Abstract
High cardiovascular risk conditions are frequently associated with altered plasma lipoprotein profile, such as elevated low-density lipoprotein (LDL) and LDL cholesterol and decreased high-density lipoprotein. There is, however, accumulating evidence that specific subclasses of LDL may play an important role in cardiovascular disease development, and their relative concentration can be regarded as a more relevant risk factor. LDL particles undergo multiple modifications in plasma that can lead to the increase of their negative charge. The resulting electronegative LDL [LDL(–)] subfraction has been demonstrated to be especially atherogenic, and became a subject of numerous recent studies. In this review, we discuss the physicochemical properties of LDL(–), methods of its detection, atherogenic activity, and relevance of the LDL electronegativity index as a potential independent predictor of cardiovascular risk.
Introduction
The risk of cardiovascular disease (CVD) development is closely associated with altered lipoprotein profile of blood plasma. Lipoprotein particles circulating in human blood vary in their chemical and physical properties. It is well documented that individuals with high CVD risk have elevated amounts of circulating low-density lipoprotein (LDL) and decreased proportion of high-density lipoprotein (HDL). LDL is the major source of cholesterol and the lipids that contribute to the development of the atherosclerotic plaque, whereas HDL is not atherogenic, and its concentrations inversely correlate with the CVD risk.Citation1 Total cholesterol and LDL cholesterol lowering with statins is therefore standard therapeutic approach for treating patients with atherosclerosis and increased CVD risk.Citation2,Citation3 This strategy, however, did not reduce the CVD risk beyond ~70% in most cases, and in some clinical studies lowering LDL cholesterol was not efficient.Citation4–Citation7 This pointed to the existence of other important factors promoting atherosclerosis development that have to be taken into account.
The LDL pool is heterogenic and consists of several subclasses that can be separated based on the differences in their density, size, chemical composition, and electrical charge.Citation8,Citation9 Among them, small dense LDL (sdLDL) was demonstrated to be highly atherogenic, and its level strongly correlated with CVD risk.Citation10–Citation12 Study of the significance of LDL subclasses for atherosclerosis progression has been hindered by the lack of standardization of the analytical methods. LDL subfraction analysis has been performed by different groups, using gradient density ultracentrifugation,Citation13–Citation15 gradient gel electrophoresis,Citation16 nuclear magnetic resonance (NMR) analysis,Citation17 homogeneous assays,Citation18–Citation20 and other methods. All these methods deliver slightly different results, and variations are possible even within one method due to the modifications of experimental conditions.Citation21,Citation22 It is, however, generally accepted that sdLDL fraction has a density of 1.044–1.063 g/mL and a particle size around 15–20 nm.Citation23 LDL subclasses analysis is currently being considered as an important diagnostic tool for improvement of CVD risk and treatment efficacy assessment.Citation24
Apart from the size and density, LDL subfractions differ by their chemical composition. LDL particles can undergo multiple modifications in the blood plasma that increase their atherogenicity.Citation25–Citation27 One of the first types of atherogenic modified LDL that has been discovered is oxidation.Citation25,Citation28 Oxidized LDL particles are recognized by a number of receptors, including CD36 and TLR-4 and can induce the immune response and inflammation that contribute to the atherosclerosis progression.Citation29,Citation30 Oxidized LDL was demonstrated to induce the lipid storage in cultured endothelial cells (ECs), but could only be prepared in vitro.Citation31,Citation32
Other forms of modified LDL have been discovered during the last two decades. Desialylated LDL could be detected in the blood plasma of atherosclerosis patients using a lectinsorbent assay.Citation33,Citation34 Desialylation of LDL particles is performed in plasma by trans-sialidase that participates in the metabolism of glycoconjugates.Citation35 Glycation of apoB lipoprotein in LDL particles has also been described.Citation36,Citation37
Another form of atherogenic modified LDL is electronegative LDL [LDL(–)] that can be distinguished using methods sensitive to the particle charge, such as agarose gel electrophoresis, isotachophoresis, or ion-exchange chromatography.Citation38,Citation39 LDL(–) is characterized by an enhanced ability to aggregate and is associated with the increased CVD risk.Citation40
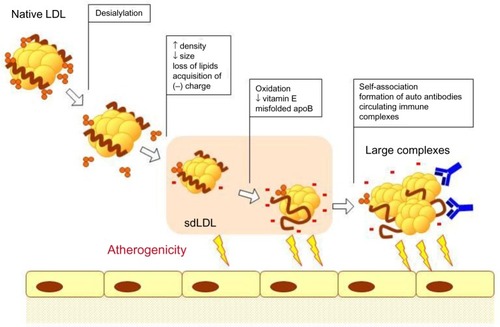
It has been demonstrated that sdLDL is especially susceptible to atherogenic modifications that occur in blood plasma. sdLDL particles have a much longer circulation time than larger LDL subfractions that are cleared from the bloodstream through interaction with the LDL receptor.Citation11,Citation41 For instance, glycation of LDL particles was observed preferentially in the sdLDL fraction, and small LDL had a decreased sialic acid content.Citation42,Citation43 sdLDL particles contained less antioxidative vitamins that make them less protected against oxidation.Citation44 Moreover, the elevated LDL(–) was associated with higher oxidized LDL and sdLDL levels.Citation45 Isolated LDL(–) particles had a decreased sialic acid content in comparison with native LDL, and this difference was more pronounced in LDL(–) fractions from patients with atherosclerosis.Citation27,Citation46 It is likely that desialylated and electronegative LDL fractions are closely linked or even identical. Converging evidence points to the existence of a cascade of multiple modifications of sdLDL particles, starting with desialylation and minimal oxidation, followed by further oxidation and formation of highly atherogenic and proinflammatory complexes.
