008Cardiovasc Diabetol2011103121496321
- Stark CasagrandeSFradkinJESaydahSHRustKFCowieCCThe prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010Diabetes Care20133682271227923418368
- TurnbullFNealBAlgertCEffects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trialsArch Intern Med2005165121410141915983291
- Norvasc® (amlodipine besylate) tablets [prescribing information]United StatesPfizer Inc., New York, NY, USA2013 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019787s053lbl.pdfAccessed May 8, 2014
- TattiPPahorMByingtonRPOutcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDMDiabetes Care19982145976039571349
- SharmaAMBakrisGNeutelJMSingle-pill combination of telmisartan/amlodipine versus amlodipine monotherapy in diabetic hypertensive patients: an 8-week randomized, parallel-group, double-blind trialClin Ther201234353755122386829
- VarroneJInvestigators of Study AML-NY-86-002The efficacy and safety of amlodipine in the treatment of mild and moderate essential hypertension in general practiceJ Cardiovasc Pharmacol199117Suppl 1S30S3316296705
- FujiwaraTIiYHatsuzawaJThe Phase III, double-blind, parallel-group controlled study of amlodipine 10 mg once daily in Japanese patients with essential hypertension who insufficiently responded to amlodipine 5 mg once dailyJ Hum Hypertens200923852152919148107
- FukutomiMHoshideSEguchiKWatanabeTShimadaKKarioKDifferential effects of strict blood pressure lowering by losartan/hydrochlorothiazide combination therapy and high-dose amlodipine monotherapy on microalbuminuria: the ALPHABET studyJ Am Soc Hypertens201261738222054782
- KarioKRobbinsJJeffersBWTitration of amlodipine to higher doses: a comparison of Asian and Western experienceVasc Health Risk Manag2013969570124235839
- HongSJAhnTHBaekSHComparison of efficacy and tolerability of amlodipine orotate versus amlodipine besylate in adult patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 8-week follow-up, noninferiority trialClin Ther200628453755116750465
- ChanISZhangZTest-based exact confidence intervals for the difference of two binomial proportionsBiometrics19995541202120911315068
- BobrieGI-COMBINE Study InvestigatorsI-COMBINE study: assessment of efficacy and safety profile of irbesartan/amlodipine fixed-dose combination therapy compared with amlodipine monotherapy in hypertensive patients uncontrolled with amlodipine 5 mg monotherapy: a multicenter, phase III, prospective, randomized, open-label with blinded-end point evaluation studyClin Ther20123481705171922853848
- KangSMYounJCChaeSCComparative efficacy and safety profile of amlodipine 5 mg/losartan 50 mg fixed-dose combination and amlodipine 10 mg monotherapy in hypertensive patients who respond poorly to amlodipine 5 mg monotherapy: an 8-week, multicenter, randomized, double-blind phase III noninferiority studyClin Ther201133121953196322136978
- Centers for Disease Control and Prevention National Center for Health Statistics Division of Health Interview StatisticsCrude and Age-Adjusted Percentage of Civilian, Noninstitutionalized Adults with Diagnosed Diabetes, United States, 1980–2011 Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figageadult.htmAccessed June 30, 2014
- GressTWNietoFJShaharEWoffordMRBrancatiFLHypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities StudyN Engl J Med20003421390591210738048
- AdlerAIStrattonIMNeilHAAssociation of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational studyBMJ2000321725841241910938049
- [No authors listed]Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study GroupBMJ199831771607037139732337
- PatelAADVANCE Collaborative GroupMacMahonSEffects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trialLancet2007370959082984017765963
- HanssonLZanchettiACarruthersSGEffects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study GroupLancet19983519118175517629635947
- ACCORD Study GroupCushmanWCEvansGWEffects of intensive blood-pressure control in type 2 diabetes mellitusN Engl J Med2010362171575158520228401
- Cooper-DeHoffRMGongYHandbergEMTight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery diseaseJAMA20103041616820606150
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group, The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack TrialMajor outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)JAMA2002288232981299712479763
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436394262022203115207952
- DahlofBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet2005366948989590616154016
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med2008359232417242819052124
- BakrisGLMaximizing cardiorenal benefit in the management of hypertension: achieve blood pressure goalsJ Clin Hypertens (Greenwich)19991214114711416606