LDL electronegativity index
LDL(–) is a heterogeneous population of particles that vary by their density and size, but share the increased negative charge.Citation47 The origins of LDL(–) are not yet clearly understood. Incubation of LDL with blood plasma at 37°C resulted in the formation of LDL(–) that could be blocked by 4-bromophenacyl bromide, an inhibitor of secretory phospholipase A2 (sPLA2), suggesting that sPLA2 plays a primary role in LDL(–) formation.Citation48 Desialylation of LDL during incubation with blood plasma has also been described.Citation35 It is likely that the acquisition of the negative charge occurs as a result of successive changes in the LDL particle, including desialylation, loss of lipids, reduction of particle size, and peroxidation in the blood plasma. A summary of such modifications leading to the formation of LDL(–) is presented in .
Figure 1 Atherogenic modifications of LDL.
Abbreviations: LDL, low-density lipoprotein; sdLDL, small dense LDL.
LDL(–) detection
The separation of LDL into electropositive [LDL(+)] and electronegative [LDL(–)] fractions was first performed by Avogaro et alCitation39 using ion-exchange chromatography. More recently, Chen et alCitation49 described five subfractions of plasma LDL with various degrees of electronegativity, from L1 (least electronegative) to L5 (most electronegative).Citation50 The authors found that the plasma levels of the most electronegative (L5) fraction were moderately elevated in individuals with high CVD risk, such as smokers, patients with hypercholesterolemia, type 2 diabetes, and myocardial infarction.Citation51–Citation53 Anion exchange chromatography remains the standard method for LDL(–) extraction for further analysis that allows a more detailed study of chemical and physical properties of these particles. Another approach to analyze the LDL subfractions based on the particle charge is the capillary isotachophoresis.Citation54 In this method, LDL(–) is detected as a fraction of fast migrating LDL, which is separated from slowly migrating LDL. Some authors used capillary isotachophoresis to analyze the sdLDL fraction obtained by heparin precipitation.Citation55 Finally, recently created monoclonal antibodies to LDL(–) allows distinguishing the LDL(–) fraction from native LDL particles by the specific epitopes.Citation9,Citation56,Citation57 Enzyme-linked immunosorbent assay (ELISA) assay with monoclonal antibodies is especially useful for fast and direct detection of LDL(–) in clinical practice.Citation58 It has to be taken into account that the existing LDL(–) detection methods are only sensitive to the electrical charge and do not distinguish the different origins of the LDL(–) particles. For instance, they cannot discriminate particles that vary by the relative amount of lipoproteins and/or nonesterified fatty acids, particles that underwent oxidation etc. Most of the available monoclonal antibodies were generated against LDL(–) particles isolated based on their charge by ion-exchange chromatography, and they do not help to overcome this problem.
Tendency to aggregate
LDL(–) particles are characterized by a number of specific chemical and physical features in comparison to native LDL. It is well known that LDL(–) is prone to aggregation.Citation39 Spontaneous aggregation of LDL(–) particles has been investigated in more detail in a recent study.Citation59 The authors report that LDL(–) formed amyloid-like structures and even possessed amyloidogenic properties, promoting native LDL particles to aggregate. Moreover, amyloid-β peptide enhanced the aggregation of LDL(–) particles. LDL particle aggregation is closely related to their atherogenic activity, and aggregated LDL(–) is characterized by an increased proteoglycan binding.Citation60,Citation61 The nature of this increased affinity is currently being investigated. Some authors proposed that the N-terminus of apoB in the LDL(–) particles play the key role as monoclonal antibodies to this region interfered with the binding of particles to proteoglycans.Citation62 Thus, this region could be an additional proteoglycan-binding site present in LDL(–), next to the constitutive binding site in apoB from native LDL.Citation63,Citation64
Misfolded apoB
The electronegative L5 fraction had a decreased content of apolipoprotein B (apoB) and increased content of other lipoproteins.Citation65 A misfolded conformation of apoB in LDL(–) particles has also been reported.Citation66,Citation67 As demonstrated by circular dichroism studies, secondary structure of apoB is disturbed in LDL(–) particles, with decreased content of α-helices and increased content of β-sheets.Citation68 Studies of tryptophan fluorescence spectroscopy also pointed to the misfolded conformation of apoB, as the fluorescence emission was decreased in LDL(–) in comparison to native LDL, indicative of the abnormal exposure of tryptophan residues to the aqueous environment.Citation59,Citation68 Another evidence of lipoprotein misfolding in LDL(–) particles comes from 2D-NMR analysis that demonstrated a number of lysine residues in LDL(–) with altered ionization status probably due to their exposure to the solvent.Citation69 Interestingly, estradiol prevented the misfolding of apoB in LDL(–) particles and decreased their aggregation ability without affecting the negative charge.Citation70 Therefore, the misfolded lipoprotein appears to be the decisive factor responsible for LDL(–) aggregation. LDL(–) fractions also demonstrate changes in their lipid composition, such as increased content of nonesterified fatty acid.Citation71,Citation72 This may explain the altered properties of LDL(–) particles’ surface and contribute to their enhanced aggregation ability.Citation73
Lipolytic activity
LDL(–) particles are characterized by the enzymatic activity that modulates their lipid composition and inflammatory properties. It has been demonstrated that the particles are enriched with platelet-activating factor acetylhydrolase (PAF-AH), a phospholipase that targets the oxidized phospholipids.Citation74 PLC-like and SMase activities have also been detected in LDL particles, although their origins remain to be elucidated.Citation75,Citation76 Proteomic studies detected a higher content of non-apoB proteins in LDL(–) in comparison to native LDL, including apoA-I, apoE, apoC-III, apoA-II, apoD, apoF, and apoJ.Citation77 Although the absolute amounts of these proteins remain very low, they could still play a role in LDL(–) properties and lipolytic activities. For instance, ApoJ acts as a chaperone that binds the misfolded proteins in blood.Citation78 Its binding to the misfolded apoB in LDL(–) particles could have a protective role against particle aggregation. The combination of PAF-AH and PLC-like activities could regulate the concentrations of proinflammatory oxidized LDL to attenuate their deleterious effects in human plasma.Citation64 More studies are needed however to determine the origin and significance of these lipolytic activities.
Inflammatory and atherogenic properties
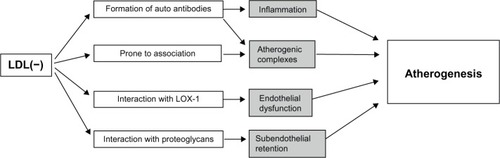
Several features of LDL(–) account for the increased atherogenicity of this LDL subtype (). Altered protein structure of the LDL(–) particles leads to a decreased affinity to the LDL receptor as compared to native LDL and prolonged circulation times of the particles.Citation79,Citation80 On the other hand, the most electronegative (L5) fraction of LDL(–) was shown to interact with the lectin-like oxidized LDL receptor 1 (LOX-1), causing endothelial dysfunction, apoptosis, and atherogenic response in cultured ECs.Citation51,Citation81 A recent study demonstrated that L5 LDL(–) fraction induced the production of reactive oxygen species and increased the C-reactive protein levels in cultured cells via LOX-1 signaling in cultured aortic ECs, which can further contribute to the atherogenesis.Citation82 Circulating LDL(–) can activate inflammatory and immune responses that contribute to the atherosclerosis progression. Because of its increased ability to bind proteoglycans, LDL(–) particles and their aggregates have a prolonged residence time in the subendothelial space. Internalized by macrophages through scavenger receptors, LDL(–) serves as a source for the lipid storage and foam cell formation in the arterial wall. The production of anti-LDL(–) autoantibodies may also play an important role in the disease progression, which currently remains to be studied.Citation9 Along with the autoantibodies to LDL, LDL-containing circulating immune complexes have been found in blood plasma of atherosclerosis patients. These complexes are characterized by smaller sizes and more negative electrical charge than native LDL and have pronounced atherogenic properties, as demonstrated by the accumulation of cholesterol esters in cultured smooth muscle cells and monocyte-derived macrophages.Citation83 In vitro studies have demonstrated that LDL(–) had a cytotoxic effect on the EC, stimulating apoptosis and cytokine production, including IL-8, MCP-1, and VCAM-1.Citation49,Citation84,Citation85 Incubation of cultured human monocytes with LDL(–), but not with LDL(+), resulted in cytokine release mediated by TLR4 and CD14, which was the main receptor recognizing LDL(–).Citation86 Similar cytokine-inducing effect has also been described for the ceramide-enriched LDL, which shares most of the properties of LDL(–).Citation87,Citation88
Figure 2 Atherogenic properties of electronegative LDL.
Abbreviations: LDL(–), electronegative low-density lipoprotein; LOX-1, LDL receptor 1.
Clinical importance of the LDL electronegativity index in prediction of CVD
Recent studies strongly suggest that LDL electronegativity can be considered as an important factor in evaluating cardiovascular risk in many pathological conditions. As mentioned earlier, the elevated levels of electronegative LDL fractions have been reported in many conditions associated with high cardiovascular risk, including hyperlipidemia, diabetes, renal disease, and coronary syndromes. In patients with hypercholesterolemia, the most electronegative, mildly oxidized L5 LDL fraction was increased in comparison with healthy individuals.Citation49 Elevated L5 LDL fraction has also been reported in familial hypercholesterolemia patientsCitation50 and in smokers,Citation51 both cases being associated with high cardiovascular risk. Recent studies demonstrated that L5 LDL fraction was significantly increased in patients with ST-segment elevation myocardial infarction, where it was demonstrated to play a role in platelet activation and thrombosis.Citation52,Citation53 Elevated levels of L5 were observed in the plasma of patients with metabolic syndrome, a group of metabolic abnormalities that are associated with CVDs, which is used to identify individuals with high cardiovascular risk.Citation89 Together these results suggest that LDL electronegativity might be considered as a novel predictor of cardiovascular risk.
The clinical importance of LDL electronegativity index has recently been explored in a study analyzing the correlation between the plasma level of L5 fraction and cardiovascular risk estimated using the Framingham risk score.Citation90 This score has been developed during a large epidemiological Framingham Heart Study and is calculated using such predictors as sex, age, blood pressure, treatment of hypertension and diabetes mellitus, smoking, body mass index, and levels of total cholesterol and HDL.Citation91,Citation92 According to this classification, “general” CVD risk included coronary death, myocardial infarction, coronary insufficiency, angina, ischemic and hemorrhagic stroke, transient ischemic attack, peripheral artery disease, and heart failure. The “hard” CVD category included coronary death, myocardial infarction, and stroke. The study was performed on patients with metabolic syndrome and healthy individuals. Comparison of these two groups revealed no statistically significant difference in total cholesterol and LDL levels, whereas L5 fraction was significantly higher in patients with metabolic syndrome. No association was registered between L5 level and total cholesterol or LDL. Regression analysis demonstrated association between L5 content and fasting plasma glucose level and body mass index. L5 level and waist circumference were associated with CVD risks, and the independent contribution of L5 content (with controlled variance of waist circumference) was 11% of 30-year “general” CVD risk and 8% of 30-year “hard” CVD risk. Therefore, L5 content was shown to strongly correlate with different CVD risk factors and with CVD risk. Moreover, the authors argued that plasma L5 levels in asymptomatic individuals with metabolic syndrome also correlated with the number of fulfilled metabolic syndrome criteria and therefore with CVD progression. However, it has not been studied, whether or not the elevated L5 fraction was accompanied by an increase of other known atherogenic LDL modifications. Future studies should compare the diagnostic values of electronegative LDL and other atherogenic LDL types, including modified LDL fractions, such as oxidized LDL and desialylated LDL. Quantitative analysis of various types of modified LDL currently remains challenging because of low amounts of circulating particles. For instance, the association of atherogenic oxidized LDL with CVD risk was difficult to establish, making its use as a biomarker inconvenient.Citation93 Therefore, LDL electronegativity was suggested as a novel index for CVD prediction because it is relatively easy to assess, although more studies are needed to strengthen this concept.
It remains to be determined whether the elevated level of L5 LDL actually plays a causative role in CVD. Nevertheless, properties of electronegative LDL attracted attention as potential direction of therapy improvement. As elevated levels of L5 are associated with such conditions as smoking, they may partly be ameliorated by lifestyle corrections that are generally recommended for individuals with high CVD risk. More directed therapeutic strategies emerge as a result of better understanding of electronegative LDL pathogenicity. In vitro studies on cultured human aortic epithelial cells explored the possibility of blocking L5 internalization through LOX-1 receptor using the naturally occurring compound sesamol. Study of the sesamol effect on Syrian hamsters fed a high-fat diet demonstrated that the compound addition could reduce plasma L5 levels and atherosclerotic lesion size.Citation94 These results encourage the search for compounds specifically targeting the electronegative LDL fraction and related signaling. In another study, the authors demonstrated a decrease of LDL(–) uptake by macrophages and foam cell formation caused by anti-LDL(–) single-chain variable antibody fragments (scFv). The exposure of macrophages to LDL(–) resulted in enhanced expression of CD36, which promoted the lipid uptake and foam cell formation.Citation95 The addition of the fragments led to a dose-dependent inhibition of the LDL(–) uptake as well as to the decrease of Cd36 expression at mRNA level. The protective effect of the fragments has also been demonstrated on animal model (Ldlr knockout mice), where it caused reduction of atherosclerotic lesion at the aortic sinus in comparison with untreated animals.Citation96 However, it is important to evaluate the effects of agents specifically targeting electronegative LDL on the production, metabolism, and uptake of other atherogenic LDL subtypes.
The increase of LDL electronegativity can therefore be considered as an emerging independent risk factor of CVD, as confirmed by the analysis of various groups of patients with elevated CVD risk. The possibility of targeted therapy aiming to reduce the LDL(–) fraction has been explored in experiments on animal models. More studies are needed however to confirm the clinical utility of LDL electronegativity index on a larger scale and to design appropriate therapeutic approaches for reducing LDL(–) fraction in humans.
Acknowledgments
The work was supported by Russian Foundation for Basic Research (Grant number 15-04-09279).
Disclosure
The authors report no conflicts of interests in this work.
References
- KraussRMLipoprotein subfractions and cardiovascular disease riskCurr Opin Lipidol20102130531120531184
- MillsEJRachlisBWuPPrimary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patientsJ Am Coll Cardiol2008521769178119022156
- GoffDCJrLloyd-JonesDMBennettG2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesJ Am Coll Cardiol2014632935295924239921
- BaigentCKeechAKearneyPMEfficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statinsLancet20053661267127816214597
- AfilaloJMajdanAAEisenbergMJIntensive statin therapy in acute coronary syndromes and stable coronary heart disease: a comparative meta-analysis of randomised controlled trialsHeart20079391492117277349
- KjekshusJApetreiEBarriosVRosuvastatin in older patients with systolic heart failureN Engl J Med20073572248226117984166
- GissiHFITavazziLMaggioniAPEffect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trialLancet20083721231123918757089
- BerneisKKKraussRMMetabolic origins and clinical significance of LDL heterogeneityJ Lipid Res2002431363137912235168
- MelloAPda SilvaITAbdallaDSElectronegative low-density lipoprotein: origin and impact on health and diseaseAtherosclerosis201121525726521292266
- RizzoMBerneisKLow-density lipoprotein size and cardiovascular risk assessmentQJM20069911416371404
- PackardCCaslakeMShepherdJThe role of small, dense low density lipoprotein (LDL): a new lookInt J Cardiol200074Suppl 1S17S2210856769
- PackardCJSmall dense low-density lipoprotein and its role as an independent predictor of cardiovascular diseaseCurr Opin Lipidol20061741241716832165
- GriffinBACaslakeMJYipBRapid isolation of low density lipoprotein (LDL) subfractions from plasma by density gradient ultracentrifugationAtherosclerosis19908359672390137
- GriffinBAFreemanDJTaitGWRole of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease riskAtherosclerosis19941062412538060384
- YeeMSPavittDVTanTLipoprotein separation in a novel iodixanol density gradient, for composition, density, and phenotype analysisJ Lipid Res2008491364137118337616
- EnsignWHillNHewardCBDisparate LDL phenotypic classification among 4 different methods assessing LDL particle characteristicsClin Chem2006521722172716740651
- OtvosJDJeyarajahEJBennettDWDevelopment of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurementClin Chem199238163216381326420
- HiranoTItoYSaegusaHA novel and simple method for quantification of small, dense LDLJ Lipid Res2003442193220112897184
- ItoYFujimuraMOhtaMDevelopment of a homogeneous assay for measurement of small dense LDL cholesterolClin Chem201157576521051530
- AlbersJJKennedyHMarcovinaSMEvaluation of a new homogenous method for detection of small dense LDL cholesterol: comparison with the LDL cholesterol profile obtained by density gradient ultracentrifugationClin Chim Acta201141255656121156166
- O’NealDHarripPDragicevicGA comparison of LDL size determination using gradient gel electrophoresis and light-scattering methodsJ Lipid Res199839208620909788255
- WitteDRTaskinenMRPerttunen-NioHStudy of agreement between LDL size as measured by nuclear magnetic resonance and gradient gel electrophoresisJ Lipid Res2004451069107614993238
- DiffenderferMRSchaeferEJThe composition and metabolism of large and small LDLCurr Opin Lipidol20142522122624811298
- HirayamaSMiidaTSmall dense LDL: an emerging risk factor for cardiovascular diseaseClin Chim Acta201241421522422989852
- SteinbergDParthasarathySCarewTEBeyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicityN Engl J Med19893209159242648148
- JaakkolaOSolakiviTTertovVVCharacteristics of low-density lipoprotein subfractions from patients with coronary artery diseaseCoron Artery Dis199343793858261211
- TertovVVBittolo-BonGSobeninIANaturally occurring modified low density lipoproteins are similar if not identical: more electronegative and desialylated lipoprotein subfractionsExp Mol Pathol1995621661728612720
- SteinbergDWitztumJLOxidized low-density lipoprotein and atherosclerosisArterioscler Thromb Vasc Biol2010302311231621084697
- MillerYIChoiSHWiesnerPOxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunityCirc Res201110823524821252151
- PentikainenMOOorniKAla-KorpelaMModified LDL – trigger of atherosclerosis and inflammation in the arterial intimaJ Intern Med200024735937010762453
- PalinskiWHorkkoSMillerECloning of monoclonal autoan-tibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasmaJ Clin Invest1996988008148698873
- ItabeHYamamotoHImanakaTSensitive detection of oxidatively modified low density lipoprotein using a monoclonal antibodyJ Lipid Res19963745538820101
- TertovVVSobeninIAOrekhovANModified (desialylated) low-density lipoprotein measured in serum by lectin-sorbent assayClin Chem199541101810217600681
- OrekhovANTertovVVMukhinDNDesialylated low density lipoprotein – naturally occurring modified lipoprotein with atherogenic potencyAtherosclerosis1991861531611872910
- TertovVVKaplunVVSobeninIAHuman plasma trans-sialidase causes atherogenic modification of low density lipoproteinAtherosclerosis200115910311511689212
- YounisNCharlton-MenysVSharmaRGlycation of LDL in non-diabetic people: small dense LDL is preferentially glycated both in vivo and in vitroAtherosclerosis200920216216818511055
- SoranHDurringtonPNSusceptibility of LDL and its subfractions to glycationCurr Opin Lipidol20112225426121734572
- HoffHFGaubatzJWIsolation, purification, and characterization of a lipoprotein containing Apo B from the human aortaAtherosclerosis1982422732977073805
- AvogaroPBonGBCazzolatoGPresence of a modified low density lipoprotein in humansArteriosclerosis1988879873341993
- Sanchez-QuesadaJLPerezACaixasAEffect of glycemic optimization on electronegative low-density lipoprotein in diabetes: relation to nonenzymatic glycosylation and oxidative modificationJ Clin Endocrinol Metabol20018632433249
- GriffinBALipoprotein atherogenicity: an overview of current mechanismsProc Nutr Soc19995816316910343354
- MatsuiHOkumuraKTokiYLow-density lipoprotein particle size as an independent predictor of glycated low-density lipoprotein levelDiabetes Care1999221220122110388998
- La BelleMKraussRMDifferences in carbohydrate content of low density lipoproteins associated with low density lipoprotein subclass patternsJ Lipid Res199031157715882246611
- TribbleDLRizzoMChaitAEnhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteinsAm J Med200111010311011165551
- HasegawaGKajiyamaSTanakaTThe alpha-glucosidase inhibitor acarbose reduces the net electronegative charge of low-density lipoprotein in patients with newly diagnosed type 2 diabetesClin Chim Acta200839011011418230353
- TertovVVSobeninIAOrekhovANSimilarity between naturally occurring modified desialylated, electronegative and aortic low density lipoproteinFree Radic Res1996253133198889495
- Sanchez-QuesadaJLBenitezSOtalCDensity distribution of electronegative LDL in normolipemic and hyperlipemic subjectsJ Lipid Res20024369970511971940
- GrecoGBaloghGBrunelliRGeneration in human plasma of misfolded, aggregation-prone electronegative low density lipoproteinBiophys J20099762863519619478
- ChenCHJiangTYangJHLow-density lipoprotein in hypercho-lesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcriptionCirculation20031072102210812695302
- YangCYRayaJLChenHHIsolation, characterization, and functional assessment of oxidatively modified subfractions of circulating low-density lipoproteinsArterioscler Thromb Vasc Biol2003231083109012689919
- TangDLuJWalterscheidJPElectronegative LDL circulating in smokers impairs endothelial progenitor cell differentiation by inhibiting Akt phosphorylation via LOX-1J Lipid Res200849334717909223
- ChanHCKeLYChuCSHighly electronegative LDL from patients with ST-elevation myocardial infarction triggers platelet activation and aggregationBlood20131223632364124030386
- ChangPYChenYJChangFHAspirin protects human coronary artery endothelial cells against atherogenic electronegative LDL via an epigenetic mechanism: a novel cytoprotective role of aspirin in acute myocardial infarctionCardiovasc Res20139913714523519265
- SchmitzGMollersCRichterVAnalytical capillary isotachophoresis of human serum lipoproteinsElectrophoresis199718180718139372273
- ZhangBKaneshiTOhtaTRelation between insulin resistance and fast-migrating LDL subfraction as characterized by capillary isotachophoresisJ Lipid Res2005462265227716061945
- DamascenoNRSevanianAApolinarioEDetection of electronegative low density lipoprotein (LDL-) in plasma and atherosclerotic lesions by monoclonal antibody-based immunoassaysClin Biochem200639283816310760
- Santo Faulin TdoEde SenaKCRodrigues TellesAEValidation of a novel ELISA for measurement of electronegative low-density lipoproteinClin Chem Lab Med2008461769177519055454
- Faulin TdoEde Sena-EvangelistaKCPachecoDBDevelopment of immunoassays for anti-electronegative LDL autoantibodies and immune complexesClin Chim Acta201241329129722037508
- ParasassiTDe SpiritoMMeiGLow density lipoprotein misfolding and amyloidogenesisFASEB J2008222350235618292214
- OorniKPentikainenMOAla-KorpelaMAggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactionsJ Lipid Res2000411703171411060340
- BancellsCBenitezSJauhiainenMHigh binding affinity of electronegative LDL to human aortic proteoglycans depends on its aggregation levelJ Lipid Res20095044645518952981
- BancellsCBenitezSOrdonez-LlanosJImmunochemical analysis of the electronegative LDL subfraction shows that abnormal N-terminal apolipoprotein B conformation is involved in increased binding to proteoglycansJ Biol Chem20112861125113321078674
- BorenJOlinKLeeIIdentification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor bindingJ Clin Invest1998101265826649637699
- Sanchez-QuesadaJLVillegasSOrdonez-LlanosJElectronegative low-density lipoprotein. A link between apolipoprotein B misfolding, lipoprotein aggregation and proteoglycan bindingCurr Opin Lipidol20122347948622964994
- KeLYEnglerDALuJChemical composition-oriented receptor selectivity of L5, a naturally occurring atherogenic low-density lipoproteinPure Appl Chem2011839
- UrsiniFDaviesKJMaiorinoMAtherosclerosis: another protein misfolding disease?Trends Mol Med2002837037412127722
- AsatryanLHamiltonRTIsasJMLDL phospholipid hydrolysis produces modified electronegative particles with an unfolded apoB-100 proteinJ Lipid Res20054611512215489541
- ParasassiTBittolo-BonGBrunelliRLoss of apoB-100 secondary structure and conformation in hydroperoxide rich, electronegative LDL(–)Free Radic Biol Med200131828911425493
- BlancoFJVillegasSBenitezS2D-NMR reveals different populations of exposed lysine residues in the apoB-100 protein of electronegative and electropositive fractions of LDL particlesJ Lipid Res2010511560156520110441
- BrunelliRBaloghGCostaGEstradiol binding prevents ApoB-100 misfolding in electronegative LDL(–)Biochemistry2010497297730220669963
- BenitezSSanchez-QuesadaJLLuceroLChanges in low-density lipoprotein electronegativity and oxidizability after aerobic exercise are related to the increase in associated non-esterified fatty acidsAtherosclerosis200216022323211755941
- GaubatzJWGillardBKMasseyJBDynamics of dense electronegative low density lipoproteins and their preferential association with lipoprotein phospholipase A(2)J Lipid Res20074834835717102149
- JayaramanSGantzDLGurskyOEffects of phospholipase A(2) and its products on structural stability of human LDL: relevance to formation of LDL-derived lipid dropletsJ Lipid Res20115254955721220788
- BenitezSSanchez-QuesadaJLRibasVPlatelet-activating factor acetylhydrolase is mainly associated with electronegative low-density lipoprotein subfractionCirculation2003108929612821559
- HolopainenJMMedinaOPMetsoAJSphingomyelinase activity associated with human plasma low density lipoproteinJ Biol Chem2000275164841648910828058
- KinnunenPKHolopainenJMSphingomyelinase activity of LDL: a link between atherosclerosis, ceramide, and apoptosis?Trends Cardiovasc Med200212374211796243
- BancellsCCanalsFBenitezSProteomic analysis of electronegative low-density lipoproteinJ Lipid Res2010513508351520699421
- WyattARWilsonMRIdentification of human plasma proteins as major clients for the extracellular chaperone clusterinJ Biol Chem20102853532353919996109
- BenitezSVillegasVBancellsCImpaired binding affinity of electronegative low-density lipoprotein (LDL) to the LDL receptor is related to nonesterified fatty acids and lysophosphatidylcholine contentBiochemistry200443158631587215595841
- UrataJIkedaSKogaSNegatively charged low-density lipoprotein is associated with atherogenic risk in hypertensive patientsHeart Vessels20122723524221491122
- LuJYangJHBurnsARMediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosisCirc Res200910461962719150883
- ChuCSWangYCLuLSElectronegative low-density lipoprotein increases C-reactive protein expression in vascular endothelial cells through the LOX-1 receptorPloS One20138e7053323950953
- SobeninIASalonenJTZhelankinAVLow density lipoprotein-containing circulating immune complexes: role in atherosclerosis and diagnostic valueBioMed Res Int2014201420569725054132
- Sanchez-QuesadaJLCamachoMAntonRElectronegative LDL of FH subjects: chemical characterization and induction of chemokine release from human endothelial cellsAtherosclerosis200316626127012535738
- BenitezSCamachoMBancellsCWide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein arrayBiochim Biophys Acta200617611014102116753331
- EstruchMBancellsCBelokiLCD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytesAtherosclerosis201322935636223880187
- EstruchMSanchez-QuesadaJLOrdonez-LlanosJCeramide-enriched LDL induces cytokine release through TLR4 and CD14 in monocytes. Similarities with electronegative LDLClin Invest Arterioscler201426131137
- EstruchMSanchez-QuesadaJLBelokiLThe induction of cytokine release in monocytes by electronegative low-density lipoprotein (LDL) is related to its higher ceramide content than native LDLInt J Mol Sci2013142601261623358250
- LeeASChenWYChanHCGender disparity in LDL-induced cardiovascular damage and the protective role of estrogens against electronegative LDLCardiovasc Diabetol2014136424666525
- HsuJFChouTCLuJLow-density lipoprotein electronegativity is a novel cardiometabolic risk factorPLoS One20149e10734025203525
- PencinaMJD’AgostinoRBSrLarsonMGPredicting the 30-year risk of cardiovascular disease: the framingham heart studyCirculation20091193078308419506114
- D’AgostinoRBSrVasanRSPencinaMJGeneral cardiovascular risk profile for use in primary care: the Framingham Heart StudyCirculation200811774375318212285
- FraleyAETsimikasSClinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular diseaseCurr Opin Lipidol20061750250916960498
- ChenWYChenFYLeeASSesamol reduces the atherogenicity of electronegative L5 LDL in vivo and in vitroJ Nat Prod20157822523325692815
- PedrosaAMFaineLAGrossoDMElectronegative LDL induction of apoptosis in macrophages: involvement of Nrf2Biochim Biophys Acta2010180143043720005974
- KazumaSMCavalcanteMFTellesAECloning and expression of an anti-LDL(–) single-chain variable fragment, and its inhibitory effect on experimental atherosclerosisMAbs2013576377523924793
